Fig. 47.1
Schematic illustrating the multistep process involved in the development of metastasis (With permission from DeVita VT Jr., Hellman S, Rosenberg SA. Cancer: Principles and Practice of Oncology, 6th ed., Lippincott Williams and Wilkins, copyright 2001)
The first step is tumorigenesis, which occurs after the initial malignant transformation. The tumor proliferates into a small mass of heterogeneous cells that are of varying metastatic or malignant potential. These tumor cells undergo multiple and sequential genetic changes, characterized by the appearance of oncogenes and a decrease in tumor suppressor genes. As the tumor grows beyond 1 mm in diameter and becomes relatively hypoxic, angiogenesis is initiated. The process of tumor angiogenesis is tightly regulated by pro- and anti-angiogenic factors secreted by both the tumor and its environment.
As tumors successfully grow, suppressors of angiogenesis are inhibited and pro-angiogenic factors predominate, resulting in neovascularity and further growth of the tumor. Some tumors may grow by utilizing other existing blood vessels in nearby tissues.
In the next step, some cells will develop an invasive phenotype. Most researchers believe that there is a selection process resulting in the clonal expansion of certain cell subpopulations with growth advantages and invasive properties.
Malignant invasion is characterized by downregulation of cell adhesion, resulting in detachment of the cell from the primary tumor mass and the extracellular matrix. Stromal invasion is accomplished through interactions with the basement membrane, including adhesion, proteolysis, and migration, ultimately resulting in detachment and invasion through the basement membrane. This invasive phenotype also enables these cells to enter thin-walled lymphatics and vasculature, allowing access to systemic circulation.
Once inside the vascular system, cells or cell clumps (emboli) are circulated and must survive hemodynamic filtering as well as immune surveillance. They must then arrest in a distant organ. There is likely a complex interaction between the malignant cell and the endothelium or exposed basement membrane, allowing cell arrest. Once arrested in a tissue bed, the cells extravasate into the tissue, enabling formation of a metastatic focus.
These metastatic cells can become dormant or proliferate; what determines this fate is not fully understood. Growth in the distant organ after deposition is a major limiting factor in the formation of metastasis.
Recent studies have shown differences in the genetic fingerprints of matched primary tumors and their lymph node metastasis suggesting that tumors may undergo continual mutagenesis.
This finding appears to confirm that there are genes specific to tumorigenesis, invasion, angiogenesis, and other steps.
These discoveries provide a sense of the future challenge in elucidating the multiple, stepwise, and specific changes that regulate a cell’s ability to metastasize. Advances in this field will have obvious and profound implications for the treatment of cancer.
Diagnosis/Staging
Spiral CT scanning of the chest/abdomen/pelvis is a highly accurate and efficient method of detecting metastases.
PET scanning detects occult disease not seen on CT scan in 20 % of stage IV patients and should be considered if such findings might affect patient management.
Increasingly, more patients are undergoing combination CT/PET scans to evaluate both the primary and metastatic lesions as this combined modality allows for better localization of tumor deposits and can assist with operative planning as well as radiation-based therapy.
Once the extent of disease workup is complete and distant metastases have been documented, the surgeon must make three important judgments.
First is whether the patient is fit for aggressive treatment. Patients with poor performance status or serious comorbidities may not tolerate chemotherapy or major surgery.
Second is whether the primary tumor presents a clinically significant risk of bowel obstruction. Symptoms, radiographic findings, and endoscopic findings are important considerations. If the proximal colon is not dilated on radiographic studies and a colonoscope can traverse the tumor, it is generally safe to begin treatment with chemotherapy.
The third determination is whether the patient’s metastases are surgically resectable, and the patient can be treated with curative intent. If complete resection of all disease can be expected, then surgical intervention should be attempted.
Multidisciplinary Evaluation
Management of patients with advanced disease is complex, and multidisciplinary evaluation can be helpful in determining initial therapy. The multidisciplinary team or “tumor board” ideally involves a surgeon, medical oncologist, radiation oncologist, pathologist, radiologist, and gastroenterologist.
Palliative Management of the Primary Cancer: Laser, Fulguration, and Stents
Incidence and Presentation
8–29 % of patients with colorectal cancer initially present with symptoms of partial or complete bowel obstruction.
In a review of 713 obstructing carcinomas, 77 % were left-sided and 23 % were right-sided cases.
The majority of patients with obstructing colorectal carcinomas have either stage III or stage IV disease.
