Colorectal Cancer: Anatomy and Staging
Ian D. Chin
Bogdan C. Paun
To treat colon and rectal cancer, some basic essential knowledge is necessary. Detailed anatomy, embryology, and histology enable surgeons and oncologists to treat patients effectively and research new therapies. The use of modern technologies allows clinicians to accurately stage patients with colorectal cancers and to focus appropriate therapies to increase survival and reduce recurrence. Staging systems help researchers communicate effectively to compare various experimental studies to provide summary analyses.
Embryology, Anatomy, and Histology of the Colon and Rectum
Knowledge of anatomy of the colon and rectum is vitally important not only for consideration of surgical technique but also for the understanding of the patterns of spread and behavior of cancer. We therefore include in this chapter a brief overview of the colon and rectal anatomy, and the reader is well advised to search more comprehensive sources for additional information (1,2,3). Short sections on embryology and histology are also included.
Embryology
By the third week of development, the primitive gut is formed from the endoderm of the yolk sac. It can be divided into the foregut, midgut, and hindgut. After the fourth week, the midgut expands faster than the abdominal cavity. The midgut is pushed into the umbilical cord as a herniated segment that extends from about the distal third of the duodenum to the proximal one-third of the transverse colon (4). This whole segment is supplied by the superior mesenteric artery, which initially forms a 90-degree axis of counterclockwise rotation. During the 10th week of development, the midgut returns to the abdomen, proximal loop first, with a further 180 degrees of counterclockwise rotation. The proximal loop passes below the mesentery of the distal loop (transverse colon). The cecum starts developing as a local enlargement in the loop posterior to the superior mesenteric artery during the period of gut herniation. The distal colon (i.e., distal one-third of transverse colon, descending colon, sigmoid colon, and rectum) is derived from the hindgut and, therefore, receives its blood supply from the inferior mesenteric artery.
The proximal anal canal, together with the rectum, is formed by the hindgut and is of endodermal origin. The distal anal canal is formed from an ingrowth of the anal pit, which is of ectodermal origin. For anatomists, the dentate line is the border between the anus and the rectum. For surgeons, the top of the muscular anal canal (anorectal ring) separates the anus from the rectum (1).
Anatomy of the Large Intestine
The large intestine extends from the terminal ileum to the dentate line in the anal canal with a length that is variable and is reported to be between 1.3 and 1.8 m (1). The diameter, which can vary with distension, gradually decreases from 7.5 cm in the cecum to 2.5 cm in the sigmoid, to expand again in the rectal vault. The colon has both inner circular and outer longitudinal muscle fibers, but some of the longitudinal fibers coalesce in three bands called the teniae coli separated by 120 degrees along the circumference. The teniae are not only thicker, but they are shorter than the intervening colonic wall. This gives rise to sacculations called haustrae separated by invaginations of the colonic wall called plicae semilunares. The plicae go partway along the colonic circumference and give it its characteristic radiologic appearance. The colon also has small fat appendages on the serosal side called appendices epiploicae.
The large bowel may be divided into several sections: cecum, ascending colon, transverse colon, descending colon, sigmoid colon, and rectum. The cecum has the largest diameter and thinnest wall. It may be quite free and completely covered with peritoneum to migrate into the pelvis, or it may be fixed to the posterior abdominal wall or iliac fossa. The appendix is a long diverticulum originating on the posterior-medial aspect of the cecum at the confluence of the three teniae. At the junction of the cecum and the ascending colon, the ileocecal valve is a protrusion of ileum in the medial aspect of the large bowel with a circular muscle layer that gives it a papillary appearance. The ascending colon is usually retroperitoneal (covered with peritoneum on its anterior and lateral surface but devoid of it posteriorly) and extends to the hepatic flexure. The hepatic flexure lies between the right lobe of the liver and the gallbladder anteriorly and the right kidney and duodenum posteriorly. The transverse colon extends from the hepatic flexure to the splenic flexure, at which points it is relatively fixed. It is invested with peritoneum and may hang in a V shape to below the umbilicus. It is adherent on its anterior surface with the greater omentum. The descending colon starts at the high splenic flexure and courses as a retroperitoneal structure to the pelvic brim. The sigmoid colon is a free intraperitoneal section of large bowel of variable length attached by its mesentery to the left pelvic wall and continues as the rectum where teniae coli coalesce.
