Eosinophilic
Allergic proctocolitis
Parasitic infection
Ischemic
Necrotizing enterocolitis
Henoch-Schonlein purpura
Hemolytic-uremic syndrome
Hirschsprung-associated enterocolitis (late)
Neutropenic colitis
Acute
Infections
Hirschsprung-associated enterocolitis (early)
Pseudomembranous
Antibiotic associated (C. difficile)
Other infections
Hirschsprung-associated enterocolitis
Hemolytic-uremic syndrome
Chronic
Ulcerative colitis
Crohn’s disease
Colitis associated with immunodeficiencies
Pouchitis
Diversion colitis
Lymphocytic epithelial infiltration
Lymphocytic colitis
Colitis associated with gluten sensitivity
Colitis associated with immunodeficiencies
Colitis associated with medication (NSAIDs)
Autoimmune enterocolitis
Graft versus host disease
Granulomatous
Crohn’s disease
Infections mycobacteria
Yersinia
Chronic granulomatous disease
In the infant, important entities include “allergic” colitis, Hirschsprung-associated enterocolitis, chronic or clinically undiagnosed infections, and necrotizing enterocolitis. In the older child, the major consideration is chronic inflammatory bowel disease (IBD), though the differential diagnosis includes those infectious etiologies which may produce a confounding clinical-pathological picture, entities which are the result of treatment (diversion colitis, pouchitis), and rarer diseases, such as “microscopic” colitis, which are occasionally encountered by the pathologist. The emphasis of this chapter is primarily on colonic disease, as those disorders involving predominantly the small bowel and characterized by chronic diarrhea and malabsorption are discussed in Chap. 4 in this book.
6.2 Major Histologic Patterns Noted in Colonic Specimens
Active colitis refers to the presence of neutrophils either in the lamina propria, in the crypt epithelium (cryptitis), or within the lumen, forming small abscesses (crypt abscesses). Neutrophils confined to the lumen of mucosal vessels are not considered part of the process of active colitis. A predominantly neutrophilic infiltrate without significant architectural changes (see below) is generally a feature of diseases with an acute course, such as infections and drug reactions. Neutrophils in these cases are frequently confined to the superficial portion of the mucosa and may be associated with small erosions or ulcers.
The features of chronic colitis, the histologic hallmark of chronic inflammatory bowel disease, are based on the recognition of changes in mucosal architecture, such as a “villiform” aspect of the surface epithelium, crypt destruction and atrophy, and shortening of the crypts with irregular branching and outline resulting in loss of their regular “test tubes in a rack” appearance. Shortening of the crypts is most often due to the presence of a basally situated chronic inflammatory infiltrate (basal plasmacytosis) which separates the base of the crypts from the muscularis mucosae. Though a chronic inflammatory infiltrate is an integral part of the process, it is the least useful histologic parameter given the wide range in numbers of lymphocytes and plasma cells in normal specimens. Chronic active colitis refers to the presence of a neutrophilic infiltrate superimposed on the above changes and is usually seen during exacerbations of IBD. Other changes seen in chronic colitis include “pyloric” and Paneth cell metaplasia. It should be noted that Paneth cells may be normally present as far as the descending colon in children.
Pseudomembranous colitis has a distinctive endoscopic and histologic appearance, referring to exudates of mucin and neutrophils coating superficially necrotic crypts, resembling erupting volcanoes. This pattern is characteristic of Clostridium difficile infections but may also be seen as a result of infection with other organisms, such as E. coli 0157:H7, and in other entities, such as Hirschsprung-associated enterocolitis (vide infra).
Eosinophilic colitis refers to a patchy or diffuse infiltrate dominated by eosinophils, usually with infiltration of the crypt or surface epithelium. Wide variations in the number of eosinophils in the normal colonic mucosa are due to differences in specimen site (greater numbers of eosinophils in the cecum as opposed to the rectum), age, and geography (Pascal et al. 1997; Lowichik and Weinberg 1996; DeBrosse et al. 2006). In infants, the main consideration is milk allergy; parasitic infection and chronic inflammatory bowel disease are also possibilities (see also Chap. 4).
6.2.1 Non-Disease-Related Artifacts (Fig. 6.1)
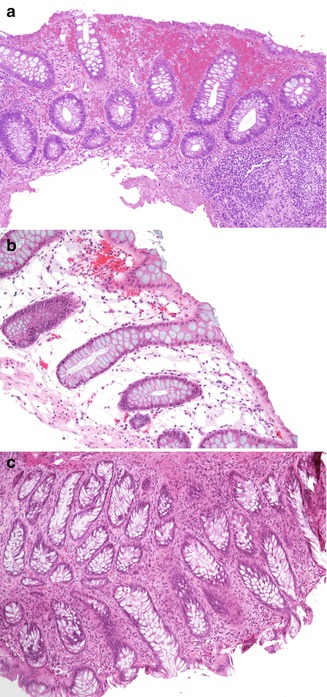
Fig. 6.1
Non-disease-related artifacts. (a) Enema effect: superficial mucosal hemorrhage and epithelial degeneration with focal goblet cell loss {hematoxylin-eosin (H + E) ×100}. (b) Pseudolipomatosis coli: air artificially introduced into the mucosa during endoscopy may suggest dilated lymphatics or excessive fat (H + E ×20). (c) Cautery artifact: focal loss of the epithelial surface and brushlike elongation of the nuclei (H + E ×20)
Non-disease-related alterations in the colonic mucosa may be induced by certain medications used in bowel preparation or by the procedure itself. For example, soap suds enemas may result in hyperemia and edema of the bowel, especially noted on endoscopy (Withers and Scott 2000). Oral sodium phosphate solutions (oral fleet enema; C.B. Fleet Co, Inc., Lynchburg, VA) may cause aphthoid-like erosions endoscopically similar to Crohn’s disease (Zwas et al. 1996). Available literature suggests that polyethylene glycol, another commonly used colonic cleansing agent for colonoscopy, is less frequently associated with mucosal abnormalities (Zwas et al. 1996). Hydrogen peroxide enemas have been associated with acute hemorrhagic colitis and with pseudolipomatosis, resulting from artificial introduction of gas into the mucosa (Withers and Scott 2000). Hypertonic saline, sodium phosphate, and bisacodyl enemas can cause mucin depletion, focal disruption of surface epithelium, mild acute inflammation, and edema, which usually resolve within 1 week (Leriche et al. 1978). Cleansing solutions used to disinfect endoscopes may produce adherent mucosal plaques, mucosal vacuolar changes, congestion, and hemorrhage (Jonas et al. 1988; Ryan and Potter 1995).
6.3 Allergic (Dietary Protein) Colitis and/or Proctitis
6.3.1 Clinical Features and Natural History
A more complete discussion of allergic enteropathy is to be found in Chap. 4, and the focus of this chapter is essentially limited to its colonic manifestations. Food allergy is believed to affect 2–8 % of children under 2 years of age (Sicherer 2000). The most common dietary proteins responsible for food allergy are cow’s milk and soy protein. In general, allergic disorders affecting the gut are non-IgE mediated (Sicherer 2000). Allergic proctitis/colitis is a disease of infants, presenting during the first 2 months of life with rectal bleeding shortly after the introduction of cow’s milk or soy protein formulae. It also commonly occurs in breast-fed infants, in whom cow’s milk protein ingested by the mother is the triggering agent in breast milk (Maloney and Nowak-Wegrzyn 2007). There is evidence that minute amounts of substances in breast milk may act as allergens in the infant (de Boissieu et al. 1997). Growth is usually not affected, and peripheral eosinophilia is variable. Exclusion of other causes of rectal bleeding, such as infection, necrotizing enterocolitis, intussusception, and anal fissure, is essential. Biopsies may be performed to rule out Hirschsprung disease in those infants presenting primarily with constipation. Endoscopic features include erythema, mucosal friability and focal erosions, and nodularity, the latter suggestive of nodular lymphoid hyperplasia (Fig. 6.2a). Disease is usually limited to the distal colon (Lake 2000). Eosinophils may be observed in stool smears, which should also be obtained to detect parasitic infection. The condition is temporary, with most patients able to tolerate milk after 2 years of age.
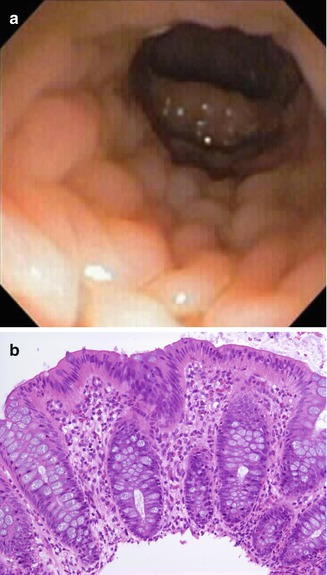

