and Andrea Bischoff1
(1)
Pediatric Surgery, Colorectal Center for Children Cincinnati Children’s Hospital, Cincinnati, OH, USA
Electronic supplementary material
Supplementary material is available in the online version of this chapter at 10.1007/978-3-319-14989-9_16.
16.1 Cloaca
16.1.1 Definition and Management
A cloaca is a malformation that affects the rectum and urogenital tract in females. These girls are born with a single perineal orifice. The vagina, urethra, and rectum are fused together inside the pelvis, creating a single common channel that opens into a single orifice in the location where the urethra normally opens (Fig. 16.1). The length of the common channel varies from case to case, from 1 to about 10 cm with an average of approximately 3 cm.
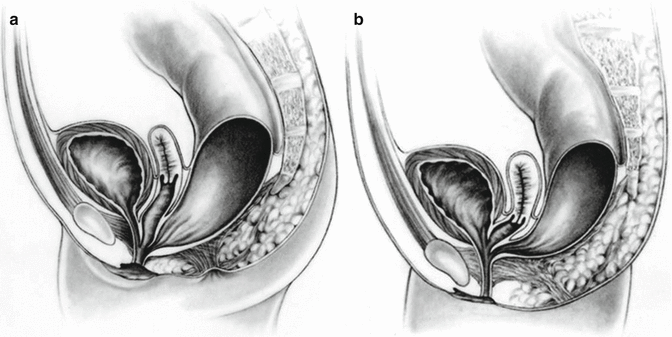

Fig. 16.1
Diagram of a cloaca. (a) Short common channel. (b) Long common channel
Thirty percent of these patients suffer in addition from a very dilated vagina full of fluid and/or mucus, called hydrocolpos [1] (Fig. 16.2). The reason why these very dilated vaginas retain fluid remains a mystery, since they are never really atretic. We speculate that there must be some sort of valve mechanism that interferes with the emptying of the fluid. Most of the patients with hydrocolpos, in addition, have duplicate Müllerian systems (Fig. 16.3).
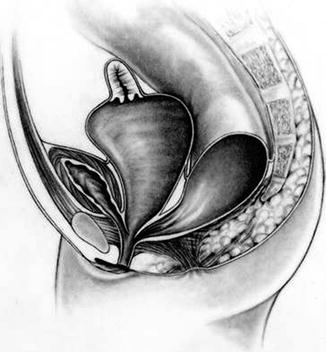
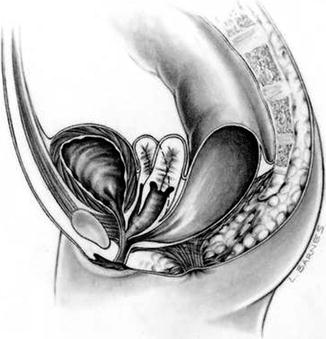

Fig. 16.2
Diagram of a cloaca with hydrocolpos

Fig. 16.3
Diagram showing a cloaca with two hemivaginas
The hydrocolpos may produce two important complications:
(a)
The first is the possibility of compressing the trigone of the bladder, producing an extrinsic ureterovesical obstruction, megaureter, and hydronephrosis.
(b)
The second possibility is that the hydrocolpos, left undrained, may become infected; creating a pyocolpos that eventually may perforate, which is a catastrophic event with risk of death. In addition, the resulting inflammation may scar the vagina and impact the future reconstruction.
Approximately 60 % of the patients with cloacas also have a double Müllerian system consisting of the presence of two hemiuteri and two hemivaginas [1]. This septation disorder may be partial or total. In addition, it can be symmetric or asymmetric. In the asymmetric types, the double Müllerian system phenomenon is frequently associated with a unilateral atresia of the Müllerian structure. When this goes unrecognized, it may produce an accumulation of menstrual blood at the age of puberty, as well as retrograde menstruation into the peritoneal cavity (Fig. 16.4) which produces rather dramatic signs of an acute abdomen and requires an emergency laparotomy. The presence of double Müllerian systems also has important potential obstetric implications that will be discussed later in this chapter.
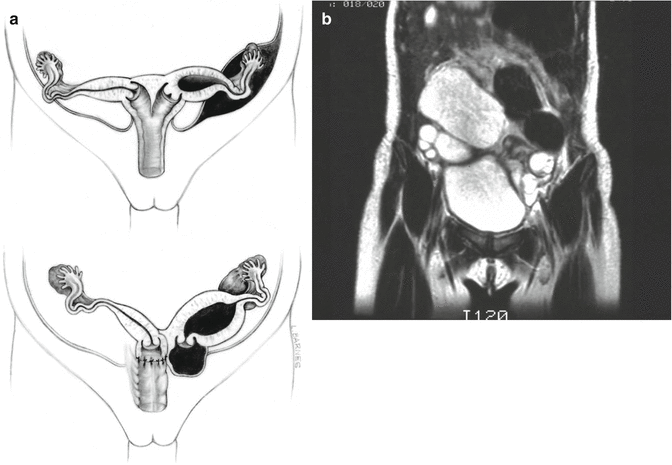

Fig. 16.4
Accumulation of menstrual blood in a patient with obstruction of the Müllerian structures. (a) Diagram. (b) MRI
Cloacas represent a very wide spectrum of defects, but the common denominator is the presence of a single perineal orifice. On the very bad side of the spectrum, one may find patients with a common channel as long as 10 cm; in such cases, usually two little hemivaginas, as well as the rectum, connect to the urinary tract at the bladder neck or above the bladder neck (at the trigone) (Fig. 16.5).
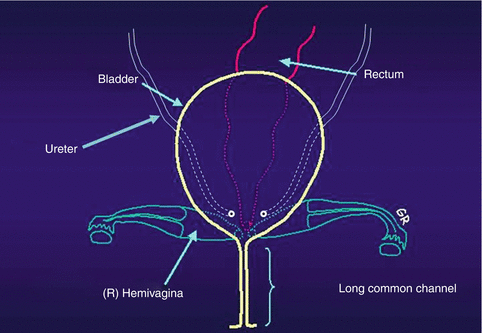