The location of the rectosigmoid junction is controversial but is probably not as important for cancer management as the distance of the cancer from the anal canal. The proximal third of the rectum is entirely covered by peritoneum, with more fat
interposing itself between the rectum and peritoneum as the rectum goes down in the pelvis. The middle third of the rectum is covered by peritoneum anteriorly. The lowermost portion of the rectum is entirely extraperitoneal. The rectum does not have a mesentery per se, but the term “mesorectum” has gained widespread use as the name for the fat surrounding the rectum containing vessels and lymphatics. It is thicker posteriorly and surrounded by a layer of endopelvic fascia. Anteriorly, the mesorectum has a fascia propria that is separate from the Denonvillier fascia covering the posterior surface of the prostate, seminal vesicles and neck of the bladder; this plane between the fascia propria and Denonvillier fascia is the anatomic dissection plane of mesorectal excision (5). Posteriorly, the mesorectum is again covered by a fascia propria that is in close apposition, but separate from, the presacral fascia (6). Laterally, the mesorectal fascia is associated with the pelvic sidewall. Older textbooks suggest that there are lateral ligaments containing the middle rectal vessels. Total mesorectal excision shows that these ligaments are really loose connective tissue, and the middle rectal vessels are usually small and close to the pelvic floor (7). In fact, the mesorectum is circumferentially enclosed in a sheath of fascia propria. When dissection is carried along this layer, the veins and nerves in the pelvis may be preserved (8).
interposing itself between the rectum and peritoneum as the rectum goes down in the pelvis. The middle third of the rectum is covered by peritoneum anteriorly. The lowermost portion of the rectum is entirely extraperitoneal. The rectum does not have a mesentery per se, but the term “mesorectum” has gained widespread use as the name for the fat surrounding the rectum containing vessels and lymphatics. It is thicker posteriorly and surrounded by a layer of endopelvic fascia. Anteriorly, the mesorectum has a fascia propria that is separate from the Denonvillier fascia covering the posterior surface of the prostate, seminal vesicles and neck of the bladder; this plane between the fascia propria and Denonvillier fascia is the anatomic dissection plane of mesorectal excision (5). Posteriorly, the mesorectum is again covered by a fascia propria that is in close apposition, but separate from, the presacral fascia (6). Laterally, the mesorectal fascia is associated with the pelvic sidewall. Older textbooks suggest that there are lateral ligaments containing the middle rectal vessels. Total mesorectal excision shows that these ligaments are really loose connective tissue, and the middle rectal vessels are usually small and close to the pelvic floor (7). In fact, the mesorectum is circumferentially enclosed in a sheath of fascia propria. When dissection is carried along this layer, the veins and nerves in the pelvis may be preserved (8).
Vascular Supply and Lymphatics
The superior mesenteric artery supplies constant blood flow to the cecum and ascending colon through the ileocolic artery. The right colic artery is variable and even absent in 30% of people (9). The transverse colon is supplied through branches of the middle colic artery (Fig. 42.1). The first branch of the inferior mesenteric artery, the left colic artery, supplies the descending colon and may form several anastomosing arches at the splenic flexure with branches of the superior mesenteric artery that are often grouped under the name of arc of Riolan. The inferior mesenteric artery also gives rise to the sigmoid artery (or arteries) that supplies the sigmoid colon and to the superior rectal artery that supplies proximal rectum (10). The rectum is also supplied by the middle rectal arteries that are small and inconstant (11), and by the inferior rectal arteries that are branches of the pudendal artery. The arteries supplying the colon form vascular arcades that anastomose and give rise to a continuous marginal artery (of Drummond). The venous drainage follows the general pattern of the arteries, such that on the right (cecum, ascending, and transverse colons) the veins join to form the superior mesenteric vein that drains in the portal vein. On the left (descending and sigmoid colons and upper rectum), the veins join to form the inferior mesenteric vein that drains into either the superior mesenteric vein or the splenic vein. The lower rectum drains into the internal iliac veins.