Fig. 6.2
An 18-day-old female with bloody diarrhea due to milk allergy. (a) Nodularity is observed by endoscopy in the distal colon. (b) The biopsy features increased number of eosinophils, frequently clustered, with infiltration of surface and crypt epithelium (H + E ×100)
6.3.2 Pathology
The most characteristic biopsy findings are acute inflammation featuring increased numbers of eosinophils, usually with neutrophils, in all compartments of the mucosa, frequently infiltrating crypts (Fig. 6.2b). The mucosal architecture is well preserved, and significant crypt architectural distortion is distinctly unusual and, if present, should suggest other disorders. Nodular lymphoid hyperplasia is a frequent concomitant finding. Increased eosinophil count to >20/HPF, peripheral blood eosinophilia, and low serum albumen are the usual diagnostic features (Machida et al. 1994). Eosinophil numbers may also vary as a result of dietary withdrawal therapies empirically attempted prior to obtaining the biopsies.
The differential diagnosis includes parasitic infections, such as Dientamoeba fragilis, which can be identified in stool smears (Cuffari et al. 1998). Please refer to the differential diagnosis of eosinophilia in the gastrointestinal tract, Table 4.12. It should be pointed out that eosinophilic infiltrates in the neonatal colon can also be seen in cases of Hirschsprung disease (Lowichik and Weinberg 1997), and that, conversely, the clinical and radiologic features of allergic colitis can occasionally mimic aganglionosis (Andiran and Dayi 2000; Bloom et al. 1999).
6.4 Vascular Disease
6.4.1 Necrotizing Enterocolitis
6.4.1.1 Clinical Features and Pathophysiology
Necrotizing enterocolitis (NEC) is the most common surgical abdominal emergency in the newborn (Ng 2001). The incidence of the disease in North America is 1–3 cases per 1,000 live births, being inversely proportional to birth weight and gestational age (Israel and Morera 2000). It occurs in approximately 10 % of babies born weighing less than 1,000 g, but the incidence ranges widely between hospital centers and across the world (Caplan and Jilling 2001). Full-term neonates account for 5–25 % of cases, with congenital heart disease, perinatal asphyxia, and maternal cocaine use believed to be significant predisposing factors in that group (Ng 2001). NEC is a disease with significant mortality and morbidity rates and is believed to be an independent predictor of neurodevelopmental abnormalities in the survivors (Caplan and Jilling 2001). Mortality is inversely related to birth weight and gestational age and is also related to the length of bowel involvement.
A discussion of the pathophysiology of NEC is beyond the scope of this chapter, and excellent reviews are available (Neu and Walker 2011). The pathogenesis of NEC is incompletely understood but is believed to be multifactorial, resulting from a combination of genetic predisposition, intestinal immaturity with inadequate motility, a heightened and inappropriate inflammatory response, abnormal microbial colonization, and an imbalance in microvascular tone (Neu and Walker 2011). Ischemic damage in NEC has been postulated to result in part from circulatory alterations diverting blood away from the abdominal organs to the heart and brain during episodes of hypoxia (the “diving” reflex). Intramural vessels are hypothesized to be the primary target, vascular damage mediated by the release of vasoconstrictor agents such as peptide endothelin-1 and free radicals such as nitric oxide (Nowicki 2005). Because NEC usually occurs after the beginning of enteral feeding, enteral alimentation has also been proposed as a contributing factor. Human milk appears to reduce the risk for NEC (McGuire and Anthony 2003). A number of different bacterial pathogens have also been associated with NEC, as well as various cytokines. Endotoxin, platelet-activating factor, and tumor necrosis factor-α have also induced ischemic damage in experimental models of NEC (Hsueh et al. 2003).
The clinical presentation varies from a relatively indolent process with ileus and diarrhea to a fulminant course characterized by sepsis and peritonitis. Early signs and symptoms of NEC may be subtle and nonspecific, such as apnea, bradycardia, and lethargy, suggesting sepsis. More specific gastrointestinal signs include abdominal distension, absent bowel sounds, vomiting, and diarrhea, with radiographic findings such as ileus and pneumatosis intestinalis. More advanced disease is characterized by a perforated viscus in a severely ill child (Neu and Walker 2011). Radiologic confirmation is based on the finding of air in the bowel wall or hepatic portal venous system (Albanese and Rowe 1998).
6.4.1.2 Pathology
The most commonly affected segments (80 % of cases) are the terminal ileum and cecum which represent a vascular watershed. Histologic features are dominated by hemorrhagic necrosis of the mucosa and varying degrees of coagulative necrosis of the muscle layers, similar to ischemic damage in older children and adults (Fig. 6.3a, b). Resected specimens may show patchy or diffuse, continuous necrosis. Transmural necrosis leads to perforation, which is frequently multifocal, and peritonitis. Vascular thromboses, often noted in the most affected areas, are most likely secondary. Inflammation, when present, is usually slight. Pneumatosis intestinalis (air in the bowel wall) is found in approximately half of the resected specimens (Fig. 6.3c, d); the air is believed to result from bacterial fermentation and is evidence for the role played by bacterial colonization (Dahms 2001). Focal reparative changes, such as granulation tissue formation, may also be found. Though some advocate the use of perioperative frozen sections to assess the viability of resection margins, the surgical practice at our institution and many others is to resect only unquestionably necrotic bowel (Albanese and Rowe 1998). Major gastrointestinal medium and long-term complications include strictures (Fig. 6.3e) in up to one-third of patients (Albanese and Rowe 1998), short gut syndrome and intestinal malabsorption, and chronic liver disease resulting from total parenteral nutrition. NEC is reported to recur in 4–6 % of cases, usually within 1 month of the initial episode (Albanese and Rowe 1998).
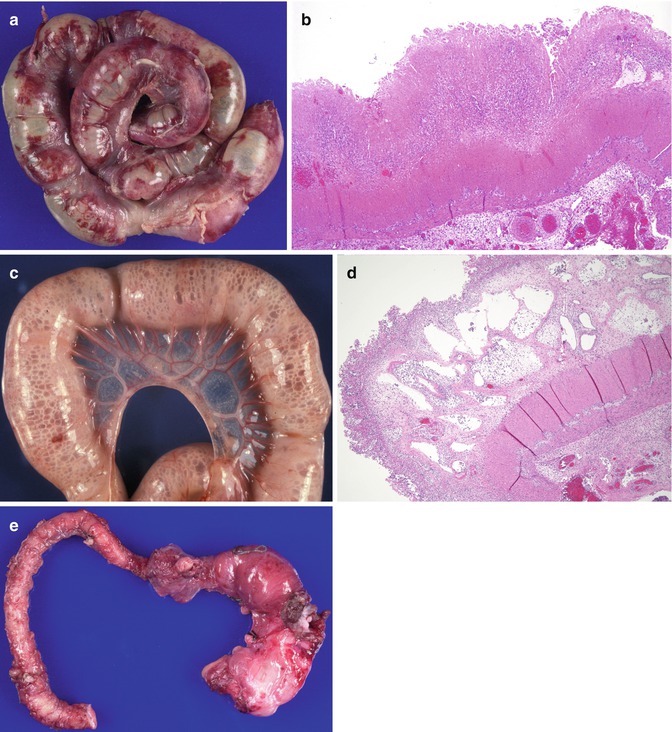

Fig. 6.3
Necrotizing enterocolitis. (a) Resected segment of necrotic bowel from a 1-month-old. (b) Hemorrhagic ischemic necrosis process involving the mucosa, submucosa, and muscular layer (H + E ×40). (c) Pneumatosis intestinalis. Numerous air-filled spaces diffusely involving the bowel wall. (d) Pneumatosis intestinalis. Low-power histologic section from (b) reveals numerous dilated spaces involving the submucosa, many of which contain neutrophils (H + E ×40). (e) NEC strictures in a resected segment of bowel
6.4.2 Henoch-Schonlein Purpura
6.4.2.1 Clinical Manifestations and Pathophysiology
Henoch-Schonlein purpura (HSP) is a systemic vasculitis whose cardinal clinical manifestations include a purpuric rash, arthritis, abdominal pain, and nephritis. It occurs worldwide, with seasonal clustering in the winter and early spring. Though it can affect persons of any age, the majority of patients are children, and it is the most common vasculitis in children. The median age at onset is 4 years (Kocoshis 1999). Pediatric cases are generally preceded by a viral or bacterial infection; adult cases have also been associated with cancer and drugs (Kocoshis 1999). It has also been observed as a complication of anti-TNF (tumor necrosis factor) therapy in Crohn’s disease (Rahman et al. 2010).
The cause of HSP is unknown, but the pathogenic process involves a disordered immune response with the deposition of immune complexes containing IgA and complement factor 3 (C3) in glomerular capillaries and in small blood vessels of the skin, gastrointestinal tract, and other organs. Genetic susceptibility is suggested by increased frequency of the disease in individuals carrying the HLA-B35 haplotype. An increased incidence of HSP has also been described in association with mutations in the MEFV gene responsible for familial Mediterranean fever (Saulsbury 2010). Increased secretory IgA with affinity to dietary antigens such as gliadin has been described in children (Moja et al. 1998).
Gastrointestinal involvement is frequent and may precede or occur after the rash. Abdominal pain and overt or occult GI bleeding are the most common intestinal symptoms. The duodenum and small bowel are the most frequently involved sites in the gastrointestinal tract (Esaki et al. 2002), and intussusception is the most significant abdominal complication (Choong and Beasley 1998). In one recent study, Zhang et al. observed GI symptoms in 90/115 patients (78 %), primarily abdominal pain. On endoscopic evaluation, they confirmed that the second part of the duodenum was the most frequently and usually most severely involved site of the upper GI tract and the rectum the most frequently involved part of the lower GI tract (Zhang and Huang 2008). Protein-losing enteropathy, perforation, pancreatitis, and cholecystitis are rarer manifestations (Bissonnette et al. 1997; Choong and Beasley 1998). Radiologic studies reveal “thumbprinting” of the intestinal wall, as found in other ischemic conditions (Esaki et al. 2002). Endoscopic examination primarily reveals purpuric lesions, erosions, and ulcers (Caplan and Jilling 2001) (Fig. 6.4a).
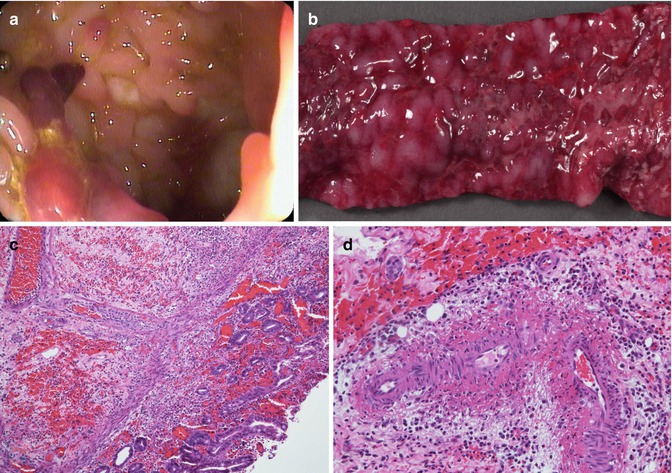