Fig. 16.5
Diagram showing a cloaca with a very long common channel
We are far from knowing the genetic causes of this condition [2]. Yet, we have never seen two cases of cloaca in the same family.
The knowledge of the intrinsic anatomic characteristics of this malformation is relatively new. We were able to detect and read old publications that we think described patients suffering from cloacas, although were not recognized as such [3–6].
We were also very impressed by the fact that most publications prior to 1982 reported high numbers of rectovaginal fistula cases and very few cloacas [7]. In retrospect, we are convinced that the authors were reporting patients suffering from cloacas as “vaginal fistulas.” We believe that because of the large number of cases of cloacas that we have seen, coming with a history of suffering from a “rectovaginal fistula,” yet, when we examined them, we found an untouched persistent urogenital sinus and a pulled-down rectum; in the medical records of those cases, the word cloaca is not present.
Most publications prior to 1982 reported very few cases of cloacas; many of them were autopsy findings. The cases that underwent an attempted repair suffered from a high mortality. The treatments used include a colostomy at birth, followed by a rectal pull-through, leaving the patient with a urogenital sinus to be repaired “later” [8–23].
The terminology used in those years was also confusing. The authors frequently published series that included cloacas and other different conditions such as “adrenal hyperplasia,” “vaginal atresia,” and “high anorectal malformation.” One particular publication from 1973 [17] is the most prominent one because it proposes the full repair of the vagina. However, looking at the diagrams, it becomes clear that the technique used by Dr. Raffensperger, the author, may be applicable only in cases with a relatively large vagina, located very low.
All pediatric surgeons as well as hundreds of patients are in debt with Dr. Hardy Hendren for his contributions in the field of pediatric surgery and pediatric urology. His seminal work on the surgical management of cloacas is the most important one that we found in our literature review [24–32]. In his initial publications, Dr. Hendren referred to this malformation as “urogenital sinus and anorectal malformation” [25]. Dr. Hendren’s contribution was particularly important in dealing with complex reoperations and repairing the challenging urologic-associated defects of these patients.
Some authors refer to cloacas with a clear embryologic bias, and therefore, they used rather confusing terms such as “urorectal septum malformation sequence” [33] or “urorectal septal defects” [34] including variants such as adrenal hyperplasia, as well as male cases [35]. Others include the cloacas as part of “Müllerian duct anomalies” [36].
16.1.1.1 Associated Defects
Twenty percent of those patients with a common channel longer than 3 cm had an absent kidney. When the common channel was shorter than 3 cm, 17 % of the patients suffered from this anomaly. Hydronephrosis occurred in 45 and 22 %, respectively, in patients with a common channel longer or shorter than 3 cm.
It is important to notice that in all other anorectal malformations, absent kidney is the most common anatomic-associated anomaly. The high incidence of hydronephrosis in this malformation is consistent with the fact that the most serious problems that these patients will suffer from (including death) are urologic.
Vesicoureteral reflux occurs in 40 and 21 %, respectively, in patients with common channel longer and shorter than 3 cm.
Most patients with hydronephrosis suffered from vesicoureteral reflux. At birth, however, some patients with hydronephrosis and megaureter seemed to suffer from a ureterovesical obstruction. In reality, the obstruction was an extrinsic one, caused by a tense hydrocolpos. When the hydrocolpos was drained, the vesicoureteral reflux becomes obvious.
Hemivertebra occurs in 13 % of cases (lumbar, thoracic, cervical, and sacral). The average sacral ratio in patients with cloacas is 0.52 AP and 0.64 lateral. Table 16.1
shows the correlation between sacral ratio and the length of the common channel.
Table 16.1
Correlation between sacral ratio and length common channel
Length of common channel | Sacral ratio | ||
|---|---|---|---|
Lateral film | Anterior/posterior film | Average | |
Less than 3 cm | 0.68 | 0.55 | 0.615 |
More than 3 cm | 0.6 | 0.53 | 0.56 |
Cardiovascular anomalies occur in 20 % of cases in cloacas. Patent ductus arteriosus occurs in 8 % of cases, atrial septum defect in 19 % of cases, ventricular septum defect in 5 % of cases, and tetralogy of Fallot in 2 % of cases. Tethered cord occurs in 36 % of the patients with cloacas.
Esophageal atresia was present in 11 % of our cloacas and duodenal atresia in 3 %.
16.1.1.2 Goals of Treatment
The treatment of cloacas represents a significant technical challenge. The final goals of treatment must result in a patient with urinary control, bowel control, sexual function, and capacity to procreate. These goals, of course, are sometimes achieved, sometimes partially achieved, and sometimes not achieved at all. For the worst scenario, we are firm in our philosophy that all patients with anorectal and urogenital malformations should be clean of stool and dry of urine in the underwear after the age of three, either because they were born with a benign malformation that was adequately reconstructed or because even when they were born with a malformation with bad functional prognosis, the patient is maintained artificially clean of stool (subjected to a successful bowel management program) [see Chap. 20] and dry of urine (subjected to intermittent catheterization) through the native urethra or through a neourethra (continent diversion).
Since we are dealing with a spectrum of defects, one should expect a spectrum of results after the treatment.
16.1.1.3 Neonatal Management
The diagnosis of a cloaca is a clinical one. It is enough to look at the patient’s perineum and make the correct diagnosis (Fig. 16.6). These patients have a single orifice, yet, the perineum has other important characteristics that help to predict the internal anatomy and the final functional prognosis. A “good-looking” perineum consists of the presence of a well-formed midline groove and a well-located and clear anal dimple, indicating that the patient has a good sphincter (Fig. 16.7). On the other hand, a “bad-looking” perineum includes a single perineal orifice but, in addition, a completely “flat bottom” with no traces of sphincter mechanism (Fig. 16.8) and most likely, a poor functional prognosis. In between those extremes of the spectrum, one can find a variety of external appearances.
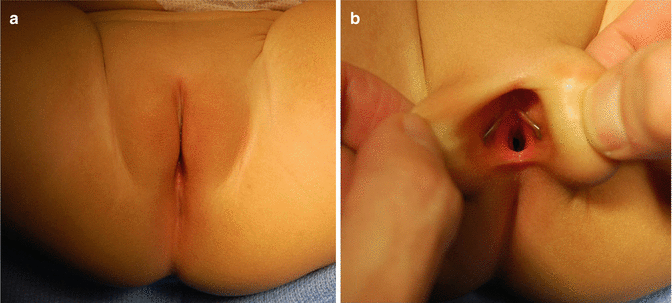

Fig. 16.6
Perineum of a patient with a cloaca. (a) Without separating the labia. (b) Separating the labia

Fig. 16.7
“Good-looking perineum.” Obvious midline groove and prominent anal dimple

Fig. 16.8
“Bad-looking perineum.” “Flat bottom,” absence of midline groove, no evidence of anal dimple
Occasionally, one can find a very large single perineal orifice, leaking urine, and with evidence of a mild separation of the pubic bones (Fig. 16.9). Those external signs correspond to a patient who has separated pubic bones and a malformation called covered cloacal exstrophy [37]. These patients have no bladder neck; their bladder is very small because it has never been full. Eventually, these girls will need a total urinary reconstruction. In addition, in these patients (covered exstrophies), it is very common to find inside the abdomen the same kind of anatomic abnormalities seen in cloacal exstrophies, mainly the presence of a very short colon with a very abnormal blood supply (Fig. 16.10). Yet, the abdominal wall is intact, which makes a difference with cloacal exstrophies (Fig. 16.11).
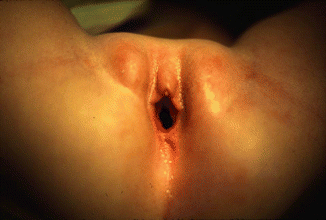
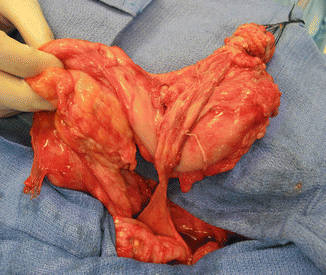
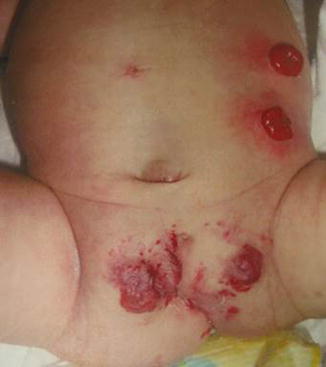

Fig. 16.9
Perineum of a patient with a large single perineal orifice. The pubic bones are separated

Fig. 16.10
Grotesque abnormal colon frequently seen in cases of cloacal exstrophies or in covered cloacal exstrophies

Fig. 16.11
External appearance of a patient with covered cloacal exstrophy. The umbilicus is frequently located lower than normal. Observe low implantation of the umbilicus and hemangiomas
It is not unusual to find hypertrophic folds of skin in the area of the single perineal orifice, which gives a false impression of a phallus (Fig. 16.12) [38], and that is why 65 cases in our series came to our institution with the misdiagnosis of intersex made at other hospitals. In our experience of over 531 cases, we only had one case of gonadal dysplasia associated with a vestibular fistula, but never with a cloaca.
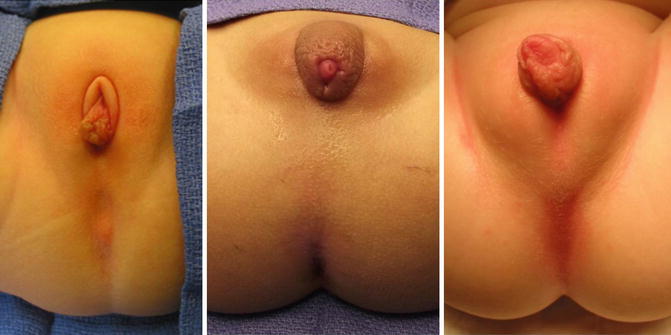

Fig. 16.12
Pictures of patients with a cloaca and a pseudophallus. Palpation of this structure allows to feel only folded skin and no real corpora
The patients that came to our institution with a cloaca and with a history of a suspected diagnosis of “intersex” described the unpleasant experience of being told that their baby had an undetermined gender. It usually took a couple of weeks, with consultation to urology, genetics, and many laboratory tests, to conclude that the patients actually were females suffering from a cloaca.
The key for the diagnosis of those cases with a pseudophallus resides in the palpation of that structure. One can feel that it is actually folded skin with no palpable corpora. Retrospectively, we found five publications referring to this condition as “pseudohermaphroditism” [39]. Some authors used the term “caudal anomalies” [40], “ambiguous genitalia with VATER” [41], or “caudal developmental field defect with female pseudohermaphroditism and VACTERL anomalies” [42] and finally “penis-like clitorises with megalourethra in non-virilized female fetus” [43]. Looking at the pictures of all those cases presented, it was obvious that all those patients suffered from cloacas with normal female gonads and chromosomes.
Figure 16.13 shows a single, very small, very narrow perineal orifice located in the tip of a pseudophallus, which usually means that the patient has other important associated urologic malformations and a long common channel.
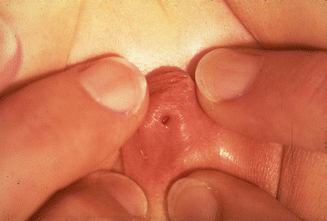

