ap < 0.05.
Abbreviations: nr: not reported; ns: not significant; adjunct: in addition to standard antibiotics (vancomycin or metronidazole).
While intravenous vancomycin has little efficacy, use of vancomycin enemas is an alternative when the oral route is not feasible, e.g. in patients with paralytic ileus. However, there are no controlled trials demonstrating efficacy of this approach. Even the case series that suggest benefit are small. A recent series of nine hospitalized patients with severe C. difficile colitis showed response to adjunctive intracolonic therapy in eight (89%) [102]. Previously pub lished data suggested an overall efficacy of 83% (from 24 prior cases). There are insufficient data on which to make a recommendation for this form of therapy.
Table 20.2 Randomized, controlled trials of treatments for patients with recurrent Clostridium difficile associated disease (CDAD).
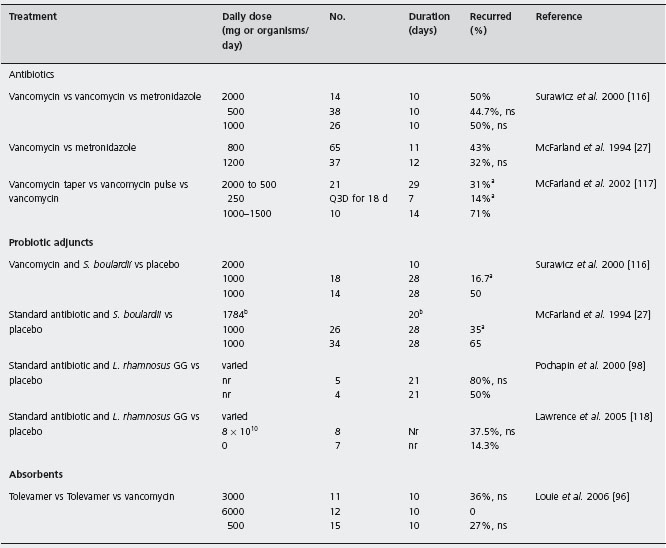
ap< 0.05
bdose or day varied, mean reported
Abbreviations: bid: twice a day; qid: four times a day; Q3D: once every three days; ns: not significant.
Metronidazole
Oral metronidazole has been used for treatment of initial CDAD, and several clinical practice guidelines suggest that it should be first- line therapy. Recommended treatment is 250–500 mg orally four times a day for 7–14 days. Studies have shown a good response rate (>95%) [88, 93]. A1c Metronidazole cure rates generally range between 76–90%, but the failure rate has increased from <16% pre-2003 to over 35% since 2004. In addition, the recurrence rate has also increased from ~20% pre-2003 to up to 47% since 2004. In 845 patients treated with metronidazole in Quebec during the 2003–2004 outbreaks, 26% failed initial treatment and 47.2% recurred within 60 days of treatment [17]. B4 One proposed explanation of a higher rate of metronidazole treatment failure is that metronidazole resistance may have developed. However, metronidazole resistance is infrequent in C. difficile isolates. Of 415 C. difficile isolates from patients in Spain, only 6% of isolates were resistant to metronidazole and 3% exhibited reduced susceptibility to vancomycin [103].
Another area of debate is the route of administration. Oral antibiotic treatment is preferred, but some patients have an ileus or toxic colon precluding the oral route. Observational evidence from case reports suggests that intravenous metronidazole is effective. In a series of 10 patients with C. difficile colitis given intravenous therapy a good response was observed in nine [104]. There are no controlled trials evaluating the efficacy of intravenous metronidazole.
Investigational treatments
As shown in Table 20.1, due to the occurrence of treatment failures and of recurrent disease , new interventions are under investigation. Tinidazole has been tested and found to be safe in phase 2 trials, but phase 3 trials are needed to demonstrate efficacy [105].