Fig. 6.4
Henoch-Schonlein purpura. (a) Ulcers and mucosal nodularity are noted on endoscopy. (b) Segmental jejunal resection from a 3-year-old girl with extensive GI bleeding reveals a diffusely hemorrhagic and extensively ulcerated mucosa. (c) Histologic section shows mucosal and submucosal hemorrhage with an acute inflammatory infiltrate and mucosal ischemic damage. Chronic vasculopathic changes are noted in a submucosal artery (H + E ×100). (d) Higher power view from another portion of the specimen shows active vasculitis of the submucosal arteries (H + E ×200)
6.4.2.2 Pathology
Endoscopic biopsies of the GI lesions are most commonly characterized by nonspecific changes, probably because most mucosal biopsies do not include the deeper vessels of the submucosa (Zhang and Huang 2008). In these cases, the value of the biopsy lies in excluding other diseases. Hemorrhagic necrosis and leukocytoclastic vasculitis of the mucosa and submucosa have been reported by other investigators (Novak et al. 1995; Esaki et al. 2002; Chesler et al. 2000) (Fig. 6.4b–d).
6.5 Hirschsprung-Associated Enterocolitis (HAEC)
6.5.1 Clinical Manifestations and Pathophysiology
A more complete discussion of Hirschsprung disease and neuromuscular intestinal diseases is to be found in Chap. 7 (Motor Disorders). Despite significant advances in our understanding and treatment of Hirschsprung disease, Hirschsprung-associated enterocolitis (HAEC) continues to be the major complication and the most important cause of mortality in this disease. There is variability in both the incidence (between 17 and 50 %) and mortality of the disease (Teitelbaum and Coran 1998). HAEC can occur before surgical treatment and from 3 weeks to 20 months after the pull-through operation (Coran and Teitelbaum 2000) and can develop in aganglionic and ganglionated bowel. Several factors have been associated with an increased risk of developing HAEC: delay in diagnosis (Teitelbaum et al. 1988), increased length of the aganglionic segment (Rescorla et al. 1992), and trisomy 21 (Caniano et al. 1990). In one series, 45 % of patients with trisomy 21 and Hirschsprung disease had enterocolitis (Teitelbaum et al. 1988). Proposed pathophysiologic mechanisms involved in the disorder include abnormal retention of neutral mucins and loss of acidic sulfomucins with an increased propensity to infection by enterocyte-adherent organisms (Teitelbaum et al. 1989) and insufficient transfer of gut mucosal IgA across the enterocyte (Wilson-Storey and Scobie 1989). The possibility of increased mucosal permeability with a resulting allergic reaction to luminal antigens is suggested by reports of eosinophilic infiltration of the submucosa and myenteric plexus even in cases not associated with enterocolitis (Lowichik and Weinberg 1997) and by the occasional overlapping clinical and radiologic features of allergic colitis and Hirschsprung disease (Bloom et al. 1999).
Patients with HAEC present with abdominal distension, vomiting, fever, lethargy, and shock (Coran and Teitelbaum 2000); abdominal radiographs reveal a distended proximal colon and absence of air in the rectosigmoid region, a useful feature which has been termed the “cutoff” sign (Coran and Teitelbaum 2000).
6.5.2 Pathology
Pathological features can vary from a mild acute cryptitis to a full-blown mucosal ischemic necrosis; some cases may have a pseudomembranous appearance (Pozo et al. 1994) (Fig. 6.5). A grading system for the histopathological findings has been proposed (Teitelbaum et al. 1989). Neonates may present with a picture suggesting necrotizing enterocolitis with pneumatosis intestinalis (Hsieh et al. 2000).
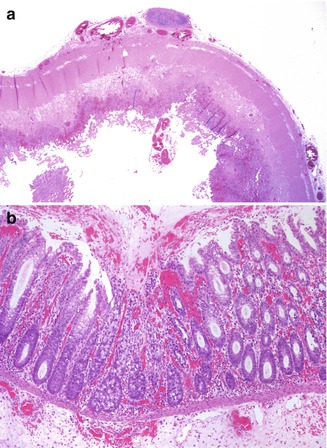

Fig. 6.5
Hirschsprung-associated enterocolitis. (a) A 5-month-old child developed severe enterocolitis 3 weeks after a Soave pull-through for aganglionosis. Diffuse ischemic necrosis was present throughout most of the colon (H + E ×20). (b) A 16-day-old child developed toxic megacolon 3 days following a diverting colostomy. The histologic pictures are that of a pseudomembranous colitis, with focal crypt necrosis and a neutrophil- and mucus-rich exudate (H + E ×100)
The mortality rates vary from 0 to 30 %, with lower rates observed in more recent studies (Teitelbaum and Coran 1998). Treatment includes rectal irrigations and antibiotics (Coran and Teitelbaum 2000). In cases of repeated enterocolitis, the possibility of persistent obstruction should be investigated, including rectal biopsies to rule out residual aganglionosis. If the biopsies are normal, the internal sphincterectomy has been performed with some success (Coran and Teitelbaum 2000).
6.6 Infections
Pathologists, especially in pediatric settings, routinely evaluate biopsies from patients at the earliest stage of IBD, when the major differential diagnosis is infectious colitis. Histologic features of initial biopsies in children are less likely to show diagnostic features of IBD than adults. Furthermore, patients may present with a concurrent intestinal infection during the initial episode of IBD (Schumacher 1993). A complete review of infectious pathology of the colon is beyond the scope of this chapter; the interested reader is referred to the excellent monograph written by Laura Lamps (2009). This section will focus on some of the infectious colitides more likely to be encountered by the pathologist in a hospital setting, emphasizing those causing more severe or prolonged clinical manifestations that may lead to a biopsy or that can mimic IBD. In industrialized countries, Shigella, Salmonella, Campylobacter, Shiga toxin-producing Escherichia coli, and Clostridium difficile are the most common pathogens causing bloody diarrhea (dysentery), and patients with bloody diarrhea are more likely to undergo endoscopic evaluations than those with non-bloody diarrhea (Pfeiffer et al. 2012). The major histologic patterns of some of the more common infections are outlined in Table 6.2
Table 6.2
Histologic features of the more common infectious colitides
Acute colitis |
Campylobacter |
Shigella |
Aeromonas |
Nontyphoid salmonella |
Cytomegalovirus |
Minimal histologic change |
Most enteric viruses |
Vibrio cholerae |
Enteropathogenic and enteroadherent E. coli |
Spirochetosis |
Pseudomembranous colitis |
C. difficile |
Enterohemorrhagic E. coli |
Granulomatous colitis |
Mycobacteria tuberculosis and avium intracellular complex |
Yersinia |
6.6.1 Salmonella
Salmonella infections in humans are major worldwide causes of diarrheal illness due to contaminated water and food and are the most frequent bacterial pathogen isolated from stools of children with diarrhea even in more affluent countries. Salmonella infections can be divided into those that cause typhoid (enteric) fever and those that do not. Typhoid fever results primarily from infection with Salmonella typhi and paratyphi (Caumes et al. 2001), is endemic in developing countries, and is most frequently due to water contaminated by human fecal material. In more affluent countries, cases of typhoid fever may result from travel or from contamination of water or food by a chronic carrier. In typhoid fever, there is prominent involvement of Peyer’s patches in the terminal ileum, which progresses through hyperplasia of lymphoid tissue, necrosis, and ulceration of the overlying mucosa. Ileocecal thickening and ulceration may suggest Crohn’s disease (Kaplan et al. 1999). In more industrialized countries, infection with nontyphoid Salmonella accounts for a significant proportion of cases of food poisoning, resulting especially from consumption of contaminated fish and poultry (Narain and Lofgren 1989; Kelly and Owen 1997). The diagnosis, in these cases, is established with stool or blood culture. Infections with nontyphoid Salmonella, rarely biopsied, are typically characterized by an acute colitis, with scattered crypt abscesses, indistinguishable from other infectious colitides (Fig. 6.6). Crypt rupture may lead to a focal histiocytic reaction. Granulomas may be rarely observed (Lamps 2009). Persistent disease with colonic involvement has been reported to mimic ulcerative colitis (Sachdev et al. 1993) or even result in obstruction suggesting Hirschsprung disease (Bhatt and Rickett 2000).
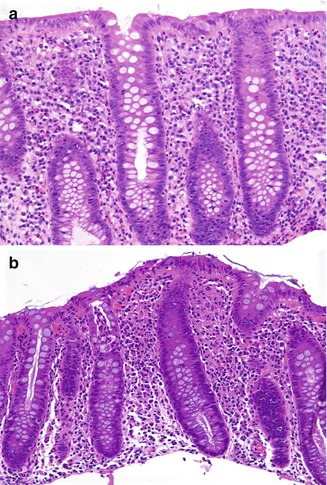