Fig. 16.13
Picture of a cloaca. The single perineal orifice is very small and is located at the tip of a pseudophallus. This kind of external anatomy is usually associated with severe urologic defects and a long common channel
Figure 16.14 shows the perineum of patients with cloaca and lipomas. Lipomas are relatively common in the perineum of patients with cloacas and do not necessarily mean that they require a more complicated type of treatment. At the time of the main repair, the lipoma can be easily excised (Fig. 16.15).
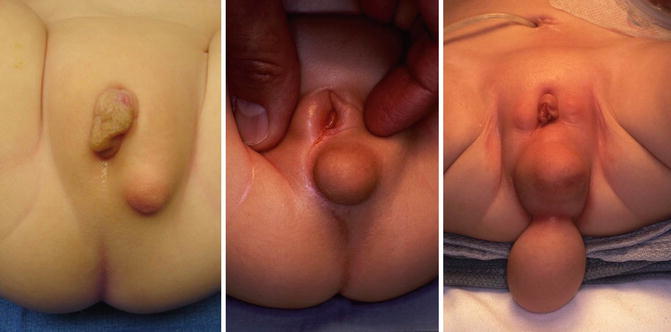
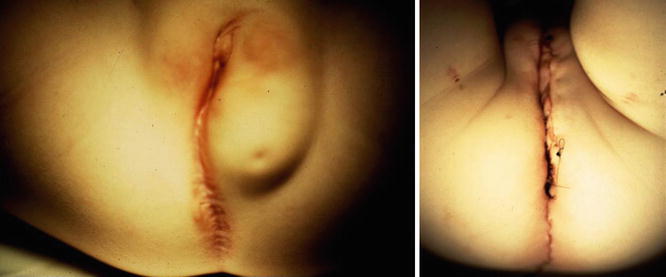

Fig. 16.14
Lipomas in the perineum of patients with cloaca

Fig. 16.15
Preoperative and postoperative appearance after a cloaca repair, including the resection of lipomas
As previously shown, a patient with a cloaca has a very high likelihood of suffering from a urologic condition. The first 24 h of life, like in all other babies with an anorectal malformation, should be used to rule out the presence of associated problems that may represent a risk for life. The most important one, in patients with a cloaca, of course, is urinary tract obstruction. The baby must have a kidney ultrasound to rule out the presence of hydronephrosis and also a pelvic ultrasound to rule out the presence of hydrocolpos and megaureter (Fig. 16.16). A plain abdominal film in a baby with a single perineal orifice may show an image of a pelvic mass, as shown in Fig. 16.17. This represents, most likely, hydrocolpos that must be drained soon. An ultrasound that shows hydronephrosis, megaureter, and a pelvic cystic mass most likely represents a hydrocolpos that is compressing the trigone and is the cause of the bilateral megaureters and the hydronephrosis.
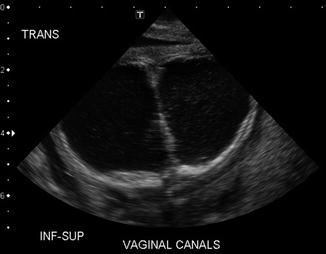
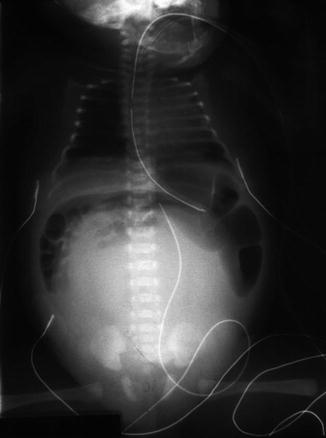

Fig. 16.16
Pelvic ultrasound of a patient with bilateral hydrocolpos

Fig. 16.17
Abdominal film of a newborn baby with a cloaca and a large hydrocolpos
Hydrocolpos as a cause of megaureters and hydronephrosis has been poorly recognized in the cases that we have received from other institutions. Many patients with hydronephrosis and megaureters were subjected to unnecessary, non-indicated ureterostomies, vesicostomies, and/or nephrostomies (Fig. 16.18). Most of the time, the simple drainage of the hydrocolpos takes care of the problem of megaureter and hydronephrosis, except in those patients who have, in addition, vesicoureteral reflux and difficulty emptying their bladder.
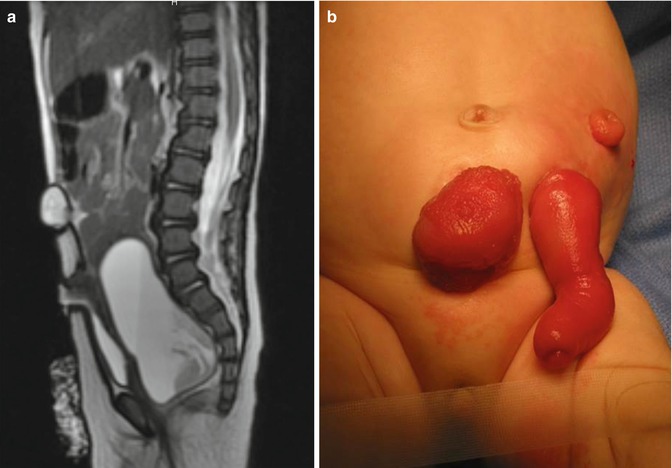

Fig. 16.18
(a) Picture of a baby with a cloaca, who underwent a colostomy and a non-indicated vesicostomy. The vesicostomy is prolapsed because it was not done correctly and the hydrocolpos remained tense and undrained. (b) MRI showing a sagittal view, the vesicostomy and the undrained hydrocolpos
Vesicostomies are occasionally indicated in these babies when we demonstrate that the common channel is too narrow and interferes with the emptying of the bladder. Also, we have seen an indication in babies with massive reflux and megaureters. However, if the patient has a hydrocolpos, the first step should be the drainage of the hydrocolpos, prior to making decisions concerning other procedures. Almost always, drainage of the hydrocolpos is all that is needed to decompress the ureters.
Also, the baby must have an echocardiogram to rule out cardiac conditions. Esophageal atresia must be ruled out in the usual manner. A spinal ultrasound is indicated to evaluate for the presence of tethered cord, and an x-ray film of the abdomen will show the characteristics of the lumbar and thoracic spine, as well as the sacrum, and a sacral ratio can be calculated.
Between 18 and 24 h after the baby is born, a decision must be made concerning the surgical treatment. These babies need a colostomy. The primary treatment of a cloaca without a colostomy has not been attempted, as far as we know. In Chap. 5 in this book, we recommended the opening of a descending colostomy with a mucous fistula, completely separated from the proximal stoma and reduced in size to avoid prolapse, since we only need that orifice to perform irrigations and diagnostic tests (high-pressure distal colostogram). In patients with cloaca, we must put more emphasis in being sure that the patient is left with a piece of colon, distal to the mucous fistula, long enough to guarantee that the pull-through will be possible in the future.
If the patient has evidence of hydrocolpos, the surgeon must be prepared to drain the hydrocolpos at the same time. We specifically recommend a midline subumbilical incision that will give the surgeons access to the entire lower abdomen. Through this incision, the surgeon can identify the junction between the descending and sigmoid colon. There, the colon is divided and the stomas are created, separated enough to be able to use a stoma bag without including the mucous fistula. The proximal stoma is created in the left flank and the mucous fistula in the left lower quadrant. At the same time, through this incision, the surgeon will be able to drain the hydrocolpos. We prefer to drain it with a tube. We have used different types of catheters for this drainage. We specifically recommend the use of a pigtail catheter that can be exteriorized through one of the lower quadrants. We like a curled catheter because it is less likely to fall out during the initial several months of life as the inflammation recedes and the vagina moves away from the abdominal wall. Since most of the patients with hydrocolpos have both hemivaginas distended, what we have done in such cases is to create a window in the septum between both hemivaginas in order to use a single tube to drain both hydrocolpos. Figures 16.19 and 16.20 show the aspect of the abdomen of one of these babies with a cloaca after the colostomy has been opened and the hydrocolpos has been drained.
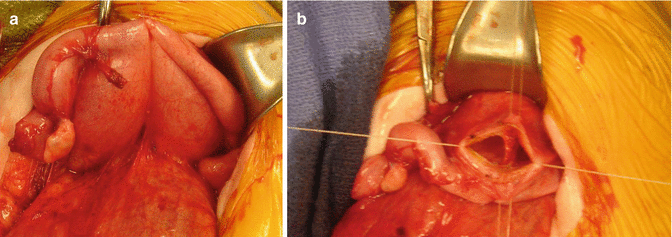
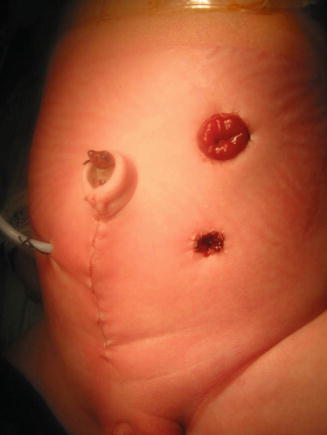