Acute malignant colon or rectal obstruction is an indication for emergent surgical intervention. However, these emergency operations are associated with a mortality rate of 15–34 % and a morbidity rate of 32–64 % despite advances in perioperative care.
Therefore, alternative palliative endoluminal strategies aimed at relieving obstruction have gained increasing popularity.
The initial symptoms of bowel obstruction include mild discomfort and a change in bowel habits. With disease progression and luminal narrowing, the symptoms may worsen ranging from crampy abdominal pain, abdominal distension, nausea, abdominal tenderness, and obstipation.
Leukocytosis is a concerning finding and may indicate a near or complete obstruction. Without treatment, the process can progress to complete obstruction, ischemia, and perforation. The risk of cecal perforation is greatest in patients who have a competent ileocecal valve, which does not allow decompression of the large intestine into the proximal small intestine.
In the setting of metastatic cancer, the clinician must first answer the following critical question, “is the colon or rectal obstruction a contraindication for systemic chemotherapy or radiotherapy?”
The degree of obstructive symptoms, endoscopic, and radiographic findings are key elements to consider when answering this question. If the patient has minimal symptoms, the cancer can be traversed endoscopically, and there is no radiographic evidence of high-grade obstruction and many patients with partially obstructing colon and rectal cancers will tolerate aggressive chemotherapy.
In those patients with partially obstructing rectal cancers, the addition of radiation therapy is also well tolerated and can be highly effective.
Patients must be instructed to monitor their symptoms closely and to report any signs of worsening obstruction immediately.
For patients with advanced obstruction, nonsurgical palliative options include laser therapy, fulguration, and colonic self-expanding metal stents. If less invasive endoluminal strategies are not successful in patients with nonresectable malignant obstruction of the colon and rectum, surgical creation of a palliative proximal diverting stoma or intestinal bypass should be performed.
Laser Therapy and Fulguration
Laser therapy has been utilized for palliation of obstructing rectal cancers. The immediate success rate in treating obstructive symptoms is in the range of 80–90 %.
However, laser therapy is practical only for treating cancers of the distal colon and rectum and is rarely used to treat proximal lesions. In addition, multiple sessions are often required in order to achieve lasting relief of symptoms.
Serious complications like bleeding, perforation, and severe pain have been reported in 5–15 % of patients, especially those undergoing multiple treatment sessions.
Self-Expanding Metal Stents
Since their introduction in 1991, colonic stents have become an effective method of palliation for obstruction in colorectal cancer patients, especially those with unresectable metastatic disease.
Stents can be placed in patients using minimal sedation and allow endoscopic assessment of the proximal colon. Moreover, these stents can be placed across relatively long lesions by overlapping stents in a “stent-within-stent” fashion.
A systematic review from 1990 to 2000 included 29 case series and evaluated technical and clinical success, complications, and reobstruction.
Stent insertion was attempted in 598 cases and was technically feasible in 551 (92 %) cases and clinically successful in relieving obstruction in 525 (88 %) cases.
Palliation of obstruction was achieved in 302 (90 %) of 336 cases. Stent placement as a “bridge to surgery” was successful in 223 (88 %) of 262 insertions of which 95 % had a one-stage surgical procedure.
There were three deaths (1 %). Perforation occurred in 22 cases (4 %). Stent migration was reported in 54 (1 %) of the 551 technically successful cases. Stent reobstruction occurred in 52 (10 %) of the 525 clinically successful cases and trended toward a higher incidence of reobstruction in the palliative treatment group.
There is limited data evaluating stent placement proximal to the splenic flexure. In a recent publication, colonic stenting was attempted in 97 patients with malignant large-bowel obstruction. Sixteen (17 %) patients had lesions proximal to the splenic flexure (eight ascending, eight transverse colon). Stenting was successful in relieving obstruction in 14 (88 %) of these patients.
Complications reported in the literature for colonic and rectal stents include stent malpositioning, stent migration, tumor ingrowth (through the stent interstices), tumor overgrowth (beyond the ends of a stent), perforation, stool impaction, bleeding, tenesmus, and postprocedure pain. Stenting of cancers in the mid to low rectum may result in urgency, pain, and incontinence. While the complications associated with stents and other less invasive endoluminal strategies should not be taken lightly, one must keep in mind that emergency operations for malignant colon and rectal obstruction have a mortality rate of 15–34 % and a morbidity rate of 32–64 %.
Surgical Management of the Primary Cancer: To Resect or Not to Resect?