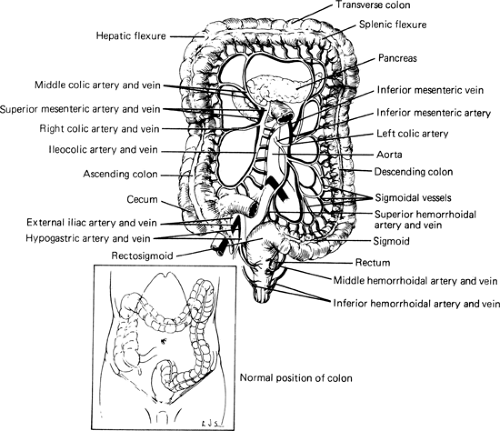 FIGURE 42.1. The vascular supply of the large bowel. The transverse colon is reflected upward. Inset shows normal position of colon within abdominal cavity. Source: From ref. 13. |
Lymph nodes are classified as epicolic (subserosal), paracolic (close to the marginal artery), intermediate (along the main arteries), and principal (root of mesentery and aortic). They usually course along with the arteries and do not form additional communications. For a good oncologic resection, it is important to include all lymphatics that may be involved by cancer, so the principle is to remove an entire section of large bowel supplied by a large, named artery with its mesentery (9).
The lymphatic drainage of the anorectal region is more complicated because of its triple vascular supply (12). The superior rectal lymph nodes first drain most of the proximal rectum to an intermediate group at the bifurcation of the superior rectal artery and then to periaortic lymph nodes. The middle rectal nodes drain to the internal iliac chains, and some inferior nodes drain through the levator ani muscle to the internal pudendal nodes. The anal canal below the dentate line is drained exclusively to the inguinal lymph nodes, while the anal canal above has dual drainage to both the inguinal lymph nodes and the internal iliac nodes.
The lymphatic drainage of the anorectal region is more complicated because of its triple vascular supply (12). The superior rectal lymph nodes first drain most of the proximal rectum to an intermediate group at the bifurcation of the superior rectal artery and then to periaortic lymph nodes. The middle rectal nodes drain to the internal iliac chains, and some inferior nodes drain through the levator ani muscle to the internal pudendal nodes. The anal canal below the dentate line is drained exclusively to the inguinal lymph nodes, while the anal canal above has dual drainage to both the inguinal lymph nodes and the internal iliac nodes.
Histology
The large bowel has the same layers as the rest of the gut: serosa, muscularis propria, submucosa, and mucosa (14). The visceral peritoneum functions as the serosa of the large bowel and is present only on the sections that are not retroperitoneal. There is some loose connective tissue and fat between the outer serosa and the inner muscularis propria, which has an outer longitudinal muscle layer (forming the teniae) and an inner circular muscle layer. The submucosa is another layer of loose connective tissue containing veins, lymphatics, and small arteries. The mucosa has an inner muscularis mucosa that has a thin layer of inner circular and outer longitudinal muscle fibers, and an epithelial lining consisting of simple columnar epithelium with interspersed goblet cells. Unlike the small bowel, the large bowel does not have villi, but has an endothelium, composed of columnar cells, that is flat with crypts.