Fig. 16.19
Intraoperative appearance of a case with two large vaginas (bilateral hydrocolpos). (a) Before drainage. (b) Creation of a “window” in the septum between both hemivaginas

Fig. 16.20
Abdomen of a patient with a cloaca showing the separated stomas and the vaginostomy tube
Figure 16.21 shows the kidney ultrasound of a baby with a cloaca born with hydronephrosis and hydrocolpos: (a) before the drainage of the hydrocolpos and (b) shows the same patient’s ultrasound after the hydrocolpos has been drained. We cannot overemphasize the importance of draining the hydrocolpos. We have received a series of patients that had a colostomy, vesicostomy, nephrostomy, or ureterostomy, but not drainage of the hydrocolpos. Those patients had multiple problems, including vesicostomy prolapse. One of the patients had a pyocolpos, and another one had a perforation of the infected vagina with severe peritonitis. Several presented with failure to thrive, acidosis, and urinary tract infections, all of which resolved once the hydrocolpos was drained.
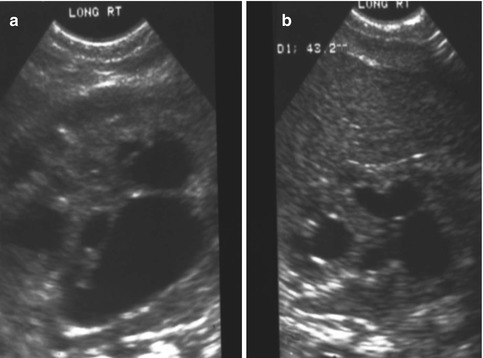

Fig. 16.21
Kidney ultrasound of a patient born with a cloaca and hydronephrosis. (a) Before drainage of hydrocolpos. (b) After drainage
If the bladder cannot empty due to the presence of a quasi-atresia of the common channel, then a vesicostomy would be indicated. Also, in the event of a patient who has a drained hydrocolpos, well-decompressed urinary tract but severe reflux, megaureter as demonstrated on a cystogram, and urinary tract infections, a vesicostomy could be indicated, with a plan for urologic reconstruction in the future. Early ureteral reimplantation of megaureters in a little baby with a bladder that most likely will have some degree of malfunction and kidneys with significant congenital damage is not recommended. We therefore prefer the opening of a temporary vesicostomy, which represents the best way to protect the kidneys.
Some of the hydrocolpos are giant and may even interfere with the respiratory function. Also, some babies with cloacas are extremely sick at birth; they have ascites, hydronephrosis, high creatinine, and severe renal damage.
The presence of calcified meconium in the abdominal film of a newborn baby with a cloaca [44] may represent a serious sign. Sometimes the meconium passes through the fallopian tubes into the peritoneal cavity producing severe peritonitis.
Once the colostomy and urogenital tract decompression has been done, the surgical emergency has been solved. Patients usually recover very well from this operation, and they start eating, growing, and developing normally. The exceptions, of course, are patients that are born with severe kidney damage and renal failure, requiring hemodialysis and consideration for a kidney transplant.
A female baby born with a cloaca that has a colostomy and is not doing well postoperatively usually has an undrained obstructed urinary tract with or without hydrocolpos. Therefore, the first study in such patients should be an ultrasound to rule out the presence of hydronephrosis, megaureter, large bladder, or hydrocolpos and act accordingly.
Another reason why these patients have sepsis and do not grow well sometimes is because the colostomy is inadequate. We are strongly opposed to the opening of loop colostomies in these babies, because that type of stoma frequently allows the passing of stool into distal bowel with direct fecal contamination of the urinary tract.
When the patients are well treated, their colostomy is adequate, and their urinary tract and hydrocolpos are well drained, they usually recover very rapidly and can go home. Within several months, they will be ready for the main repair.
16.1.1.4 Main Repair
In June 1982, for the first time, we had the opportunity to use the posterior sagittal approach to repair a cloaca under direct vision. Fortunately, that first cloaca was what we now consider a “benign type” of malformation, meaning that the common channel was relatively short (less than 3 cm), and therefore, we were able to repair the malformation successfully. That particular patient today has urinary control, bowel control, sexual function, and already has successfully delivered a baby by cesarean section.
During the first few years after 1982, our approach for the repair of cloacas consisted of separating the rectum from the urogenital tract like in all other malformations, followed by the separation of the vagina from the urethra and bladder, reconstruction of what used to be the common channel as a neourethra, mobilization and dissection of the vagina to be able to pull it down to be placed posterior to the urethra, and performing a pull-through of the rectum to be placed within the limits of the sphincter [45] (Fig. 16.22). Soon enough, we learned that that approach was highly successful in a certain type of malformations that now we call “benign,” but was not successful in other more complex types. The main lesson learned during the last 32 years is that we are dealing with a wide spectrum of defects [1, 46]. It has been an eye-opening, constant learning experience. The more experience we develop, the more we understand that the spectrum seems to be wider and wider. As will be shown in this chapter, the learning process allowed us to design surgical maneuvers applicable to different anatomic variants of these defects. The posterior sagittal anorectovaginourethroplasty is the name that we gave to the repair when it was done in the way that was already described, meaning separation and mobilization of the three structures (rectum, vagina, and urethra), done posterior sagittally.
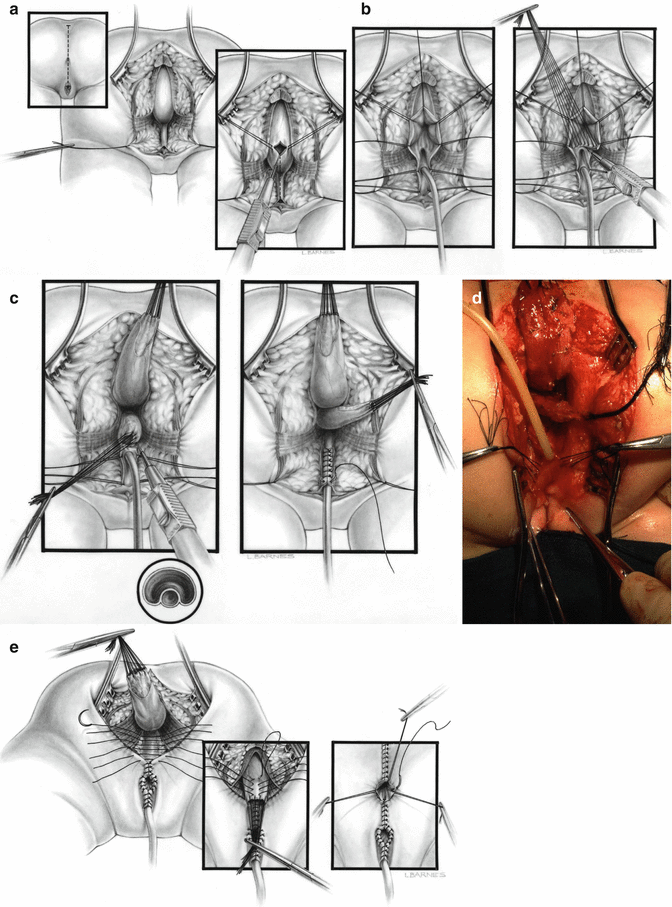

Fig. 16.22
Diagrams and pictures showing the technique originally used by us, before the advent of the total urogenital mobilization. (a) Opening. (b) Diagram showing the separation of the rectum from the vagina. (c) Diagram showing the vagina being separated from the urethra. The old common channel is reconstructed as a neourethra. (d) Intraoperative picture showing rectum and vagina separated. (e) Diagram showing the reconstruction being completed
In many patients, the posterior approach was not enough to repair the malformation, and it was necessary to open the abdomen to complete the repair.
In 1996, for the first time, we used an innovative surgical maneuver that we called “total urogenital mobilization,” which allows us to reduce the operative time about 70 %, significantly reduces the blood loss, makes the operation more reproducible, and renders better cosmetic and functional results in the management of cloacas [47] (Fig. 16.23) (Animations 16.1 and 16.2). Subsequently, we found that the total urogenital mobilization was not enough to repair more complex types of defects, and, therefore, we designed the “transabdominal extended total urogenital mobilization,” which allows us to repair cloacas with common channels between 3 and 5 cm. Yet, even with the use of an “extended transabdominal” approach, some cloacas required further technically demanding maneuvers, including the complete separation of bladder and urethra from the genital tract (Animation 16.3). In order to do that, we had to open the bladder and pass feeding tubes through the ureters to avoid their injury. In addition, in some patients, we perform a maneuver called “carving the pubic cartilage,” in order to create a shorter trajectory for the urethra and vagina to be pulled down behind the pubis and to be sutured next to the clitoris. In some specific type of cases, we apply a maneuver called “vaginal switch” that will be described below [48]. In another group of cases, we have to replace the vagina totally or partially, and we perform that with the rectum, colon, or small bowel. Finally, there is a group of cloacas with an extremely long common channel (more than 5 cm), in which we leave intact the common channel to be used eventually as a conduit for intermittent catheterization, and we go directly through the abdomen to separate the vagina(s), and the rectum, from the trigone or the bladder neck.
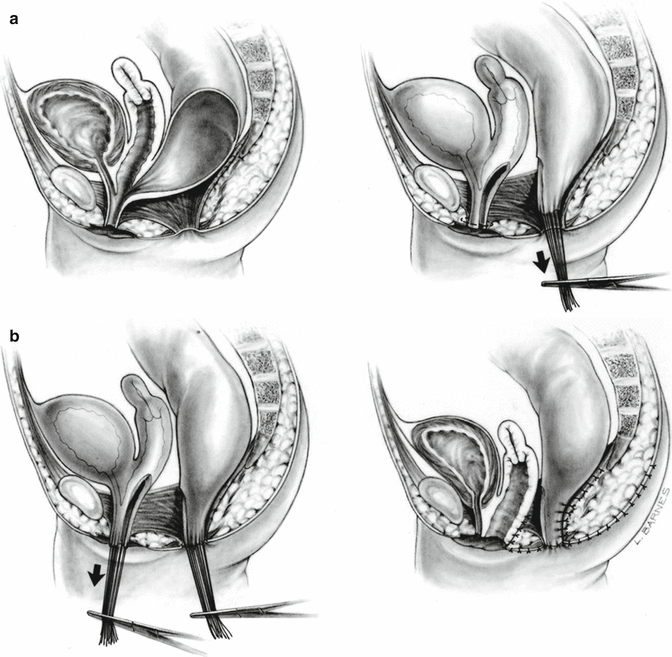