Fig. 6.6
Colitis due to Salmonella. (a) An 11-year-old boy had a 2-weeks episode of bloody diarrhea due to infection by Salmonella enteritidis. There is hemorrhage and a neutrophil-rich inflammatory infiltrate, primarily involving the upper portion of the mucosa. There is minimal crypt architectural distortion (H + E ×200). (b) Colonic biopsies from a 14-year-old boy with several months’ symptomatology in whom Salmonella was cultured from stools. There are superficial microabscesses and a small superficial granuloma (H + E ×100)
6.6.2 Shigella
Shigella infections are also endemic in countries with inadequate sanitation and water treatment. The virulence of the organism results from a combination of enterocyte invasion and damage and the elaboration of a potent toxin (Shiga toxin) (Kelly and Owen 1997). In contrast with Salmonella, the left colon and rectosigmoid are most frequently involved (Speelman et al. 1984), though pancolitis is seen in fatal cases (Butler et al. 1989). Histopathological findings are primarily those of an acute colitis (Sachdev et al. 1993); some studies in endemic areas have reported mild mucosal distortion suggesting ulcerative colitis (Anand et al. 1986). Diffuse ulcerations with crypt abscesses, goblet cell depletion, and crypt branching may be seen in severe infections, so that distinction from fulminant ulcerative colitis may be difficult without microbiologic confirmation (Butler et al. 1989).
6.6.3 Campylobacter
Campylobacter infections affect both small and large intestines and are one of the most frequently found organisms isolated from patients with colitis (Price et al. 1979; Schumacher 1993). Campylobacter is the most common cause of bacterial dysentery in the first year of life (Pfeiffer et al. 2012). C. jejuni accounts for greater than 90 % of infections in humans, and other species such as C. coli, C. fetus, and C. upsaliensis account for the rest. Campylobacter are commensals in the intestines of many species. Infection is acquired through ingestion of raw meat, poultry, fish, and water. Histopathological features in colonic biopsy samples are similar to those encountered in Salmonella and Shigella infections, including edema resulting in crypt separation and a gap between their base and the muscularis mucosae, focal collections of polymorphs in the lamina propria, and incipient crypt abscesses with polymorphs mainly between crypt epithelial cells (Price et al. 1979).
6.6.4 Escherichia coli Infection and the Hemolytic-Uremic Syndrome
Escherichia coli are gram-negative lactose-fermenting bacilli that consist of many strains, ranging from those that make up the normal gut flora to highly pathogenic strains. They can usually only be distinguished from each other by immunochemical or genotyping methods. The major categories of diarrhea-causing E. coli include enterotoxigenic (traveler’s diarrhea), enteroinvasive (a major cause of dysentery), enteropathogenic (diarrhea in infants), enterohemorrhagic, and enteroaggregative E. coli (the latter also referred to as enteroadherent E. coli) (Levine 1987). Enterohemorrhagic E. coli include the serogroup O157: H7, the most common serogroup responsible for enterohemorrhagic colitis. E.coli O157:H7 is estimated to cause 10,000–20,000 infections each year in the United States and 250 deaths annually (Boyce et al. 1995). E.coli O157:H7 is among the three most common bacterial enteropathogens (trailing Salmonella and Campylobacter) and is the most frequently isolated pathogen from stool of patients with frank hematochezia (Griffin et al. 1990; Pai et al. 1984). Infections can be sporadic or epidemic, especially in institutional settings (Griffin 1995). Hemolytic-uremic syndrome (HUS) occurs in about 12 % of children with E.coli O157:H7 infection (Ostroff et al. 1989). Infections with other E.coli serotypes, Shigella, and other Shiga toxin-producing microbes can also cause the syndrome. Infections occur throughout the year but seem to have a predilection for warmer months (Ostroff et al. 1989). Summer predilection may be associated with higher consumption of improperly cooked ground beef (Le Saux et al. 1993). Outbreaks related to ground beef consumption in restaurants have been highly publicized (Bell et al. 1994; Wells et al. 1983), but a greater number of cases actually occur in the home (Le Saux et al. 1993). Individuals at extremes of age appear most susceptible, with children younger than 4 years at greatest risk for infections and susceptibility to hemolytic-uremic syndrome (Ashkenazi et al. 1991; Cimolai et al. 1990; Spika et al. 1986). The pathogenesis of the disorder involves adhesion to and invasion of mucosal epithelial cells with production of Shiga toxin. A subunit of the toxin binds to the membranes of glomerular, colonic, and endothelial cells, is endocytosed, and causes cell death by interfering with ribosomal function. In addition, Shiga toxin induces endothelial cells to produce excess von Willebrand’s and platelet-activating factors, as well as causing platelet activation (Moake 2002).
The incubation period for E.coli O157:H7 is 3–4 days, and the average duration of illness is 7 days (Fagundes Neto et al. 1989; Carter et al. 1987; Ostroff et al. 1989). Bloody diarrhea, vomiting, and fever usually manifest by the third to fifth day of the illness (Ostroff et al. 1989). Non-bloody E.coli O157:H7 infections tend to be less severe and of shorter duration. Fecal leukocytes are present in the majority (Tarr 1995). Colonoscopic findings demonstrate nonuniform and nonspecific mucosal erythema and edema mainly in the ascending and transverse colon (Griffin et al. 1990). Barium enema demonstrates signs of bowel edema and submucosal hemorrhage, such as thumbprinting. Stool culture is the primary means of identifying E.coli O157:H7 (March and Ratnam 1986).
Histopathological changes include features of both ischemic and acute infectious colitis. With associated HUS, the changes predominantly affect the left colon and are dominated by hemorrhage, mucosal necrosis, and fibrin thrombi in mucosal and submucosal vessels. Severe cases requiring surgery or observed at autopsy are noted to have a swollen, hemorrhagic mucosa, frequently ulcerated, and often partially covered by pseudomembranes (Fig. 6.7). An acute colitis pattern with involvement primarily of the right colon is seen in those cases without associated HUS, featuring a variably intense neutrophilic infiltrate with pericryptitis, crypt microabscesses, and, less commonly, pseudomembranes (Murray and Patterson 2000).
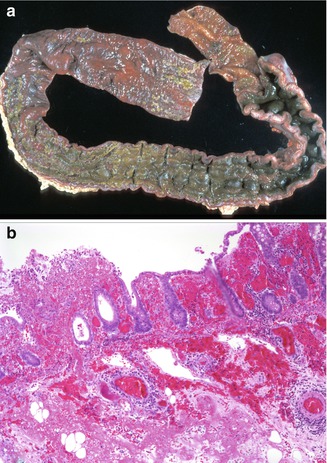

Fig. 6.7
Hemolytic-uremic syndrome. (a) A 4-year-old male, who after presenting with vomiting and watery diarrhea that progressed to bloody diarrhea, developed renal failure, seizures, and eventual brain death. (a) At autopsy, the mucosa of the colon appeared markedly swollen and hemorrhagic with a lobulated, cobblestone-like appearance of the mucosa. (b) Histologic section of the above specimen is characterized by ischemic necrosis with extensive hemorrhage and foci of ulceration. Thrombi are observed in mucosal and submucosal vessels (H + E ×100)
Major gastrointestinal complications of HUS include stricture, perforation, intussusception, hemoperitoneum, and pancreatitis (Brandt et al. 1990).
6.6.5 Yersinia
Yersinia infections (yersiniosis) are caused by two pathogenic types, Yersinia enterocolitica (YE) and Yersinia pseudotuberculosis (YP). Both can cause mesenteric lymphadenitis, appendicitis, and terminal ileitis, which can mimic acute appendicitis or stricturing Crohn’s disease of the terminal ileum (Lamps et al. 2001). Infection with Y. enterocolitica is more common in colder climates and during winter (Abdel-Haq et al. 2000); it usually causes an acute self-limited colitis and is an important bacterial cause of diarrhea in Scandinavian countries and Canada. Exposure to undercooked pork is a predisposing factor (Abdel-Haq et al. 2000). As the organism is dependent on a rich iron source for growth, children administered frequent blood transfusions, such as those affected by thalassemia, are at increased risk (Hansen et al. 2001). Y. pseudotuberculosis is also a food-borne pathogen that has been associated with community outbreaks (Press et al. 2001). It has, in addition, been reported as a cause of acute interstitial nephritis (Kobayashi et al. 2000) and may result in systemic manifestations similar to Kawasaki disease (Uchiyama and Kato 1999). Severe infections with Y. enterocolitica can result in a florid lymphoid hyperplasia of the mucosa and submucosa of the terminal ileum and cecum, eventuating in ulceration of the overlying mucosa (Fig. 6.8). Granulomatous inflammation of the bowel and mesenteric lymph nodes is characteristic of Y. pseudotuberculosis but can also be seen with Y. enterocolitica (Lamps et al. 2001). Gram-negative bacilli may be observed on occasion. The differential diagnosis includes mycobacterium and Crohn’s disease. Though distinguishing between yersiniosis and Crohn’s disease may be difficult, evidence of chronic disease, such as crypt distortion, muscular hypertrophy, and neural hyperplasia, would favor the latter. Serology and cultures are helpful when the diagnosis is in doubt; polymerase chain reaction (PCR) may prove a useful diagnostic adjunct (Lamps 2009).
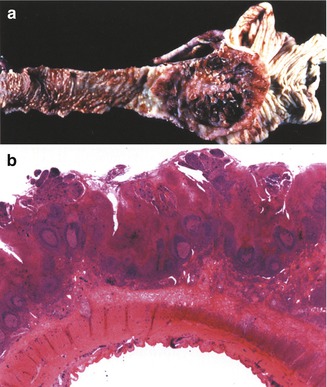