Absorbents
Another approach is the administration of an agent that may bind C. difficile toxins within the intestinal lumen. In a phase 2 randomized double-blind trial, tolevamer in doses of 3 or 6g/d) was compared to vancomycin (500mg/d) for 10 days in patients with initial or recurrent CDAD [96]. For patients with initial CDAD, the rates of recurrence were not significantly different by treatment group: 4/30 (13%) in the 3g tolevamer group, 5/39 (13%) in the 6g tolevamer group and 10/56 (18%) in the vancomycin group. A1d Phase 3 trials were recently completed, but the results have not yet been published.
Bacitracin
Bacitracin is an orally administered non-absorbable antibiotic, that is characterized by an unpleasant taste and is relatively expensive. Two uncontrolled studies in 1980 showed response to therapy in small numbers of patients (two and four patients each) [106,107]. More recently a double-blind randomized, controlled trial compared bacitracin (80,000 units daily) with vancomycin (500 mg daily) in 42 patients. Clinical response (decreased diarrhea at four days) was similar in the two groups. A1d However, the bacitracin treated patient had higher rates of persistent C. difficile positive cultures in the stools [89]. The authors recommended bacitracin as a first-line alternative to vancomycin, A second randomized, controlled trial in 24 patients also showed similar efficacy of bacitracin and vancomycin, although vancomycin was more effective in clearing C. difficile and toxin from the stool [90]. A randomized trial com paring metronidazole and bacitracin would be important and has not been performed.
Fusidic acid
Fusidic acid is considered to be an L-selectin blocker that inhibits leukocyte extravasation into inflamed sites [108]. In a small randomized trial in which 40 patients were randomized to one of four oral agents: fusidic acid, metronidazole, vancomycin and teicoplanin the response rates were similar for all four groups (93–96%). The authors recommend metronidazole because of its lower costs, reserving other drugs for patients who do not tolerate or respond to metronidazole [93]. A randomized, controlled trial in 131 patients with initial episodes of CDAD comparing fusidic acid (750mg/d) and metronidazole (1200mg/d) for seven days showed no statistically significant difference in clinical cure rate [97]. A1d
A phase 3 trial with patients randomized to fusidic acid (n = 59) or metronidazole (n = 55) for seven days found equivalent rates of cure and recurrences. A1d Resistance to fusidic acid was found in 1% of colonic isolates obtained from pre-treatment patients and from 11 /20 (55%) of isolates in those patients who remained culture positive after treatment with fusidic acid [109].
Probiotics
Probiotics are defined as live microorganisms that confer a health benefit on the host when administered in adequate amounts [110]. Probiotics have no proven role in treatment of initial CDAD. In a placebo controlled randomized trial of a probiotic mixture for the prevention of antibiotic-associated diarrhea, a secondary outcome was the prevention of CDAD [111]. Patients were randomized to receive either a probiotic mixture of Lactobacillus casei, Lactobacillus bul-garicus and Streptococcus thermophilus (Actimel drink) at a dose of 2.2 × 108cfu/day or a placebo drink for the duration of antibiotic therapy plus one additional week. Of the 113 subjects completing the trial, 0/56 (0%) developed CDAD in the probiotic group compared to 9/53 (17%) in the placebo group, p < 0.05. A1c The estimated cost of preventing one case of CDAD with probiotic was $120.00. Currently, several probiotics are under development for CDAD including Lactobacillus acidophilus, Saccharomyces boulardii and a non-toxigenic strain of Clostridium difficile.