Fig. 16.23
Total urogenital mobilization (diagrams showing the basic concept). (a) Separation of the rectum. (b) Total urogenital mobilization
As we learned more about the complexity of cloacal malformations, we developed a serious concern about the reproducibility of some of the techniques used to repair complex cloacas. Fortunately, more than 50 % of the cloacas have a common channel shorter than 3 cm. This means that they can be repaired posterior sagittally, without opening the abdomen and using the maneuver called “total urogenital mobilization.” We believe that the total urogenital mobilization is highly reproducible, and we believe that most pediatric surgeons can learn to do it well. On the other hand, we believe that those cases of cloacas with a common channel longer than 3 cm must be repaired by those surgeons specially dedicated and experienced in dealing with these malformations.
Figure 16.24 shows the different steps of the decision-making algorithm in the repair of cloacas. We will describe each one of them.
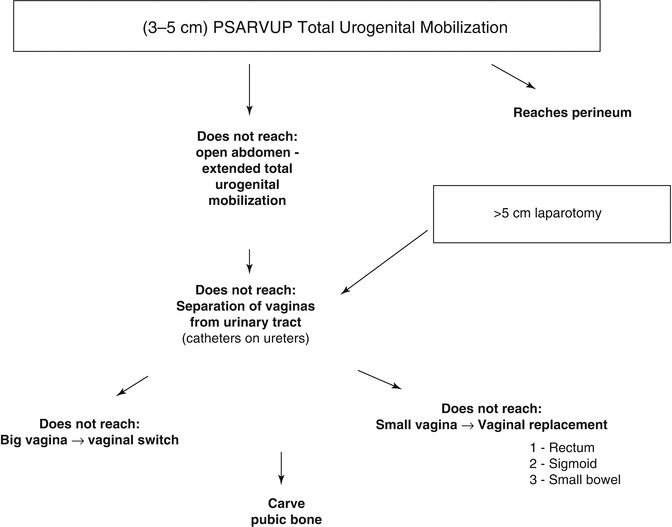

Fig. 16.24
Decision-making algorithm to repair cloacas with a common channel longer than 3 cm
Cloacas with a Common Channel of Less Than 1 cm
Figure 16.25 shows a cloaca with a short common channel. In these cases, we recommend a relatively simple procedure that we call a posterior sagittal anorectovaginoplasty. The urethra is left untouched. Basically, what we do in these cases is to separate the rectum from the vagina the same way that we do in cases of vestibular fistulas (see Chap. 15). Next to that, rather than separating the vagina from the urinary tract or performing a total urogenital mobilization, we mobilize only the lateral and posterior walls of the vagina, enough as to be able to suture the edges of the vagina to the skin of the neolabia (Fig. 16.25b). By doing that, we do not disturb the urethra or the common wall between the vagina and urethra, which is a high-morbidity type of maneuver. The cosmetic effect of this operation is excellent. The patients look and behave basically like a patient operated on for a rectovestibular fistula. We call this type of cloaca “cloaca type 1.” The results in terms of bowel and urinary control are not different from those of patients with rectovestibular fistulas when they have a normal sacrum. Figure 16.25c shows the final result after one of these introitoplasties. These patients may have mild female hypospadias, which is irrelevant because they do not need intermittent catheterization and because the urethral meatus is readily visible.
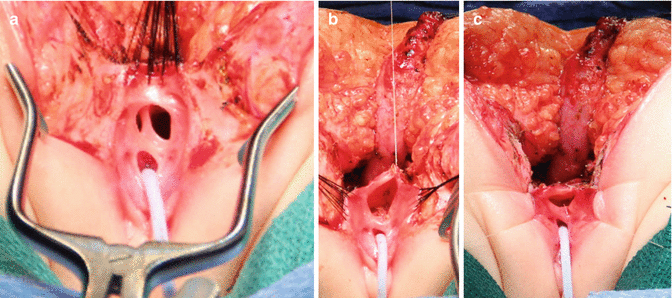

Fig. 16.25
Intraoperative picture of a cloaca with 1 cm common channel. (a) Exposure – multiple silk sutures in the rectum. Observe the vaginal septum. (b) Sutures placed in lateral vaginal walls. The rectum has been already separated, and the vaginal septum has been resected. (c) Repaired introitus – the lateral walls of the vagina are sutured to the labia. The introitus has been enlarged
Cloacas with a 1–3 cm Common Channel
Fortunately, 66 % of our patients with cloacas belong to this type. These patients have, in general, a good prognosis. Twenty-eight percent of them will require intermittent catheterization after the reconstructive operation, and the bowel function depends very much on the quality of the sacrum and spine [1, 46].
The procedure to repair these malformations was performed by us any time from 1 to 12 months of age. If a baby happens to be born in our institution and is growing and developing normally, we do it between 1 and 3 months of life. Most of our patients, however, come from other institutions, and, therefore, we have experience doing this procedure at different ages.
We start the operation by performing vaginoscopy and cystoscopy. We strongly recommend for the general pediatric surgeon to do the vaginoscopy and cystoscopy as a separate setting. By doing that, he or she will be able to measure the length of the common channel and based on that to:
Determine whether or not he is capable of doing that operation or if the patient should rather be referred to another center.
Determine whether or not it will be necessary to open the abdomen for the reconstruction. This represents important information for the anesthesiologist as well as the entire operating team. It helps with equipment needs, predicting operating time, etc.
Determine the final functional prognosis.
Determine whether or not the patient needs a total bowel preparation, in case some form of vaginal replacement with bowel is necessary.
When the vaginoscopy and cystoscopy shows that the patient has a common channel of 1–3 cm, we can be confident that we can repair that malformation using only the posterior sagittal approach and total urogenital mobilization, without opening the abdomen. The operation will take us approximately 3 h. The cosmetic result is excellent, and the function, in general, is very good.
The patient is placed in prone position with the pelvis elevated and is washed, prepped, and draped in the usual manner. A posterior sagittal incision is used, running from the middle portion of the sacrum down to the single perineal orifice. We divide the skin, subcutaneous tissue, parasagittal fibers, and the entire sphincter mechanism precisely in the midline (Fig. 16.26). The common channel is opened exactly in the midline, including the vagina and the rectum (when it is found), exposing the internal anatomy of the malformation (Fig. 16.27). This step is facilitated by placing a mosquito clamp in the single perineal orifice, to help guide the midline incision of the posterior aspect of the common channel.
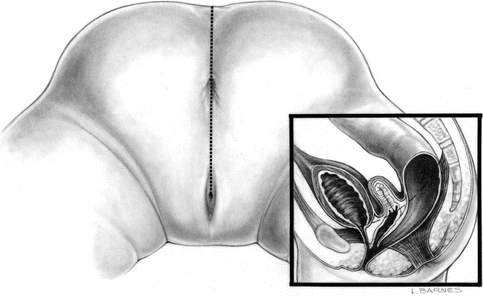
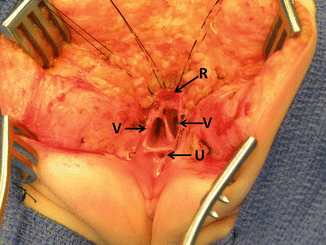