Fig. 6.8
Yersinia enterocolitis. (a) A large area of ulceration is noted in the ileocecal valve. Nodular excrescences can be observed along the mucosa of the ileum proximal to the area of ulceration, due to lymphoid hyperplasia. (b) Extensive necrosis and hemorrhage of the mucosa overlying diffuse lymphoid hyperplasia with reactive germinal centers. Necrotizing granulomas were also observed in the bowel and lymph nodes (not shown) (H + E ×40) (Case courtesy of Dr Ronald Jaffe, Children’s Hospital of Pittsburg)
6.6.6 Clostridium difficile Infection and Pseudomembranous Colitis
Clostridium difficile is a gram-positive, anaerobic bacillus pathogen that accounts for up to 25 % of cases of antibiotic-associated diarrhea and most cases of antibiotic-associated pseudomembranous colitis. Though most frequently associated with C. difficile infection, pseudomembranous colitis can be observed with other entities, such as necrotizing enterocolitis and Hirschsprung-associated enterocolitis (Table 6.3). Less than 1 % of healthy adults are C. difficile carriers, although 25 % of adults recently treated with antibiotics appear to be colonized (Johnson et al. 1990). Over 50 % of healthy infants are asymptomatic carriers and appear to approach adult carrier status by 1 year of age (Johnson et al. 1990). Treatment does not reduce the carrier state (Johnson et al. 1992).
Table 6.3
Diseases associated with formation of colonic pseudomembranes
Clostridium difficile infection |
Escherichia coli O157:H7 infection |
Ischemia |
Necrotizing enterocolitis |
Fungal infections |
Neutropenic colitis |
Hirschsprung-associated enterocolitis |
Amebiasis |
Host and bacterial factors which determine C. difficile colitis and asymptomatic carriage are not clear. It appears that antecedent disruption of normal colonic bacterial flora is a risk factor for C. difficile-induced colitis. Any antibiotic therapy is capable of producing infection by C. difficile; however, those antibiotics which alter intestinal flora appear to be most responsible. Independent of antibiotic usage, additional risk factors for C. difficile colitis include Crohn’s disease, ulcerative colitis, general anesthesia, chemotherapeutics, neutropenia, Hirschsprung disease, and cystic fibrosis.
C. difficile infection may present a continuum of colonic symptoms, from mild diarrhea and abdominal cramping with a lack of systemic symptoms to toxic megacolon, perforation, and peritonitis. Recently, an epidemic strain, the NAP1 strain, has been associated with increased virulence and also antibiotic resistance (McDonald et al. 2005). Symptoms of C. difficile infection usually begin during or shortly following antibiotic therapy but may be delayed for several weeks. Diarrhea may become severe and debilitating with systemic symptoms including fever, malaise, anorexia, and dehydration. Occult or frank intestinal bleeding may be present. Macroscopic endoscopic findings reveal characteristic yellow plaques 2–10 mm in diameter interspersed with normal-appearing mucosa (Fig. 6.9a, c).
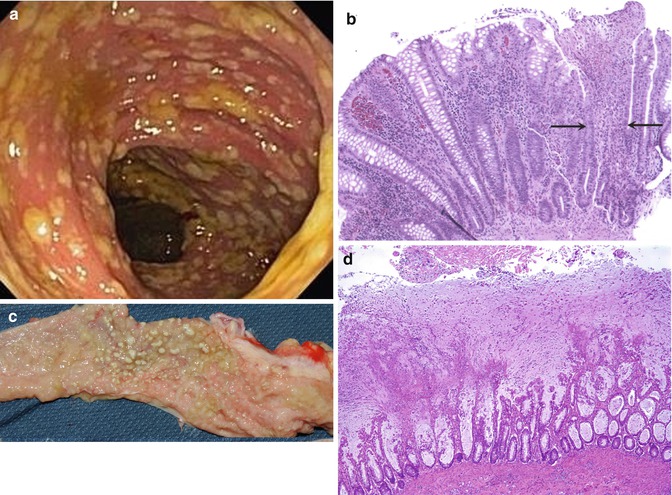

Fig. 6.9
Pseudomembranous colitis. (a) Endoscopic findings are characteristic yellowish pseudomembranes that bleed easily. (b) The early lesion consists of focal epithelial and crypt necrosis (between the arrows). An overlying neutrophilic exsudate is present. (c) A 19-month-old child developed severe bloody diarrhea and shock due to Clostridium difficile following a 2-weeks course of antibiotic therapy. The mucosal surface of the colon is studded with nodular whitish plaques. (d) Histologic section from (c) reveals extensive crypt disruption and widespread pseudomembranes composed of mucus, neutrophils, and cellular debris (H + E ×40)
The earliest histologic lesions consist of foci of epithelial necrosis with a neutrophilic infiltrate and eventuate into epithelial loss and disrupted crypts (Fig. 6.9b). The pathognomonic volcano-like membranes consist of epithelial debris, mucus, and neutrophil-rich exudates. Late lesions consist of extensive mucosal necrosis and destruction (Fig. 6.9c, d), essentially indistinguishable from ischemic colitis or severe IBD.
A demonstration of C. difficile toxins in the stool is the diagnostic gold standard with high sensitivity (94–100 %) and specificity (De Girolami et al. 1992; Doern et al. 1992). PCR, however, is being utilized more commonly. Stool culture is not the method for establishing diagnosis as culture is difficult, and many C. difficile strains are not toxicogenic.
6.6.7 Adenovirus
Adenovirus infection can cause severe colitis in immunocompromised patients, especially those with AIDS, although patients with solid organ and bone marrow transplants are also at risk (Baldwin et al. 2000). Typical intranuclear inclusions can be seen in infected epithelial cells, which can be confirmed by immunohistochemistry, in situ hybridization, or electron microscopy. Adenoviral inclusions can be found in epithelial cells of the appendix in cases of intussusception (Berrebi et al. 1997; Yunis et al. 1975) (Fig. 6.10).
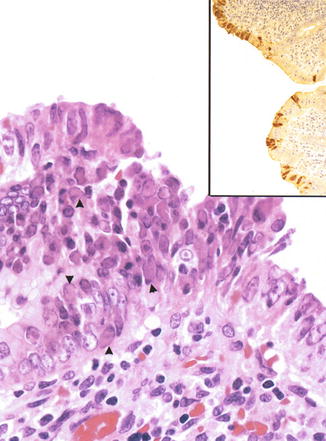

Fig. 6.10
Adenovirus. Appendix from a 2-year-old child with ileocecal intussusception. Numerous intranuclear inclusions can be observed in the focally heaped-up epithelium. Inset: immunohistochemistry with an antibody against adenovirus reveals extensive staining of the surface epithelium
6.6.8 Cytomegalovirus (CMV) (Fig. 6.11)
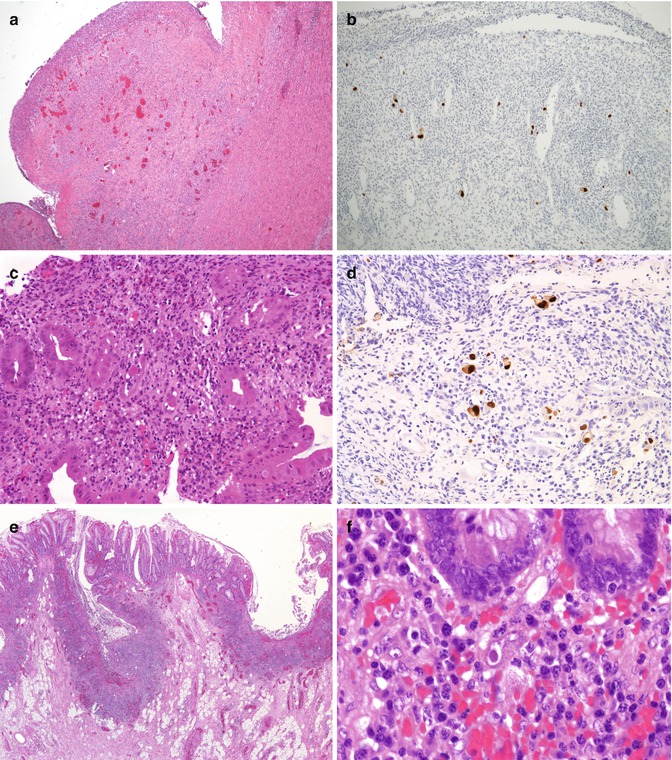
Fig. 6.11
Cytomegalovirus. (a) Congenital CMV. Large heaped ulcers were noted throughout the GI tract (H + E ×4). (b) Immunostaining for CMV from section (a) (hematoxylin counterstain ×100). (c) Posttransplant CMV infection. Colonic biopsies from an 8-year-old boy who developed CMV infection following bone marrow transplantation show extensive crypt-destructive inflammation and viral inclusions mainly in endothelial cells. (d) Immunostaining for CMV predominates in endothelial cells (hematoxylin counterstain ×200). (e) CMV infection in IBD. Severe active disease is present with numerous ulcers in this 15-year-old patient with ulcerative colitis of several years duration (H + E ×20). (f) Typical purplish intranuclear inclusions surrounded by a clear halo are noted in enlarged nuclei of stromal, endothelial, and epithelial cells. These should be carefully sought in any patient undergoing immunomodulator therapy for IBD who develops a sudden flare-up of the disease
CMV infection of the gastrointestinal tract occurs almost exclusively in immunocompromised patients, particularly those with AIDS, those transplant recipients (Einsele et al. 1994; Toogood et al. 1996), and those receiving immunosuppressive therapies. CMV infection can complicate the clinical course of patients with ulcerative colitis and Crohn’s disease, in whom the virus may present as steroid-resistant colitis (Cottone et al. 2001; Pfau et al. 2001). CMV enterocolitis has also been reported in premature infants (Fournier et al. 1989) and in apparently healthy, full-term infants in whom it may result in protracted diarrhea (Quiros-Tejeira et al. 1999). Patients with CMV enterocolitis present with abdominal pain, bloody diarrhea, perforation, and peritonitis. In the most severe cases, evidence of systemic infection is also present. Viral inclusions are usually observed in the nuclei and cytoplasm of epithelial and stromal cells, particularly in endothelial cells, with concomitant endotheliitis, thrombosis, and resultant ischemic damage (Yost 2002). In the neonate, infection of enteric ganglion cells has been postulated to result in motility disorders.
6.6.9 Amebiasis
Infections with Entamoeba histolytica occur primarily in Africa, Indochina, and Central and South America where it is second only to malaria as a cause of protozoan infection-related death (Yost 2002). In the United States, infection occurs in immigrants from endemic areas, in institutionalized persons, or as a result of travel (Yost 2002). The diffuse colonic inflammation resulting from acute infection may be difficult to distinguish from ulcerative colitis and Crohn’s disease endoscopically and histologically (Fig. 6.12). Granular, shallow ulcers of the colon, occasionally extending through the entire thickness of the wall, with necrotic exudates are the major histopathological features (Katz and Taylor 2001). The characteristic 3- to 5μm-long trophozoites, which resemble histiocytes and typically contain ingested red blood cells, are best identified in specimens which include the exudates and ulcer margins (Katz and Taylor 2001).
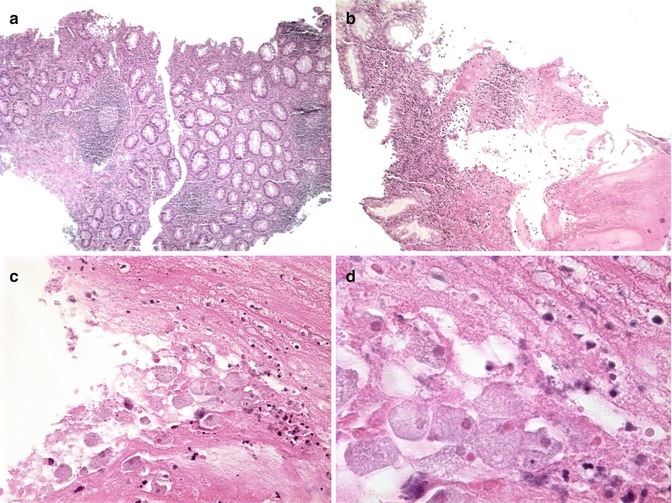