Ramoplanin
This antibiotic targets bacterial DNA-dependent RNA polymerase and inhibits cell wall synthesis. In a phase 2 open-label trial of 86 CDAD patients were treated with 10 days of either ramoplanin (200 mg bid or 400 mg bid) orease vancomycin (125mg qid) [94]. The rates of cure were similar in all groups: 83% and 85% for patients receiving 200 mg or 400 mg of ramoplanin and 86% for patients receiving vancomycin. A1d
Rifampin
In a phase 2 study of Rifampin 39 patients were randomized to receive either metronidazole 500 mg tid or met-ronidazole plus rifampin 300mg bid for 10 days. The recurrence rates were similar by day 40 (38% and 42% respectively) [95]. A1d
Teicoplanin
De Lalla et al. reported a randomized, controlled trial in which 46 patients with CDAD received either teicoplanin (200mg) or vancomycin 2g daily for 10 days [92]. The initial cure rates for teicoplanin and vancomycin were 96% and 100%, and the recurrence rates were 7.7% and 20%. These differences were not statistically significant. However, teicoplanin appeared to be significantly more protective against recurrent disease than fusidic acid (RR = 0.19; 95% CI: 0.04 to 0.99) (Table 20.1) [93]. A1d
Tiacumicin B
Tiacumicin B (also known as OPT-80 and PAR-101) is a macrocyclic antibiomicrobial that targets RNA polymerase. Early phase 1 studies showed it was well tolerated in healthy volunteers at doses up to 450mg/day [112]. In a phase 2 dose ranging study in 49 patients with mild to moderate CDAD [105] the cure rates were similar in patients receiving 100 mg (86 %), 200 mg (87%) or 400 mg (100%). The recurrence rates for these treatments were also similar: 8%, 0% and 6%. Phase 3 trials are in progress.
Treatment for recurrent CDAD
Most patients (76–98%) with their first episode of CDAD are cured after treatment with either vancomycin or metronidazole [9, 99]. However, a proportion of patients develop recurrent episodes of CDAD that may last for years, despite antibiotic treatment [9, 60]. Once a second episode of CDAD occurs, 60% of patients continue to experience subsequent episodes and are considered to have an especially difficult form of CDAD, designated “recurrent CDAD” [9, 60]. Treatment of recurrent CDAD has traditionally relied upon antibiotics (usually vancomycin or metronidazole), toxin binding resins, fecal enemas and the use of biotherapeutic agents [13, 26, 27,113–115].
Vancomycin or metronidazole treatments
There are a limited number of randomized trials comparing antibiotic treatments in patients with recurrent CDAD (in which patients with an initial episode have been specifically excluded) (Table 20.2) [27, 96, 98,116–118]. Most trials have been in patients with initial disease, or have not specifically included or excluded patients with prior CDAD episodes. In a randomized, double-blind, placebo-controlled trial of an investigational probiotic treatment patients with recurrent CDAD were randomized to receive 10 days of either high dose vancomycin (2g/d), low dose vancomycin (500mg/d) or metronidazole (1 g/d) plus either the probiotic or a placebo. In the three antibiotic treatment groups that received the probiotic placebo the observed recurrence rates were similar: high dose vancomycin 50%, low dose vancomycin 45% and metronidazole 50%. An earlier double-blind, placebo-controlled trial compared the efficacy of these antibiotics in various doses with a placebo in 102 patients. [27]. The observed recurrence rates of 43% for vancomycin-treated patients and 32% for metronida-zole-treated patients were not significantly different. A1a
A series of 163 patients with recurrent CDAD who were followed prospectively documented the rate of CDAD recurrences over a two-month period in patients who were treated with a variety of strategies using either vancomycin or metronidazole [117]. Of the 125 patients treated with a variety of doses and durations of vancomycin, 46% subsequently developed recurrent CDAD. This recurrence rate was not significantly different from the rate of 42% in the 38 patients treated with metronidazole (p > 0.05). B4
Similar rates of recurrence following therapy with vancomycin and metronidazole are seen despite the differences in the pharmacokinetics of these agents in the intestine. In the healthy intestine, metronidazole is rapidly absorbed from the feces, and the concentration within the lumen of the gut is low. Once an acute episode of CDAD occurs, high concentrations of metronidazole have been documented in the infected intestine [119]. Vancomycin is bacteriostatic on Clostridium difficile organisms, thus allowing the persistence of vegetative cells of Clostridium difficile and the rapid increase in Clostridium difficile after discontinuation [120]. The time required for spore germination, C. difficile overgrowth and acute toxigenic symptoms may be extremely short (usually 3–5 days) once the treatment antibiotics have been discontinued. In one long-term follow-up study 97% of recurrences were observed within four weeks of stopping antibiotics (median of seven days) [14]. The short time between the end of antibiotic therapy and the recurrence of symptoms was confirmed by two other studies of patients with recurrent CDAD [9, 99]. Delayed onsets of new episodes (four to eight weeks later) reported in some studies may be due to the exposure to exogenous spores or germination of asymptomatic carriage of Clostridium difficile during the time when the normal colonic flora has not yet recovered. A previous study has shown that antibiotics may disrupt the normal flora for up to six weeks after discontinuation [121]. Continuation or restarting inciting antibiotics after successful treatments has been shown to increase the risk of recurrence [122].