Fig. 16.26
Diagram of a posterior sagittal incision to repair a cloaca

Fig. 16.27
Picture showing the anatomy of the most common type of cloaca. R rectum, V vagina, U urethra
The first step, as in all cloacas, consists of separating the rectum from the vagina. When the patient has two Müllerian systems, the rectum is found in the middle of both hemivaginas. Usually, it opens in a little orifice located in the posterior aspect of the vaginal septum. Multiple 5-0 silk stitches are placed around the rectal opening in order to apply uniform traction (Fig. 16.28). Special emphasis is placed on creating a plane of dissection in the common wall existing between the rectum and the vagina(s). The use of uniform traction is highly recommended in order to achieve this. We must keep in mind that these structures (rectum and vagina(s)) share a common wall without a natural plane of dissection. Once the rectum and vagina(s) are fully separated, a circumferential dissection is performed, applying uniform traction on the rectum, dividing the bands and vessels that hold the rectum in the pelvis. As we progress with this dissection, we keep gaining length until we have enough rectum to comfortably reach the perineum within the limits of the sphincter (Animation 16.2)
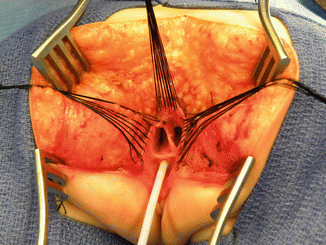

Fig. 16.28
Multiple fine sutures are placed on the edges of the rectal wall, the lateral walls of the vagina, and the common channel
After we finish that part, in the past (before 1996) [1, 45, 46], we used to separate the vagina from the urinary tract, which was a technically demanding maneuver that we do not do anymore in this type of malformation. It took many hours to do this, and over 10 % of our patients suffered from vaginal strictures and/or urethrovaginal fistulas as a consequence of that separation [47]. Because of that, in 1996, we switched to the total urogenital mobilization. For this we place multiple 5-0 silk sutures in the edges of the common channel and the lateral walls of the vagina to apply a uniform traction (Figs. 16.28 and 16.29). Another set of sutures is placed in a horizontal, transverse fashion, about 5 mm from the clitoris (Fig. 16.30). The common channel is divided distal to the transverse line of sutures between the clitoris and the sutures using the needle-tip cautery. The incision includes the full thickness of the common channel. A plane of dissection exists between the pubis and the common channel (Animation 16.2). The separation of the common channel from the posterior aspect of the pubis is a very easy maneuver because there is an obvious plane, and within a couple of minutes, we can reach the upper part of the pubis. Once there, it is relatively easy to identify white, avascular bands that represent the suspensory mechanism of the bladder, vagina, and urethra (Fig. 16.31). These are divided with the cautery as well as their lateral attachments on both sides of the vagina. When we divide these suspensory ligaments of the vagina and urethra, one can see a characteristic fat herniating through the fascia. This is a characteristic retropubic fat pad that indicates that we are in the right plane (Fig. 16.32). The suspensory ligaments of urethra and vagina extend onto both lateral walls of the vagina and must be divided, trying to preserve the blood supply of the vagina. By doing this division of the suspensory ligaments, we gain approximately 2 cm of length in the common channel. We then go to the dorsal part of the vagina(s) and divide the bands, holding them posteriorly and laterally. By doing that, we usually gain another centimeter. As a consequence, in most instances, the total urogenital mobilization allows the mobilization of the vagina and urethra with a common channel of 3 cm comfortably (Fig. 16.33). Occasionally, we were able to totally repair cloacas with up to a 4.5-cm common channel only posterior sagittally, using this maneuver. Other times, we do not know why, we can only gain 2 cm of length, due to lack of elasticity and an inflammatory process that we find in some of these patients.
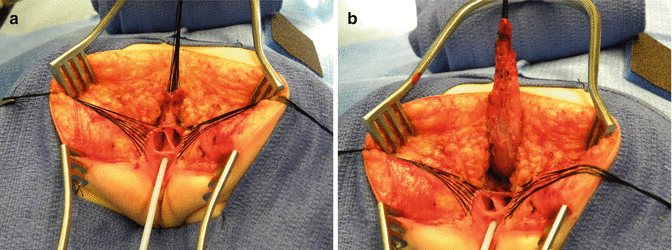
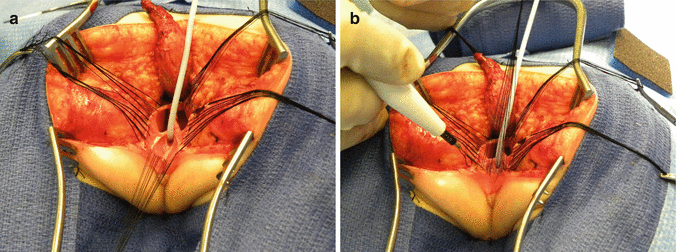
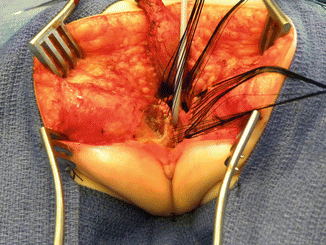
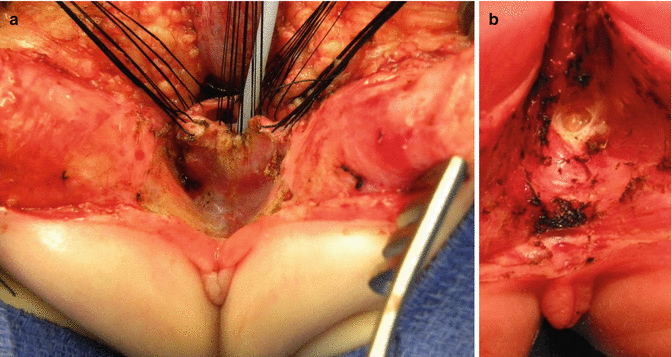
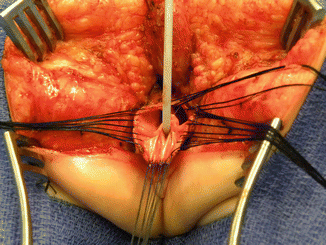

Fig. 16.29
Rectal dissection. (a) The beginning of the separation of the rectum from the vagina. (b) Rectum fully separated

Fig. 16.30
Picture showing another set of sutures places horizontally, approximately 5 mm proximal to the clitoris. (a) Sutures in place. (b) The urogenital sinus is divided between the clitoris and the sutures

Fig. 16.31
Picture showing the lateral dissection of the common channel and vagina

Fig. 16.32
The suspensory ligaments of urethra and vagina. (a) Exposure – observe whitish fascia. (b) Divided suspensory ligaments (observe retropubic fat)

Fig. 16.33
Picture showing a fully mobilized urogenital sinus
Once we mobilize the urogenital sinus, we then split in the midline of what used to be the common channel, into two lateral flaps (Fig. 16.34). By doing that, we can suture the urethral meatus to the tissue behind the clitoris with interrupted 6-0 Vicryl sutures (Fig. 16.35). The two flaps that we develop from what used to be the common channel now become part of the neolabia that is sutured to the skin with interrupted 6-0 Vicryl sutures (Animation 16.2). The lateral walls of the vagina(s) are sutured to the neolabia until we create a nice-looking introitus (Fig. 16.36). The electrical stimulator is then used to determine the limits of the anal sphincter, which are marked with temporary silk stitches. The perineal body is reconstructed between the posterior limit of the vagina and the anterior limit of the sphincter. We use 4-0 Vicryl or 5-0 Vicryl sutures to bring together the tissue of the perineal body. These stitches are very important because they represent the main supporting mechanism to avoid dehiscence of the perineum. The skin of the perineal body is sutured usually with interrupted 6-0 Vicryl or 5-0 Vicryl sutures. By doing this, we bring together the anterior limits of the anal sphincter (Animation 16.2). The rectum then is placed within the limits of the sphincter and in front of the levator mechanism. The posterior edge of the levator muscle is sutured with interrupted 5-0 Vicryl sutures in the same way that it is done in all other malformations. The posterior edge of the muscle complex on each side is sutured together in the midline, taking with the same stitches a bite of the posterior rectal wall. The ischiorectal fossa and the subcutaneous tissue are both closed with interrupted 5-0 Vicryl sutures, and the skin is closed with subcuticular 5-0 monofilament, absorbable sutures. The anoplasty is performed with 16 circumferential stitches of 6-0 long-term absorbable sutures after we resect the excessive rectal tissue, usually between 5 and 10 mm (Fig. 16.37).
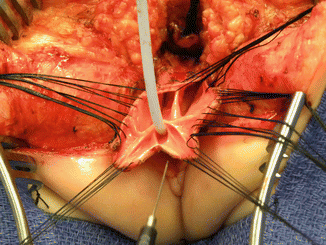
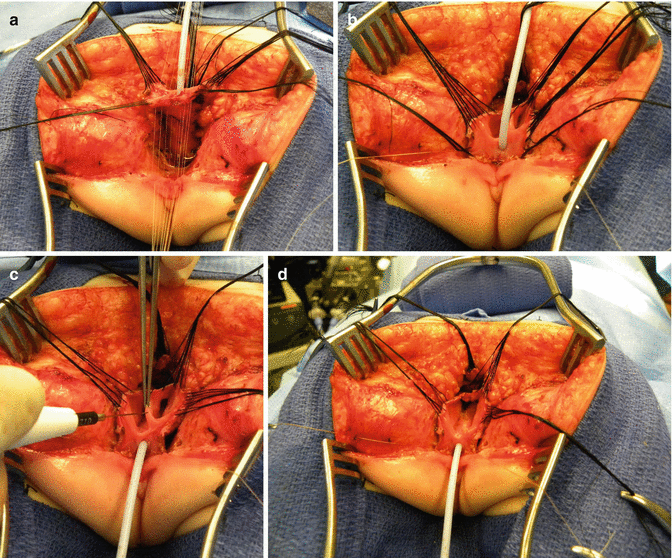
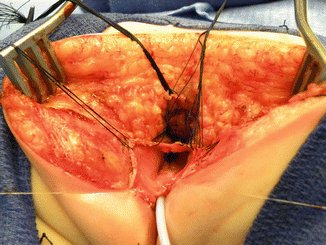
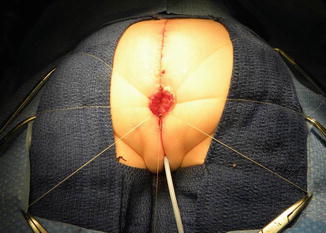