Fig. 6.12
Amebiasis. (a) Colonic biopsy characterized by superficial ulcers and a neutrophilic infiltrate admixed with a mononuclear infiltrate in the lamina propria (H + E ×50). (b) Amorphous eosinophilic debris typical of E. histolytica infection (H + E ×100). (c) The amount of debris may overshadow the presence of organism which resemble foamy macrophages (H + E ×400). (d) Ingestion of red blood cells is a typical feature of infection by E. histolytica (H + E, oil immersion) (Case courtesy of Dr Elisabeth Furth, Hospital of the University of Pennsylvania)
6.6.10 Schistosomiasis
Schistosomiasis is the second most prevalent tropical disease after malaria with a wide geographical distribution. The incidence of schistosomiasis appears to be rising in non-endemic countries as a result of immigration and travel to endemic areas. Most patients in endemic areas have chronic schistosomiasis and remain relatively asymptomatic. Gastrointestinal symptoms, when present, usually consist of bloody diarrhea, anemia, and weight loss (Lamps 2009). The colonic complications of infestation by S. mansoni, S. intercalatum, and others are related to the deposition of viable eggs in the venules of the colonic mucosa, resulting in an immune response in the host with eosinophilic and granulomatous inflammation (Lamps 2009; Sharaiha et al. 2010). If a patient undergoes colonoscopy during the course of investigation, the endoscopic features may suggest idiopathic proctitis or colitis (Sharaiha et al. 2010). Biopsies reveal a granulomatous inflammation that may be rich in eosinophils associated with the eggs or parts of the eggs (Fig. 6.23d).
6.7 Chronic Inflammatory Bowel Disease (IBD)
6.7.1 General Remarks
Once considered rare in pediatric patients, IBD is now recognized with increased frequency in children and adolescents. In fact, approximately 20–25 % of IBD patients are diagnosed by 20 years of age. In North America, it is estimated that there are 100,000 children suffering from IBD at any given time (Kugathasan et al. 2003). On a clinical and histopathological basis, IBD is commonly classified as either ulcerative colitis or Crohn’s disease. The disease pathogenesis is not fully understood by these broadly based phenotypes, which more than likely involve multiple, distinct pathophysiologies. In general, IBD appears to result from an inappropriate immune response to antigens, such as gut microbes or ill-defined food antigens, in a genetically susceptible host. The observation that IBD occurs more frequently in industrialized, as opposed to developing, countries has led to the “hygiene hypothesis.” The hygiene hypothesis suggests that in parts of the world where humans are exposed to fewer microbes, the intestinal immune system might not be as prepared to respond to antigens. This could lead to the development of IBD in a genetically susceptible individual.
Genetics is clearly a risk factor for inflammatory bowel disease. Familial aggregation studies demonstrate an increased disease risk in first-degree relatives of affected patients (Orholm et al. 1991). The incidence of IBD in first-degree relatives among patients younger than 5 years of age is 56 % and younger than 20 years of age is 30 % (Polito et al. 1996). Additionally, monozygotic twins have a higher concordance rate than dizygotic twins (Tysk et al. 1988). However, the etiology of IBD cannot be explained by genetics alone, and the absence of simple, Mendelian inheritance suggests that more than a single genetic abnormality may be present. Recent genome-wide association studies (GWAS) utilizing high-density SNP array technology have identified 40 loci associated with Crohn’s disease and 17 loci associated with ulcerative colitis (Shikhare and Kugathasan 2010). Additionally, in 2008, susceptibility loci for pediatric-onset IBD specifically were reported on 20q13 and 21q22 (Kugathasan et al. 2008).
One of the gene candidates which has been proposed in Crohn’s disease is the nucleotide-binding oligomerization domain-containing protein 2 (NOD-2) gene. The NOD-2 gene encodes a protein which senses peptidoglycan, a component of the bacterial cell wall (Abraham and Cho 2009). There are many theories regarding how mutations in the NOD-2 gene contribute to the inflammation of Crohn’s disease, but it is thought to involve abnormalities in the innate immune system. Individuals with a homozygous NOD-2 variant may have a 20-times greater risk of developing ileal or fibrostenosing Crohn’s disease (Abreu et al. 2002; Ahmad et al. 2002; Cuthbert et al. 2002; Lesage et al. 2002). There are many other genes which have been linked to the pathogenesis of Crohn’s disease and ulcerative colitis—these diseases are believed to be polygenic. Still, it is believed that known genetic associations account for only 20 % of the genetic variance in IBD (Abraham and Cho 2009).
Available literature suggests that the genetic abnormalities in inflammatory bowel disease allow for abnormalities in the host immune system’s response to luminal antigens (Sartor and Muehlbauer 2007). These antigens are most likely the endogenous microflora, which provide constant stimulation of the intestinal immune system. This observation is supported by numerous genetically altered animal models in which intestinal inflammation does not develop in the absence of commensal flora (germ-free animals) (Elson et al. 1998; Rath et al. 2001). The animal observations parallel human clinical observation in which the intestinal inflammation in IBD can be treated with antimicrobial therapy or by altering the intestinal flora with probiotics (Swidsinski et al. 2002). Additionally, eliminating food antigens by surgical diversion of the fecal stream significantly improves disease activity (Sartor and Muehlbauer 2007). The use of defined formula diets in the treatment of IBD also suggests a role for diet or gut microbiota in the inflammatory process. Enteral nutritional therapy is a treatment modality which requires that a significant percentage of a patient’s calories come from a defined formula. Defined formula diets are a commonly prescribed therapy for Crohn’s disease worldwide, and there is a significant body of evidence supporting this approach (Critch et al. 2012). For example, a recent small, randomized, controlled trial demonstrated that enteral nutritional therapy may be more effective than corticosteroids in achieving mucosal healing in Crohn’s disease. The mechanism of action of defined formula diets in IBD is an active area of research, although modulation of the gut microbiota has been suggested (Leach et al. 2008). These observations emphasize the importance of understanding interactions between the intestinal immune system and luminal bacteria.
Regardless of genetic or environmental effects, the net result in IBD is continued and inappropriate activation of the intestinal immune system. More specifically, overactivity of certain T-helper cells results in poorly regulated secretion of proinflammatory cytokines. Examples of these cytokines include tumor necrosis factor alpha (TNF-α), interleukin-1 β, interferon γ, and cytokines of the interleukin 23-Th 17 pathway. Also, interleukin-10, an anti-inflammatory mediator, may be deficient in certain patients with inflammatory bowel disease (Abraham and Cho 2009). IL-10-deficient mice develop chronic IBD with a transmural leukocyte infiltration (Elson et al. 2005). Also, loss-of-function mutations in a component of the IL-10 receptor were recently identified in a small cohort of pediatric patients presenting with severe, early-onset intestinal inflammation and perianal fistulae (Glocker et al. 2010). The role of the abnormal immune response in inflammatory bowel disease is also illustrated by the IBD-like clinical and histopathological presentation of several primary immunodeficiencies (Table 6.4), more fully discussed in Chap. 5.
Table 6.4
Congenital immunodeficiencies that may have an IBD-like presentation
X-linked agammaglobulinemia |
Common variable immunodeficiency |
IgA deficiency |
Wiskott-Aldrich syndrome |
Chronic granulomatous disease |
Glycogen storage disease type Ib |
6.7.2 Clinical Manifestations
The clinical manifestations of IBD are dependent upon the disease distribution and the intensity of intestinal inflammation. For example, in ulcerative colitis (UC), which involves the colon exclusively, the most common presenting symptoms are abdominal pain, diarrhea, and rectal bleeding. In pediatric UC, 83–95 % of patients present with rectal bleeding (Bousvaros et al. 2007). While isolated left-sided colitis is common in adults, most children with UC present with inflammation extending proximal to the splenic flexure. A statewide epidemiological study in Wisconsin evaluating pediatric patients newly diagnosed with IBD found left-sided colitis in only 10 % of children with UC at the time of presentation. Conversely, 90 % of children newly diagnosed with UC presented with pancolitis (Kugathasan et al. 2003). Children with UC often present with more severe symptoms than adult patients, and this may be related to the frequently observed more extensive disease distribution.
In contrast to ulcerative colitis, Crohn’s disease may involve any portion of the gastrointestinal tract. Again, the location and the extent of the disease determine the presentation. The majority of children present with disease of the terminal ileum (50–70 %) (Lenaerts et al. 1989). Ten percent to 20 % of children have isolated colonic disease, and 10–15 % have diffuse small bowel disease of the more proximal ileum or jejunum (Lenaerts et al. 1989). Isolated gastroduodenal disease is observed in less than 5 % of patients; however, there may be endoscopic and histologic evidence of gastroduodenal inflammation in up to 30–40 % of children with Crohn’s disease (Lenaerts et al. 1989). Crohn’s disease involving the small intestine usually presents with evidence of malabsorption, diarrhea, abdominal pain, growth deceleration, weight loss, and anorexia. Initially, these symptoms may be quite subtle. Growth failure is usually insidious and may precede the onset of intestinal symptoms (Hildebrand et al. 1994). Colonic Crohn’s disease may be clinically indistinguishable from ulcerative colitis with symptoms of bloody abdominal pain, diarrhea, rectal bleeding, urgency, and tenesmus. Aside from intestinal inflammation, perianal disease affects approximately 15 % of pediatric patients with Crohn’s disease and includes simple skin tags, fissures, abscesses, and fistulae (Markowitz et al. 1984). Finally, approximately one-fourth of pediatric patients with IBD experience extraintestinal manifestations (see next section) (Dotson et al. 2010). Some of the salient differences in clinical presentation between pediatric- and adult-onset IBD are summarized in Table 6.5.
Table 6.5
Contrasting features between IBD in children and IBD in adults
Genetic factors important in early-onset IBD |
Family history of IBD present in 40 % of children |
Crohn’s disease |
Children more often present with colitis |
Children have more inflammatory disease than adults as per Vienna classification (stricturing/fistulizing/inflammatory) |
Growth failure is major presenting feature |
Isolated ileal disease less frequent in younger children (increases with age) |
Ulcerative colitis |
Pancolitis more frequent at presentation in children than adults |
More frequent progression to extensive disease in children with initially limited disease |
6.7.3 Extraintestinal Manifestations of IBD
Twenty-five to 35 % of patients with inflammatory bowel disease have at least one inflammatory disorder outside of the gastrointestinal tract. The extraintestinal manifestations include arthritis, dermatopathies, ophthalmologic abnormalities, and hepatobiliary disease.
Arthritis is the most common extraintestinal manifestation in children and adolescents, occurring in 7–25 % of pediatric patients (Frayha et al. 1975). Arthritis is usually a transient, nondeforming synovitis which is asymmetrically distributed and commonly involves the knees. Arthritis may precede gastrointestinal symptoms (Lindsley and Schaller 1974). Ankylosing spondylitis, an HLA-B27-associated disease, occurs in 2–6 % of patients with IBD (Palm et al. 2002).
Skin manifestations of IBD include erythema nodosum and pyoderma gangrenosum. Erythema nodosum affects 3 % of pediatric Crohn’s disease patients and usually occurs in the setting of active disease (Dotson et al. 2010). Erythema nodosum lesions are macular, erythematous, tender nodules that appear primarily on anterior surfaces of the legs. Pyoderma gangrenosum affects less than 1 % of pediatric IBD patients (Dotson et al. 2010) and appears as an indolent chronic ulcer, which may occur in disease remission.
The incidence of ophthalmologic manifestations of inflammatory bowel disease is 4 % in adults and is presumed to be lower in pediatric patients (Hofley et al. 1993). Episcleritis and anterior uveitis are the most common ocular findings. Uveitis is commonly symptomatic resulting in pain and/or diminished visual acuity.
Several hepatobiliary diseases have been reported in pediatric inflammatory bowel disease patients including hepatitis (autoimmune or granulomatous), primary sclerosing cholangitis, hepatic steatosis, amyloidosis, cholelithiasis, and pancreatitis (Dotson et al. 2010). The most common, serious, hepatobiliary manifestation of IBD in children is primary sclerosing cholangitis (PSC) (Jose and Heyman 2008). PSC occurs in fewer than 10 % of patients (Dotson et al. 2010).
6.7.4 Pathology
Endoscopic GI biopsies remain the gold standard for the diagnosis of IBD. Concomitantly, the marked increase in the use of endoscopy in routine pediatric GI practice during the past few decades presents the pathologist with more extensive sampling of the gastrointestinal tract, with samples from earlier stages of disease or from disease with atypical clinical features. The purpose of the next few sections is thus to remind the pathologist of the features that distinguish IBD from infectious and other colitides and that distinguish ulcerative colitis from Crohn’s disease, emphasizing atypical features of ulcerative colitis that may confound the two entities.
6.7.4.1 Distinguishing Acute Self-Limited Colitis from IBD
Endoscopic features alone may not reliably distinguish acute self-limited colitis (ASLC) from IBD. Stool cultures and duration of diarrhea may help, as patients without an identifiable pathogen or in whom diarrhea lasts more than several weeks are more likely to have IBD. However, microbiologic investigations can reveal a colitis-causing pathogen such as Salmonella, Campylobacter, and Yersinia in up to 15 % of patients with IBD (Schumacher 1993). ASLC is characterized by a predominantly neutrophilic infiltrate without significant crypt architectural changes. Neutrophils in these cases predominate in the superficial portion of the mucosa and may be associated with small erosions or ulcers (Jenkins et al. 1997). Neutrophils may also invade the crypt epithelium (cryptitis) or form small abscesses within the crypt lumen (crypt abscesses). Although numerous crypt abscesses suggest UC, they may be noted in CD as well as in infections and Clostridium difficile-related injury. The histologic diagnosis of IBD rests on the recognition of chronic mucosal damage, chronic colitis. Multiple biopsy studies of new-onset IBD in adults have shown that histologic features can reliably distinguish IBD from self-limited colitis (Dundas et al. 1997; Jenkins et al. 1997; Nostrant et al. 1987; Tanaka et al. 1999). These include irregular branching of the crypts with loss of their regular outline, shortening of the crypts, and areas of crypt loss (Fig. 6.13). Shortening of the crypts most often results from the presence of a basal chronic inflammatory infiltrate (basal plasmacytosis) between the base of the crypts and the muscularis mucosae. The presence of an increased mononuclear inflammatory cell infiltrate alone is the least useful histologic marker given the variable numbers of lymphocytes and plasma cells in normal specimens.
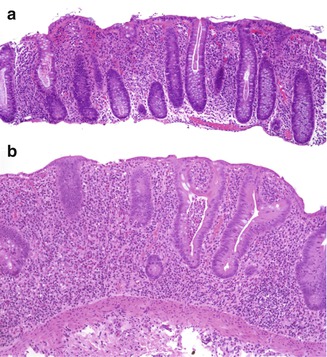