The role of surgical resection of the primary colon or rectal cancer in patients with unresectable metastases is controversial, and no randomized controlled trials have demonstrated a survival benefit for bowel resection in stage IV patients.
Randomized trials of 5FU-based chemotherapy vs. best supportive care, conducted in the 1990s, have shown that stage IV patients receiving systemic chemotherapy have increased length and quality of life.
At this time, standard management for patients with unresectable metastatic colorectal cancer is systemic chemotherapy, at least initially.
The proper use of elective colon and rectal resections in nonobstructed patients is a source of continuing debate. Loss of performance status, risk of surgical complications, and delay in chemotherapy are potential downsides to palliative surgical resection.
On the other hand, elective operations have a far lower morbidity than emergency surgery. In addition, there are increased risks and potential complications associated with operations performed on patients who develop large-bowel obstruction while receiving chemotherapy or who present with more advanced disease after multiple cycles of ineffective chemotherapy.
From the limited data in the literature, it is clear that initial colon resection is frequently practiced, particularly for patients with colon primaries and with less extensive metastatic disease. However, it is difficult to assess the impact of colon and rectal resection on symptom control, tolerance to subsequent chemotherapy, quality of life, or survival from these studies.
A recent meta-analysis evaluating patients with stage IV colorectal cancer treated with chemotherapy combined with and without surgical resection revealed prolonged survival in patients undergoing palliative surgical resection and chemotherapy when compared to chemotherapy alone. Chemotherapy regimens included 5-flourouracil, oxaliplatin, and irinotecan.
Eight retrospective studies with a sum total of 1,062 patients met the inclusion criteria for this study. The median survival for palliative surgical resection combined with chemotherapy ranged from 14 to 22 months (data extracted from studies with 100 % patient participation in systemic chemotherapy). The median survival for chemotherapy alone was 6–15 months.
The estimated standardized median difference in survival was 6.0 months in favor of palliative surgical resection (standardized difference 0.55; 95 % CI 0.29, 0.82; p < 0.001). In addition, patients managed with chemotherapy alone were more likely to experience a complication related to the primary tumor (95 % CI 1.7, 34.4; p = 0.008). There was no difference in the incidence of metastatic disease tumor burden becoming more favorable and amenable to curative resection after systemic chemotherapy in either group (0.85; 95 % CI 0.40, 1.8; p = 0.662).
There are obvious limitations of this meta-analysis given the retrospective nature of the studies available for review as well as the chemotherapy regimens utilized in these studies not being equivalent to current regimens.
There is little published data evaluating the effectiveness of radiotherapy in palliative management of stage IV rectal cancer. Crane and colleagues reported 55 patients who received chemoradiotherapy and 25 patients who received chemoradiotherapy followed by surgery. The majority of both groups received systemic therapy (78 % of patients).
Pelvic symptom control was high (81 %) in the chemoradiotherapy group but not as high as in the chemoradiotherapy combined with surgical resection group (91 %). There was limited data on the durability of symptom control over time.
To summarize the treatment options for stage IV patients with unresectable metastases, treatment algorithms are shown for patients with stage IV colon cancer (Fig. 47.2) and stage IV rectal cancer (Fig. 47.3). The algorithms show multiple treatment options, reflecting the heterogeneity of disease presentation. The major variables to consider are location of the primary tumor, degree of colon and/or rectal obstruction, extent of metastatic disease, and fitness of the patient for surgery.
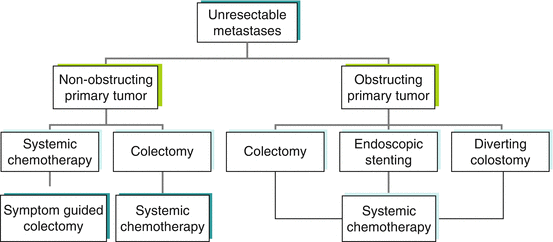
Fig. 47.2
Treatment algorithm for patients with stage IV colon cancer: use of palliative colon resection
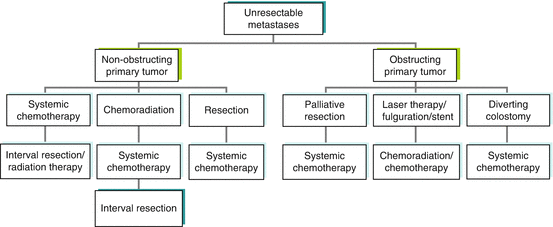
Fig. 47.3
Treatment algorithm for patients with stage IV rectal cancer: use of palliative rectal resection
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