Fig. 16.34
The urogenital sinus (original common channel) is divided in the midline

Fig. 16.35
Urethral opening repositioning and resection of the vaginal septum. (a) Fine long-term absorbable sutures are used to anastomose the urethral opening immediately behind the clitoris. (b) Sutures are tied. (c) Resecting the vaginal septum. (d) Vaginal septum resected

Fig. 16.36
Suturing vaginal walls to the neolabia

Fig. 16.37
Final external aspect of a repaired cloaca
These patients do very well and can eat the same day of surgery. The patients stay in the hospital approximately 48 h. A Foley catheter remains in place for approximately 2 or 3 weeks. We must keep in mind that about 20 % of these patients may eventually require intermittent catheterization, and, therefore, we leave the Foley catheter until the postoperative inflammatory process allows us to see where the urethral meatus is located, in case the patient needs intermittent catheterization, before we pull the catheter out. Two weeks after surgery, the parents come to the clinic, we teach them how to dilate the anal orifice, and they do it following our protocol of anal dilatations as previously described.
Prior to the colostomy closure and under the same anesthesia, a vaginoscopy and cystoscopy are performed to confirm that the urethra and vagina are patent and healthy. In the event of finding problems with these, they have to be taken care of, prior to the colostomy closure. If, for instance, at the time of colostomy closure, we find a narrow ring-like, vaginal opening with a wide, deep compliant vagina, we may do nothing at this age. On the other hand, if the orifice is too narrow and we believe it is at risk of closing completely, then we perform an external vaginoplasty to make the orifice larger and postpone the colostomy closure for a month (Figs. 16.38 and 16.39). On the other hand, if the patient has a long, narrow vaginal stricture, she may need a complete reoperation. We frequently saw this kind of complication prior to the advent of the total urogenital mobilization. The total urogenital mobilization prevents this complication from happening most of the time since it preserves a very good blood supply for the vagina and the urethra.
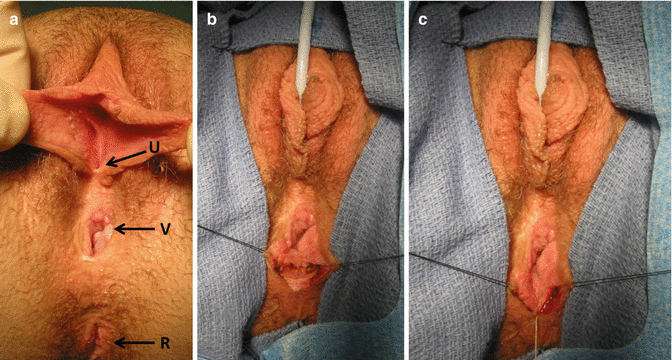
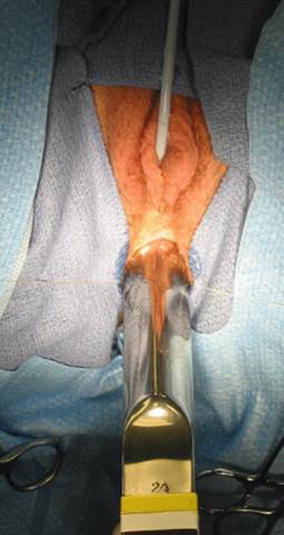

Fig. 16.38
External vaginoplasty to enlarge a strictured vaginal orifice. (a) Narrow vaginal orifice R rectum, U urethra, V vagina. (b) Longitudinal incision of the posterior aspect of the anal opening. (c) Horizontal suture

Fig. 16.39
Enlarged vaginal orifice. Large Hegar dilator inserted
After the total urogenital mobilization, some patients leak urine for a period of several weeks. A voiding cystourethrogram at this point may show an image consistent with an absent bladder neck, due to the pulling of the whole urogenital sinus from below. This happens mainly when the repair required a significant traction of the urogenital tract. However, most of these patients recover normal urinary function after a few weeks. The colostomy can be closed following the same principles that we mentioned in the chapter related to colostomy closure.
Cloacas with a 3- to 5-cm Common Channel (Animation 16.3)
When the endoscopy allows us to determine that the patient has a common channel length of 3–5 cm, we perform a total body preparation as described in Chap. 11 because we know that most likely it will be necessary to open the abdomen, in addition to the posterior sagittal approach, to repair the malformation. We tell the anesthesiologist about these findings and the possibility that the operation will go for a longer period of time than one that could be done posterior sagittally only. After we perform a total body preparation, we put the patient in the prone position with the pelvis elevated and perform the same posterior sagittal incision as previously described. The internal anatomy of the malformation is exposed; the rectum is separated from the urogenital tract in the same way as previously described. A total urogenital mobilization is performed as previously described. Occasionally, we are happily surprised to find out that it is possible to reconstruct the urethra, vagina, and rectum without opening the abdomen. However, most of the time, the total mobilization is not enough to repair the malformation. In such a case, the patient is turned to the supine position and the abdomen is opened with a midline infraumbilical incision.
The next step is to perform what we call “extended transabdominal urogenital mobilization.” Traction is applied to the dome of the bladder; the lateral attachments of the bladder are divided to obtain a better exposure (Fig. 16.40). The midline incision is extended all the way down to the pubis. Between the bladder and posterior aspect of the pubis, one can see the space that we created from below with the total urogenital mobilization. The urogenital sinus is then brought up through this space between the bladder and pubis (Fig. 16.41). At this point, we divide all the pelvic avascular attachments of the bladder and urethra. Through the laparotomy, these attachments are easily seen and divided. Usually this maneuver allows us to gain extra length on the urogenital mobilization. If that is enough to complete our repair, we then go ahead and pull through back down the urogenital complex and repair the urethra and vagina as previously described. If that is not enough to achieve a tension-free anastomosis between the urethra and clitoris and vagina and neolabia, a maneuver called “carving of the pubic cartilage” is indicated.
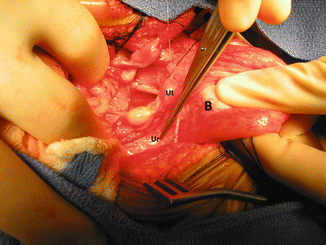
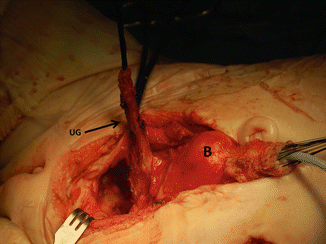

Fig. 16.40
Intraoperative picture showing the bladder pulled caudally to have a better access to the pelvic floor. B bladder, Ut uterus, Ur ureter