Fig. 6.13
Acute self-limited colitis versus IBD. (a) A 13-year-old female with infectious colitis. The inflammatory infiltrate is predominantly neutrophilic and is present mostly in the upper portion of the mucosa (“top-heavy”). The architecture of the crypts is well preserved. (b) A 4-year-old girl with failure to thrive, abdominal pain and diarrhea of several months’ duration. This biopsy shows features of IBD: there is crypt distortion with shortening of the crypts, in association with a predominantly mononuclear inflammatory infiltrate distributed throughout the mucosa. A crypt microabscess is present
6.7.4.2 Histologic Features of Early-Onset IBD
Despite the importance of recognizing chronic mucosal changes in the biopsies of patients with IBD, it has been well documented that initial colonic or rectal biopsies from 10 to 34 % of pediatric patients ultimately shown to have UC lacked architectural distortion or other histologic features of chronic colitis (Escher et al. 2002; Glickman et al. 2004; Konuma et al. 1995; Markowitz et al. 1993; Robert et al. 2004a; Washington et al. 2002). This is seen particularly in younger patients (<10 years) and may be due to shorter duration of symptoms or longer progression to chronicity in children (Robert et al. 2004b). Focal active colitis may be a feature of self-limited colitis but may also be an early manifestation of IBD (Xin et al. 2003). Close follow-up and repeat biopsies may be necessary in these cases (Fig. 6.14). Increased mucosal eosinophils may be seen in the earliest biopsies of children eventually proven to have IBD, prompting a diagnosis of food allergy. In a recent case series of IBD diagnosed in 16 children less than 2 years of age, 6 children had an initial diagnosis of allergy (Cannioto et al. 2009). On the other hand, histologic features similar to IBD may be seen in patients with primary immunodeficiencies and autoimmune enteropathy (Daniels et al. 2007). These conditions should always merit consideration when clinical manifestations of IBD occur in younger children. Histologic features which may point to a correct diagnosis in these patients include lack or paucity of plasma cells in the inflammatory infiltrate (as in common variable immunodeficiency or severe combined immunodeficiency), extensive crypt apoptotic activity, or absence of goblet and Paneth cells (as in autoimmune enteropathy). Mutations in genes coding for cytokines and immune response may underlie cases of IBD manifesting in infancy or early childhood (Glocker et al. 2010).
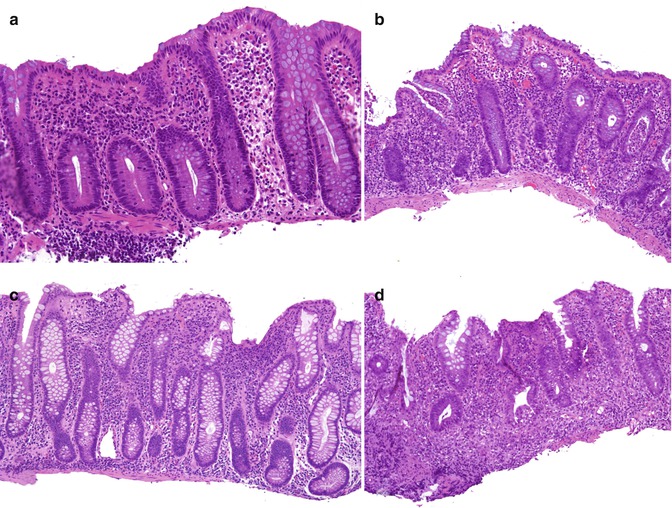