Staging of Colon and Rectal Cancers
Currently, colorectal cancer (CRC) is staged primarily by pathological assessment of the resected specimen (15). Staging the cancer provides information as to the likely behavior of the cancer and will, therefore, guide further management. Staging also provides a common language in cancer research and is essential to the advancement of knowledge in oncology. Numerous predictors have been found for CRC, including local behavior of the tumor, surgical adequacy, and molecular characteristics of the tumor, but these have not been incorporated into the mainstream staging systems or used as standard guides for therapeutic intervention. Several imaging modalities are used to estimate the pathological stage of CRC preoperatively, which include computed tomography (CT), magnetic resonance imaging (MRI), and endorectal ultrasound (ERUS). The development of several neoadjuvant therapies, especially for rectal cancer, has provided a great impetus to improving and introducing these imaging modalities into widespread use (16). The introduction of novel imaging techniques, such as positron emission tomography (PET), or novel predictors of outcome, such as molecular markers, may revolutionize colon cancer care and improve outcome.
Pathological Staging
The first staging system, based on depth of penetration, was reported in 1926 by Lockhart-Mummery. He followed 200 consecutive patients with rectal cancer and reported survival data for three classes (A, B, or C) (17). At the Mayo Clinic, Rankin and Broders believed that the local spread of rectal cancer was less important as an outcome measure in nonmetastatic disease than the microscopic behavior of the cancer cells and proposed a staging system based on the grade (1,2,3,4) of tumor (18). In 1932, Cuthbert Dukes, at St. Mark’s Hospital in London, suggested an improvement on the Lockhart-Mummery staging system (19) and defined rectal cancers as Dukes A if confined to the rectal wall, Dukes B if spread by direct extension to extrarectal tissue, and Dukes C if the regional lymph nodes were involved. He reported a 3-year survival of 80%, 73%, and 97% for the Dukes A, B, and C stages, respectively. Dukes also looked at the grades of cancers according to Broders’ method and indeed found a correlation between higher grades and decreased survival. The majority of his patients were in the intermediate-grade 2 category, and there was a relationship between his stages and the grades of the cancers; therefore, Dukes concluded that grading alone would be a less useful method of staging. Later, the group at Mayo accepted the principle of the Dukes system but insisted that the grade of tumor be added for prognostic accuracy and be used as well (20).
Several modifications of the Dukes staging followed, including that of Dukes himself in 1935 that came after more detailed study of the lymphatic spread of the cancer (21). They reported on 100 cases, 62 of which were C cases, and included 24 beautiful drawings of the lymphatic spread in abdominoperineal specimens. It was found that the cancers spread to the lymph nodes in an orderly and predictable manner first to the perirectal nodes and then to the nodes higher up on the superior rectal artery, without downward or lateral spread, unless all lymph channels were blocked. They suggested that cases with involved lymph nodes close to the bowel wall should be designated C1, and those with nodes higher up on the rectal artery be C2. In 1949, Kirklin, Dockerty, and Waugh (22) modified the Dukes staging as follows:
| A | Cancer limited to mucosa |
| B1 | Cancer into, but not through, muscularis propria |
| B2 | Cancer through muscularis propria |
| C | Any depth with lymph node involvement and showed penetration of the peritoneal reflection did not impact outcome |
Astler and Coller took it one step further and proposed another modification to the Dukes staging, which is still in widespread use today, by further subdividing the C stage into C1—limited to bowel wall with positive nodes and C2—through muscularis propria with positive nodes (23). Furthermore, they applied this system to both colon and rectal carcinomas and showed a gradually decreasing 5-year survival from 100% to 22% for stage A to C2, respectively.
A clinicopathological system of staging (A—tumor confined to bowel wall, B—tumor extension to pericolic fat, C—lymph node involvement, and D—cancers with liver, lung, bone, or peritoneal metastases, or cancers that were not resectable because of parietal or adjacent organ involvement) was suggested in 1967 in a paper advocating the no-touch isolation technique of colon cancer resection (24). This was validated and compared to the old Dukes and Astler-Coller modification to show that it more closely correlated with survival (25).