Thus, the efficacy of treatment for recurrent CDAD may be influenced by the presence of residual Clostridium difficile spores in the intestinal tract and the interval over which the intestine is susceptible to Clostridium difficile overgrowth. Short duration antibiotic treatments may be effective in initially resolving the symptoms of diarrhea, but may be ineffective during the “window of susceptibility”, i.e. the interval during which the intestinal micro-flora needs to be re-established in order to resist the overgrowth of Clostridium difficile and recurrence of disease. Several strategies have been tested, including providing extended protection by tapering the dose or providing “pulsed” doses of antibiotics, use of biotherapeutic agents (“beneficial microbes”) or restoration of intestinal micro-flora using fecal infusions of bacteria or normal stool contents.
Vancomycin taper/pulse
There have been two studies of patients with recurrent CDAD treated with either tapering or pulsed dosing of vancomycin. In an observational study of 163 patients with recurrent CDAD who were treated with a variety of strategies that included either vancomycin or metronidazole and followed for two months the overall recurrence rates for all patients receiving the two antibiotics were not significantly different [117]. B4 Vancomycin tapering and vancomycin pulsed dosing were shown to be effective for reducing the frequency of recurrences. The recurrence rate for 14 patients treated with vancomycin in doses between 1 and 1.5g/d for 10 days was 71% compared to a rate of 31% in 29 patients treated with a tapering dose of vancomycin (over a mean of 21 days), and there was only one recurrence in seven patients treated with a pulsed dosing of vancomycin (125–500 mg pulse every three days over a mean of 18 days). Although these patients were studied using standardized protocols this was not a randomized trial and the results are suggestive, but not definitive.
Tedesco et al. reported an uncontrolled study of 22 patients who had recurrent CDAD and were treated with a tapering dose of vancomycin for 21 days (500mg/d for one week, 250mg/d for the second week, 125mg/d for the third week, and 125 mg on alternate days for a fourth week) and a pulse dose (125mg every third day for 21 days). There were no recurrences, but the results of this uncontrolled study do not constitute definitive evidence [123]. B4
Adjunctive intracolonic vancomycin appeared to be beneficial in a case series of nine patients (three of whom had recurrent CDAD) but the results have not been confirmed in a randomized trial [102].
Other investigational treatments
In contrast to patients with initial CDAD, no randomized, controlled trials of teicoplanin, bacitracin, or rifampin have been performed for patients with recurrent CDAD.
Absorbents
Another approach that has been investigated in a phase 2 randomized double-blind trial is administration of an agent that may bind C. difficile toxins within the intestinal lumen. Tolevamer in doses of 3 or 6g/d was compared to vancomycin 500mg/d for 10 days in patients with initial or recurrent CDAD. In patients with recurrent CDAD, the rates of recurrence were: 4/11 in the 3g tolevamer group, 0/12 in the 6g tolevamer group and 4/15 in the vancomycin group [96]. These differences were not statistically significant, but this small study lacked power to demonstrate true differences in efficacy of these regimens, should they exist.