Fig. 6.14
Early IBD. (a) This 3-year-old girl was biopsied for rectal bleeding. The biopsy shows minimal inflammation with a mild eosinophilia, which prompted a diagnosis of possible food allergy. A single bifid crypt is noted to the right. (b) One year later, she underwent colonoscopy for recurrent rectal bleeding associated with failure to thrive, at which time a diffuse colitis was noted. A biopsy from the right colon reveals active chronic colitis with crypt architectural distortion and microabscesses. (c) This girl was biopsied at 4 years of age for diarrhea and failure to thrive. The lamina propria is hypercellular, and there is mild crypt architectural distortion. (d) Repeat biopsies 4 months later for continued symptoms show active chronic inflammation with marked crypt architectural changes
6.7.5 Ulcerative Colitis
The macroscopic and microscopic features which distinguish UC and Crohn’s disease are, in most respects, similar in children and adults and are outlined in Table 6.6. However, biopsies in the early stages of disease, even in combination with clinical and radiologic features, may not allow distinction between these two entities. Absence of ileal involvement does not rule out CD and appears to be more frequent in younger patient with CD than older children or adults (Guariso et al. 2010). Similarly, diffuse colitis may be a manifestation of both CD and UC in children. Distinguishing between the two early on, however, may not be critical as the initial medical therapy is similar.
Table 6.6
Distinguishing features of ulcerative colitis and Crohn’s disease
Ulcerative colitis | Crohn’s disease | |
|---|---|---|
Macroscopic | ||
Rectal involvement | Yesa | Variable |
Distribution | Diffusea | Segmental or diffuse |
Terminal ileum | “Backwash” ileitis | Often thickened and stenosed |
Serosa | Usually normal | “Creeping fat” |
Bowel wall | Normal thickness | Frequently thickened |
Mucosa | Hemorrhagic | Cobblestone and ulcers linear |
Pseudopolyps | Frequent | Less common |
Strictures | No | Common |
Fistulas | No | Common |
Involvement of gut proximal to colon | Nob | Common |
Microscopic | ||
Inflammation | Confined to mucosa and superficial submucosa | Transmural |
Lymphoid hyperplasia | Infrequent | Common |
Crypt abscesses | Extensive | Focal |
Mucus depletion | Frequent | Infrequent |
Deeply situated sarcoid-like granulomas | No | Yes |
Fissures and sinuses | No | Yes |
Villous surface transformation | Common | Infrequent |
Submucosal fibrosis | Rare | Common |
Neuromatous hyperplasia | Rare | Common |
UC is classically defined as diffuse chronic mucosal inflammation limited to the colon, which invariably affects the rectum, and extends proximally in a symmetric uninterrupted pattern to involve part or all of the large intestine. The mucosa characteristically exhibits a diffuse hemorrhagic appearance (Fig. 6.15a).
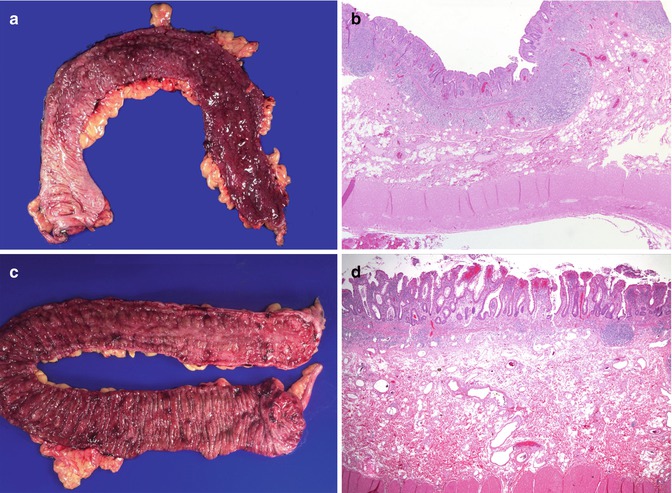

Fig. 6.15
Ulcerative colitis. (a) Colectomy specimen from a 4-year-old girl with bloody diarrhea and failure to thrive who failed medical therapy. There is continuous and diffuse involvement of the mucosa from the distal portion of the colon, on the right of the photograph, with decreasing involvement toward the proximal colon (on the left side of the photograph). (b) Inflammation is limited to the mucosa and superficial submucosa. Small superficial ulcers are noted. (c) Colectomy specimen from a 13-year-old girl. Although the entire mucosal surface is hemorrhagic, disease predominates in the distal colon where the mucosa is cobblestoned and ulcerated. The mucosal surface in the more proximal colon is hemorrhagic, but there is preservation of the haustral folds. (d) Low-power histologic section highlights a superficial inflammatory infiltrate with a villiform epithelial surface and crypt architectural distortion
Microscopically, ulcerative colitis is characterized by inflammation limited to the mucosa and superficial submucosa (Fig. 6.15b); deeper layers of the bowel are only exceptionally involved, as in toxic megacolon. Neutrophilic mucosal infiltration, cryptitis, crypt microascesses, epithelial degeneration and goblet cell depletion are characteristic though relatively nonspecific microscopic feature of active UC. Chronicity, as previously defined, is characterized by crypt architectural changes such as irregular branching and atrophy, usually accompanied by a mononuclear inflammatory infiltrate. Increased crypt epithelial turnover in UC results in goblet cell depletion and Paneth cell metaplasia (Tanaka et al. 2001), less frequently observed in CD. The latter must be interpreted with caution in pediatric cases, as Paneth cells can be present in the distal colon in normal young children. Crypt abscesses are not specific, but when diffuse are suggestive of UC, whereas they tend to be more isolated in Crohn’s disease (Riddell 2000). Rupture of crypt abscesses into the lamina propria or erosions may result in collections of histiocytes which may simulate but should be distinguished from true granulomas (Fig. 6.16).
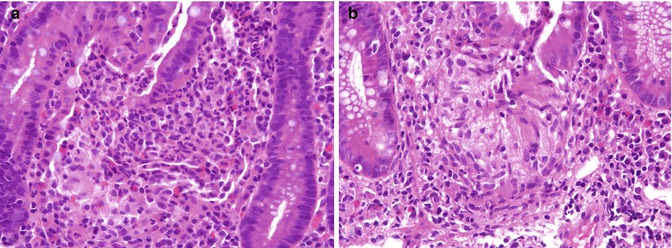

Fig. 6.16
Ulcerative colitis. (a) Histiocytoid reaction at the base of a ruptured crypt in this biopsy from a 16-year-old boy with disease limited to the left colon does not represent a true granuloma. (b) A mucin granuloma at the base of a crypt from an 8-year-old girl with a diagnosis of ulcerative colitis. Contrast with Fig. 6.23a
Pseudopolyps, more commonly found in UC than CD, are discrete areas resulting from surviving islands of mucosa or heaped-up granulation tissue. The latter are more accurately referred to as “inflammatory polyps.” Occasionally, regenerating mucosa within such an inflammatory polyp may form irregular, dilated glands, which bear a marked resemblance to retention or “juvenile” polyps (Riddell 2000). In contrast to adenomas, pseudopolyps have a short stalk and are generally smooth surfaced (Fig. 6.17). Extensive arborization and fusion of the polyps may result in mucosal bridging.
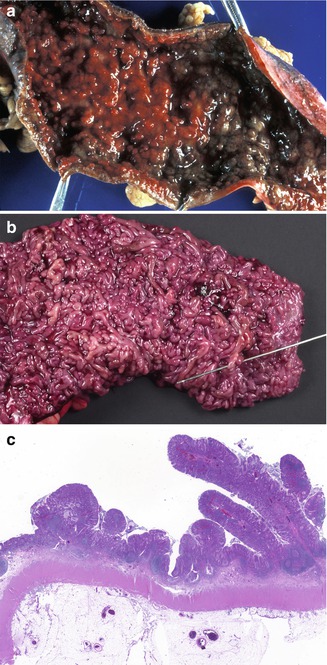

Fig. 6.17
Pseudopolyps. (a) Colectomy specimen from a 14-year-old male with severe ulcerative colitis characterized by a hemorrhagic mucosa with numerous pseudopolyps. (b) A 17-year-old female with long-standing ulcerative colitis. This is an unusual example of extensive polypoid transformation of the colonic mucosa. Fusion of the polyps has resulted in mucosal bridges as demonstrated by the probe. (c) Low-power section of “a.” “Filiform” elongated polyps with a narrow stalk as well as the more usual broad-based polyps are seen
6.7.6 “Atypical” Features in the Diagnosis of Ulcerative Colitis
6.7.6.1 Rectal Sparing and Patchiness
Although ulcerative colitis is traditionally considered to be a diffuse process that begins in the rectum and extends proximally in a continuous fashion, a number of studies suggest that initial rectal biopsies in children with UC may not demonstrate mucosal architectural changes as consistently as in adults or may even be “normal” (rectal sparing). An unequivocal diagnosis of IBD may be more difficult in these cases, as may be the distinction between UC and CD.
Five of 12 children with untreated UC in one study were found to have mild patchy inflammation or normal histology in the rectum (Markowitz et al. 1993), whereas relative rectal sparing compared to adults was found in one study of 53 children (Washington et al. 2002). In one study, “absolute” rectal sparing, in which evidence of both inflammation and chronicity is absent, is nonetheless infrequent in children with UC (4 % of 73 pediatric cases), though “relative” rectal sparing, defined as the presence of inflammation without changes of chronicity, is more frequent, noted in 26 % of cases (Glickman et al. 2004). Faubion et al. identified a 27 % prevalence of rectal sparing in children with IBD and sclerosing cholangitis, suggesting the possibility that rectal sparing may be more common in this subset of patients (Faubion et al. 2001). Moreover, discontinuous involvement and rectal healing have been reported during the course of long-standing disease in adults, which likely result from treatment effect or natural variation in the course of disease and also reflect the current clinical practice of sampling multiple mucosal biopsies over time (Kim et al. 1999; Kleer and Appelman 1998). Medical therapy can have a profound but variable effect on mucosal histology, ranging from decreased intensity of the inflammatory infiltrate to complete normalization of the mucosa, including discontinuity of mucosal disease in UC (Geboes and Dalle 2002). Quiescent colitis is characterized by mucosal atrophy and crypt architectural changes in the absence of acute inflammation, ulceration, and mucus depletion seen in the active phase (Fig. 6.18).
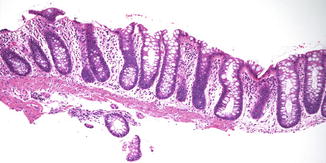





Fig. 6.18
Treated colitis. Rectal biopsy from an 11-year-old boy with a history of ulcerative colitis following treatment for several months. There is no significant inflammation and only mild crypt architectural changes are noted
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