Tumor, Node, Metastasis Staging
The numerous ABC staging systems and their modifications became confusing and were slowly replaced with the tumor, node, metastasis (TNM) staging system. It is quite complex and is constantly being updated, which makes for both its greatest strength and its greatest disadvantage. The initial TNM system was established in the 1940s by Dr. Pierre Denoix and then developed under the auspices of the Union Internacional Contra la Cancrum (26). The American Joint Committee on Cancer (AJCC) was established in 1959 and started publishing separate definitions for the TNM classes. The two systems were unified in 1987, and the latest iteration of classifications was published in 2002 (15).
Table 42.1 Tumor, Node, Metastasis Classification for Adenocarcinoma of the Colon or Rectum | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The TNM system may be used for both clinical and pathological staging, although the latter may be designated by the prefix p. It classifies tumor according to the variables of tumor invasion, lymph node involvement, and distal metastasis, but although it mentions other variables such as grade, resection margin, lymphovascular invasion, and molecular markers, it does not include them in the staging system (Table 42.1).
Surgical Resection Margins
The completeness of surgical extirpation of colon and rectal cancer is of utmost importance in effecting a cure. This is assessed by examining the margins of the resected specimen; the resection is classified as Ro if it is clear, R1 if there is microscopic evidence of tumor cells at the margin, and R2 if there is gross evidence of tumor at the margin. The proximal resection margin of colon and rectal cancers is never really of concern during surgical excision. Distally, the margin is also generous for colon cancers but becomes of great concern when dealing with rectal cancers because sphincter preservation is an important goal.
Initially, abdominal perineal resection (APR) was widely practiced. As surgical techniques improved, more sphincter-saving procedures were used, and the caveat of a 5-cm distal resection margin was employed (27). Research and technical advancements made possible low anastomoses and allowed sphincter-saving resections of rectal cancers very close to the anus with <5-cm margins. This renewed interest in the area and the original observations of Dukes (21) that rectal cancers rarely spread distally were confirmed (28) by showing advanced cancers that block the proximal lymphatics will allow distal lymphatic spread. This was confirmed by examining resected APR specimens and measuring the distal histologic spread of cancer cells. The cancer-specific survival did not seem to be adversely affected by distal margins of 2 cm (29) or even 1 cm (30). However, decreased survival and increased recurrence rates were found when the distal margin was <0.8 cm. Patients with advanced rectal cancers that had undergone preoperative chemoradiation also did not seem to benefit from a distal margin >1 cm (31). These findings were confirmed in a group of 270 patients in whom a margin of <1 cm was not an adverse prognostic factor (32). Some groups can get even closer to the anal canal by removing the internal sphincter en
bloc with the tumor (33,34). However, opinion is not uniform, and some studies suggest longer mural distal margin and longer distal mesorectal margin (3 [35] or 4 [36] cm).
bloc with the tumor (33,34). However, opinion is not uniform, and some studies suggest longer mural distal margin and longer distal mesorectal margin (3 [35] or 4 [36] cm).
Historically, only the proximal and distal margins of cancers were reported. Faced with variable and high rates of local recurrence, several studies started reporting on the circumferential margin (37,38,39). A positive circumferential margin is defined as tumor within 1 mm of the cut surface, and it was found to predict much higher rates of local recurrence and poorer survival rates. Not all cases of local recurrence occurred in patients with positive circumferential margin, and reports of discontinuous tumor spread in the mesorectum seemed to be the explanation (40). Based on this observation, Heald and Ryall introduced the concept of total mesorectal excision (TME) for rectal cancer (the entire mesorectum is removed within its fascial envelope) and showed the extremely low local recurrence rate of 3.7% at 5 years using this technique (41). Using TME, all lymphatic-bearing tissue around the rectum is removed, and only the most advanced cancers involve the circumferential margin. Therefore, a positive margin is a predictor of poor overall outcome rather than local recurrence (42). Involvement of the radial margin continues to be a poor predictor of outcome in patients that receive postoperative radiation (43) or preoperative chemoradiation (44).