Ion exchange resins
Although in vivo studies of two resins, cholestyramine and colestipol, demonstrated binding of cytotoxin (as well as some binding of vancomycin), and cholestyramine delayed death in the hamster model of clindamycin-induced cecitis [124] trials of ion exchange materials to bind toxins in humans have produced negative results. Colestipol was compared to placebo in a randomized, controlled trial in 38 patients with postoperative diarrhea. There was no difference in fecal excretion of C. difficile toxin, and treatment with ion exchange resins currently is not recommended [125]. A1d The study, however, lacks adequate power to show true differences between the treatment groups, should they exist.
Probiotics
A meta-analysis of six randomized, controlled trials using probiotics combined with one of the two standard antibiotics to treat CDAD found that probiotics significantly reduced the risk of CDAD (combined RR = 0.59; 95% CI: 0.41, 0.85; p = 0.005) [126]. A1c Although a variety of probiotic strains have been tested, most studies were not large randomized, controlled trials. It is important to note that the term “probiotic” covers a wide variety of bacterial and yeast strains, and clinical efficacy may be linked to a specific strain. For example, not all Lactobacilli strains are equally effective probiotics for specific diseases. When considering efficacy of probiotics for any disease, it is important to link the clinical evidence with specific strains.
Saccharomyces boulardii
There have been two double-blind randomized, controlled trials for the use of Saccharomyces boulardii and antibiotics for patients with recurrent CDAD (Table 20.2). In a trial in 168 patients with recurrent CDAD, standard antibiotics were combined with Saccharomyces boulardii or placebo [116]. Three antibiotic regimens were used for 10 days: vancomycin in doses of 2 g or 500mg/d or metronidazole 1 g/d. At the end of the 10-day period either Saccharomyces boulardii or placebo (1g/d for 28 days) were added to the antibiotic regimen. The patients were followed for two months for subsequent Clostridium difficile recurrences. A significant decrease in recurrences was observed only in patients treated with the high dose vancomycin and Saccharomyces boulardii treatment (16.7%) compared with patients who received high dose vancomycin and placebo (50%, p = 0.05). A1c No significant reductions in recurrence rates in either the low dose vancomycin or metronidazole treatment groups, were observed in Saccharomyces boulardii treated patients, compared to placebo controls. No serious adverse effects were noted in any of these patients.
An earlier study had also indicated the efficacy of the combination treatment using a standard antibiotic (vancomycin or metronidazole) and Saccharomyces boulardii for patients with CDAD [27]. In this trial patients who received vancomycin or metronidazole in doses determined by the physician were randomized to receive either Saccharomyces boulardii (1g/d) or placebo for 28 days. All patients were followed for two months for subsequent recurrences. Approximately half of the enrolled patients were experiencing their first episode and half had recurrent CDAD. In the 60 patients with recurrent CDAD the recurrence rate in patients who received Saccharomyces boulardii was 35% compared to 65% for patients in the placebo group (p = 0.04). A1d In this small study vancomycin was not shown to be more effective than metronidazole, regardless of the dose or duration of treatment.
Lactobacillus rhamnosus GG
Lactobacillus GG is another probiotic that has been reported to reduce CDAD recurrences in several case series and case reports [113,127,128]. A small randomized trial comparing L. rhamnosus GG (8 x 1010 organisms/day) to placebo as an adjunctive to standard antibiotic therapy with vancomycin or metronidazole was terminated due to poor enrollment. The observed recurrence rates were 3/8 and 1/7 in the Lactobacillus and placebo groups [118].
Fecal biotherapy
In an effort to replace the microflora disrupted by recurrent CDAD and antibiotic treatments, fecal enemas of normal stool contents have been administered to patients with recurrent CDAD [129–133]. However, no randomized, placebo-controlled trials have been performed. In the case reports describing the use of fecal enemas in a total of 87 patients with recurrent CDAD, 77 (89%) of the patients responded to fecal biotherapy [134]. B4
Immunoglobulin
The use of immunoglobulin for patients with recurrent CDAD has been reported in the literature in small case series or case reports [135–137]. A phase 2 study of 79 patients who had toxin positive CDAD compared results in 18 patients given standard antibiotics and IV IgG treatment (200–300 mg/kg/d) with 61 controls who received standard antibiotics only. No significant differences in mortality (three died in each group) or colectomy (three in each group) were observed [138]. B4
Whey protein made from cows immunized with C. difficile is also being tested. In a phase 2 trial 77 CDAD patients were given anti-C. difficile whey protein concentrate in a dose of 5g tid for 14 days after 10 days of treatment with vancomycin. No adverse effects were observed. Four of 63 (6.3%) patients experienced relapse within 46 days of completing this treatment [139]. Phase 3 trials are in progress. Another investigational approach is the use of a targeted immunoglobulin product specifically directed against C. difficile toxin. The safety and kinetics of neutralizing human monoclonal antibody against C. difficile toxin A is under study. In a phase 2 dose ranging study in 30 CDAD patients no serious adverse effects were noted [140]. Phase 3 trials are in progress.
Treatment of asymptomatic carriers
Asymptomatic carriers of Clostridium difficile have been shown to be a source of new nosocomial cases of CDAD. In order to control the spread of Clostridium difficile, a policy of treating asymptomatic carriers has been tested by several investigators. However, treatment of asymptomatic carriers has not been found to reduce the incidence of CDAD and has not been shown to reduce the frequency of nosocomial outbreaks [141]. Bender et al. demonstrated that treatment of carriers with metronidazole was ineffective in reducing the incidence of new CDAD cases at a chronic care facility [142]. Delmee et al. were able to reduce the CDAD frequency in patients on a leukemia unit from 16.6% to 3.6% after all patients with Clostridium difficile were treated with vancomycin [143]. However, this study was not randomized and both symptomatic and asymptomatic Clostridium difficile cases were treated. Johnson et al. treated 30 asymptomatic carriers of Clostridium difficile in a randomized, placebo-controlled trial with either vancomycin (1 g/d), metronidazole (1g/d), or placebo [85]. Although vancomycin significantly reduced Clostridium difficile carriage at the end of the 10 days of treatment (10%) compared to either metronidazole (70%, p = 0.02) or placebo (80%, p = 0.005), the recurrence rate at the end of the two-month follow-up period was significantly higher (67%) in the vancomycin group compared to the placebo group (11%, p < 0.05). A1d Although there are small randomized, placebo-controlled trials demonstrating the effectiveness of treating asymptomatic carriers, the overall evidence is not sufficiently strong to support a recommendation for the use of this intervention.
Prevention
Infection control
The importance of infection control practices for the prevention and control of nosocomial infections of CDAD and recurrent CDAD has been well documented [13, 26]. Studies documenting that 48–56% of clinical recurrences are new infections with a different strain of Clostridium difficile also add support to the importance of disrupting the nosocomial acquisition of new strains of Clostridium difficile in the hospital environment [15, 64, 144].
The most important intervention for prevention of CDAD is the interruption of the horizontal transmission of Clostridium difficile. Five aspects of infection control practices have been investigated: (1) environmental disinfection of contaminated surfaces or fomites, and medical equipment or use of disposable instruments, (2) reducing hand carriage by hospital care personnel, (3) isolation or cohorting of infected patients, (4) treatment of asymptomatic carriers and (5) multi-disciplinary approach using a combination of the above. None of these infection control practices has been evaluated in randomized, controlled trials, but most have been evaluated in the hospital environment using a defined intervention and have compared infection rates before and after the introduction of the intervention or have compared the results between a ward in which the intervention was introduced with those in a control ward in which the new intervention was not used.
Another strategy under investigation is vaccination of newly admitted patients at health care facilities using a vaccine against the toxins of C. difficile. One such vaccine, developed by Acambis, is currently under investigation in phase 3 trials. A small phase 2 study in 30 subjects showed that this vaccine was not associated with any serious adverse effects [145].
Environmental disinfectants
Contamination of environmental surfaces by Clostridium difficile
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