Lymph Node Metastases
Presence of lymph node metastases in CRC is an important predictor of outcome as recognized by the TNM classification. There is an inverse relationship between the number of involved lymph nodes and the 5-year survival. The best single dichotomization is obtained by dividing patients into three or less and four or more lymph nodes (45). Furthermore, lymph node metastases in the pathological specimen are the main determinants of the need for postoperative chemotherapy. The number of lymph nodes that are retrieved from the specimen is highly variable, depending on the surgical technique and on the method of handling the specimen. This affects the sensitivity of detection of metastases. By using careful pathological dissection, more lymph nodes may be retrieved, upstaging the tumor, allowing the benefit of chemotherapy, and improving outcome (46). Traditional dissection in the gross lab, usually done with a pair of scissors by sight and palpation, is the easiest, inexpensive, and most commonly employed method. Some groups have good results with this method (47), although in usual clinical practice the results are less than ideal (48). More elaborate lymph node retrieval techniques, such as fat clearance, are expensive and time consuming, but invariably obtain more lymph nodes and may upstage the tumor (49). Most laboratories cannot routinely employ these techniques. A minimum number of examined lymph nodes retrieved to accurately stage the tumor needs to be employed; the latest edition of the AJCC Cancer Staging Manual recommends that 7 to 14 nodes need to be recovered, which is a change from the 12-node requirement of the previous edition.
Two new techniques have been developed to improve the lymph node metastasis detection rate: ultrastaging and sentinel lymph node (SLN) mapping. Usually, lymph nodes are embedded after being retrieved and several sections are assessed on hematoxylin and eosin (H&E) staining. Using more specialized staining techniques (ultrastaging), small nests of cancer cells (micrometastases) that would not otherwise be detected on standard H&E staining can be seen. The significance of these micrometastases is difficult to determine because the literature in this area is still immature. Several studies have examined negative H&E-stained lymph nodes using an ultrastaging method to subclassify cancers into separate groups with different survival rates. Immunohistochemical staining for carcinoembryonic antigen (CEA) (50) and anticytokeratin (51) failed to show a difference. However, using a CEA-specific nested reverse-transcriptase polymerase chain reaction (RT-PCR) resulted in a 40% 5-year survival difference (52). Another study, using immunohistochemical staining for CEA and CK20, also showed a 5-year survival difference (53).
Another technique used to reduce the sampling error for lymph nodes is SLN mapping, which is based on the experience of melanoma and breast cancer (54). This technique commonly uses isosulfan blue dye injected around the tumor in vivo to detect the first lymph nodes draining the tumor bed within 10 to 15 minutes. Because these nodes are few and most likely to harbor cancer cells, they can be serially sectioned (every 20–40 μm) and studied with H&E, immunohistochemical staining, or RT-PCR technique, which would improve diagnostic accuracy. SLN mapping has been shown to be feasible, without greatly adding to operating time or adding to morbidity (55), but the technique suffers from an unclear definition for positive nodes (on H&E staining or ultrastaging) and from a false-negative rate of 4% to 5% (56) (on ultrastaging). Some of the results of SLN mapping are good, with 32% of colon and 17% of rectal cancers being upstaged (57). It is unclear at this point whether decisions about adjuvant chemotherapy may be made based on ultrastaging, SLN mapping, or a combination of the two.
Other Prognostic Factors
As mentioned previously, the tumor grade can be used as an indicator of future metastases and survival (18,58). Although there may be a problem with interobserver variability, the terms “low grade” and “high grade” may be used instead of the four classes of Broders to reduce reporting variability. The grading of rectal cancer may be used to judge the adequacy of local excision (i.e., high-grade cancer needing further surgery) (59,60) because it predicts probability of lymph node metastasis.
Invasion of nerves and lymphatic or blood vessels has been associated with a poor prognosis. Neural invasion seems to be associated with increased local recurrence (61,62) and decreased survival (63). It is often difficult to differentiate between lymphatic and venous channels because invasion in both cases appears as cancer cells within endothelial-lined spaces. So, they are described together as lymphovascular invasion (64). Lymphovascular invasion is associated with increased local recurrence (59,62), lymph node metastases (65) and distant recurrence (61), and decreased survival (63,66). Presence of lymphovascular invasion decreases the survival of node-negative disease to almost the survival of node-positive disease in rectal cancer.
Molecular Markers
Numerous tumor markers have been studied extensively in an effort to improve our prognostic ability and better target therapy. Although many are promising, none have really entered into common clinical practice or have yet been proven effective as part of an adjuvant therapy trial. Nevertheless, it is important to have some general knowledge of the advances made.
p53 is a tumor-suppressor gene on chromosome 17 that undergoes mutation commonly in CRC (∼50%). Loss of the function of p53 interferes with DNA repair and apoptosis, leading to cell proliferation and genomic instability. The mutated gene has a longer half-life and can be detected by immunohistochemistry. Nuclear overexpression of p53 actually indicates mutation in the gene. Some studies have shown that p53 overexpression leads to decreased survival (67,68,69,70), whereas others have not shown such an effect (71,72). p53 overexpression may be associated with tumor sensitivity to radiation (73), although not consistently (74), and may be used as an indication
for chemotherapy for stage II CRC (75). K-ras is an oncogene that encodes for a small protein believed to be involved in transduction of extracellular mitogenic signals. Mutations in this gene are found less commonly (∼25%–30%) in CRCs than p53 mutations. Detection of K-ras mutation may improve the prognostic accuracy of detection of p53 mutations (69,76). Vascular endothelial growth factor (VEGF) is associated with angiogenesis, and its presence in stage II CRC is associated with increased recurrence (77). Indeed, a monoclonal antibody against VEGF, bevacizumab, has now been shown to be effective in metastatic colon cancer (78). Thymidylate synthase (TS) is an enzyme that catalyzes the methylation of dUMP to dTMP and is a critical target for 5-fluorouracil. CRCs that overexpress this enzyme tend to be resistant to 5-fluorouracil–based chemotherapy (79). Patients with stage III CRC that have increased polymorphism of the TS gene have worse survival (80). Similarly, patients with lymph node metastases that have high levels of TS expression also have a worse survival when receiving chemotherapy (81). Multiple other molecular markers have been associated with decreased survival in CRC, including cyclooxygenase-2 (82) and cyclin A (83).
for chemotherapy for stage II CRC (75). K-ras is an oncogene that encodes for a small protein believed to be involved in transduction of extracellular mitogenic signals. Mutations in this gene are found less commonly (∼25%–30%) in CRCs than p53 mutations. Detection of K-ras mutation may improve the prognostic accuracy of detection of p53 mutations (69,76). Vascular endothelial growth factor (VEGF) is associated with angiogenesis, and its presence in stage II CRC is associated with increased recurrence (77). Indeed, a monoclonal antibody against VEGF, bevacizumab, has now been shown to be effective in metastatic colon cancer (78). Thymidylate synthase (TS) is an enzyme that catalyzes the methylation of dUMP to dTMP and is a critical target for 5-fluorouracil. CRCs that overexpress this enzyme tend to be resistant to 5-fluorouracil–based chemotherapy (79). Patients with stage III CRC that have increased polymorphism of the TS gene have worse survival (80). Similarly, patients with lymph node metastases that have high levels of TS expression also have a worse survival when receiving chemotherapy (81). Multiple other molecular markers have been associated with decreased survival in CRC, including cyclooxygenase-2 (82) and cyclin A (83).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