Fig. 16.41
Intraoperative picture showing bladder and urogenital sinus out of the pelvis. Us urogenital sinus, B bladder
Carving of the Pubic Cartilage Maneuver
Under normal circumstances, the urogenital sinus is located behind the pubic cartilage; it runs below the cartilage and up, anterior to the cartilage to connect to the clitoris. Resecting approximately 50 % of the lower portion of the pubic cartilage does not compromise the pelvis stability, and yet, it allows a straighter trajectory of the urogenital sinus. The resection of the cartilage can be done easily, with the needle-tip cautery on “cutting” mode in babies. In older patients, this can be done with a “rongeur” type of instrument. This maneuver may allow for a tension-free anastomosis between the urethra and vagina with the clitoris and neolabia (Fig. 16.42). This maneuver may work in cases that need 0.5–1 cm of extra urethra and vaginal length to achieve a tension-free anastomosis. If that is not enough, then the next step must be the separation of the vagina from the urinary tract.
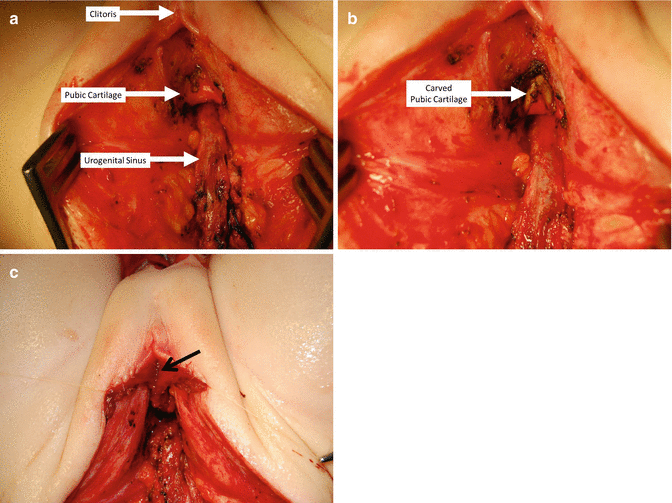

Fig. 16.42
Carving the lower part of the pubic cartilage to create a shorter trajectory of urethra and vagina. (a) Before carving. (b) After carving. (c) Urethra sutured – arrow in urethral opening
Separations of Vagina(s) from the Urinary Tract (Animation 16.3)
This is the most technically demanding maneuver of the entire repair of cloacas. This procedure, in the past, before the total urogenital mobilization, was attempted from below, but it was very difficult to do. Now that we do the total urogenital mobilization, we do not have to separate the vagina from the urinary tract in cases with common channel shorter than 3 cm. Yet, in cases with longer common channel, we must separate both structures through the abdomen. The separation is done through a laparotomy but with bladder and vagina(s) fully mobilized and out of the abdomen. The bladder is opened in the midline, and feeding tubes are introduced through each one of the ureters (Fig. 16.43a). We must keep in mind that in cloaca patients, both ureters pass through the common wall between the vagina and the bladder. The separation of these two structures may include the skeletonizing and dissection of both ureters. If the patient suffers from reflux, this is a golden opportunity to perform a ureteral reimplantation or, if appropriate, a cutaneous ureterostomy; otherwise, to do it later would represent a technically more demanding procedure. The assistant puts two fingers inside the bladder and applies traction caudally into the bladder (Fig. 16.43b). Vicryl sutures are used to pull the uterus or hemiuterus in the opposite direction. A plane is created in the middle of the wide common wall that exists between the vagina(s) and the urinary tract. This common wall extends from the urethra and includes the bladder neck, trigone, and part of the bladder. In general, the most technically demanding steps of the operations designed to repair anorectal malformations is actually the separation of the structures (rectum, genitalia, and urinary tract). We are supposed to separate them without damaging them. This is difficult to achieve for several reasons:
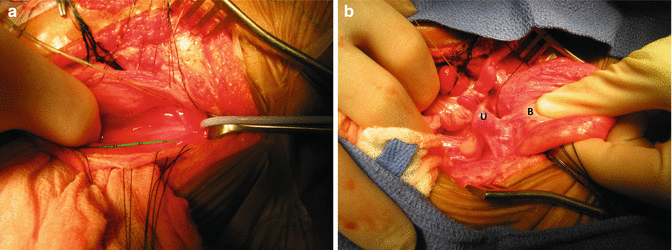

Fig. 16.43
Intraoperative pictures taken during the separation of vagina from the urinary tract. (a) Bladder open and catheters placed in the ureters. (b) The assistant puts fingers into the bladder and thumb outside the bladder, pulling it caudally. Traction sutures are placed in the uterus to apply traction and facilitate the dissection. B bladder, Ut uterus
(a)
Those structures are congenitally fused without a plane of separation.
(b)
The common wall has a very rich blood supply.
(c)
The exposure is difficult because these structures are located in a place difficult to reach from below or from above.
(d)
The ureters run through this common wall.
The separation of structures usually takes about 70 % of the total operative time. To achieve a good repair, it is necessary to achieve a good separation of these structures with minimal or no damage. The previously described “extended transabdominal total urogenital mobilization” allowed us to perform the separation of structures basically outside the abdomen. The surgeon works from above between the bladder and the vagina(s). We perform the entire dissection with a fine needle-tip cautery. During the dissection, the surgeon must stop frequently to verify that the thicknesses of the vaginal wall, as well as that of the bladder, are equal. In other words, the surgeon does this to be sure that neither of those walls are becoming too thin. Intermittently, this dissection is interrupted to palpate the location of the ureters (previously catheterized). Also, it is convenient to perform part of the dissection backwards, meaning from caudal to cephalad, since the common channel is exposed and brought up through the incision. We keep dissecting a little bit from below and then from above, until both dissection planes meet. At that point, the only structures that join the genitourinary structures to the patient’s body are the ureters, the ovarian vessels, and the internal iliac vessels. These vessels must be kept in mind and carefully respected.
Figure 16.44 shows the posterior aspect of the bladder and trigone. Both ureters can be seen intact.
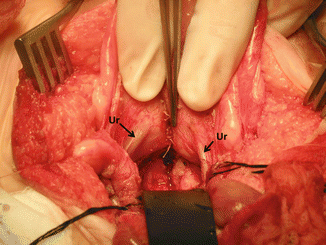

Fig. 16.44
Picture taken after the bladder and vagina have been separated. The ureters can be seen intact. Arrows on ureters. Black retractor pushing down uterus and vagina
Once the separation has been achieved, the surgeon can plan the type of reconstruction that is best for the patient’s specific anatomic variant. The first possibility is that after the separation, one becomes happily surprised to find that the vagina(s) actually reaches the perineum. This is the ideal time to remove the vaginal septum that separates two hemivaginas (if present) to trim off damaged vaginal tissue and tubularize the available vaginal tissue in preparation for the pull-through.
The total separation of vagina(s) and urinary tract runs with the implicit risk of devascularization of the distal urethra. It is necessary to be sure that the urethra that is sutured immediately behind the clitoris has a good blood supply. At least ten of our patients suffered from an ischemic-acquired urethral atresia after one of these operations performed by us.
If the urethra’s blood supply is severely deficient, the surgeon must make a decision about the possibility of permanently closing the bladder neck. In that case, the patient needs a vesicostomy; subsequently, an artificial conduit for bladder catheterization will be done (Mitrofanoff principle) [49]. Later in the patient’s life (3–4 years), the functional and anatomic characteristics of the bladder can be studied to determine its capacity, detrusor activity, compliance, as well as the presence of vesicoureteral reflux, and a final reconstruction can be planned, which may include a bladder augmentation and a Mitrofanoff type of procedure.
Planning the reconstruction of the vagina, urethra, and rectum, after the total separation of the structures, the surgeon will face one of several scenarios, and based on those, he/she will make a decision. The first possibility will be that the patient has anatomic characteristics that make her suitable for a surgical maneuver called “vaginal switch.”
Vaginal Switch
This maneuver is applicable only when the patient has a specific type of anatomy (Fig. 16.45). These patients have two hemivaginas very separated, as well as the hemiuteri with a vaginal septum and originally two large hydrocolpi. If we can estimate that the distance between one hemiuterus and the other is longer than the vertical length of both hemivaginas, then the patient may be a candidate for the vaginal switch maneuver [48]. As can be seen in Fig. 16.46, the maneuver consists in sacrificing one of the hemiuteri, being careful enough to preserve the ovary and its blood supply. The vaginal septum is removed. Both dilated hemivaginas are tubularized into a single vagina, and what used to be the dome of one side, the place where we resected the hemiuterus, becomes the lowest part of the new switched-down vagina (Fig. 16.46) (Animation 16.4). This maneuver works, and we have several patients menstruating through this type of repair, but it is only applicable if the patient has the specific type of anatomy already described. If, early in the operation, one estimates that the anatomy of the patient belongs to this category, one has to separate completely only one hemivagina from the urinary tract and try to preserve the blood supply of the opposite side. In this type of maneuver, the blood supply of the entire switched vagina will depend on the preservation of the blood supply of the opposite hemivagina. If one can see that the distance between both hemiuteri is not long enough, then we have to separate both hemivaginas completely from the trigone and urinary tract as previously discussed.
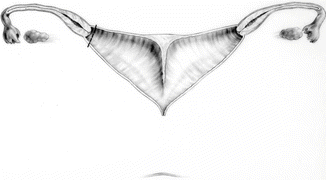
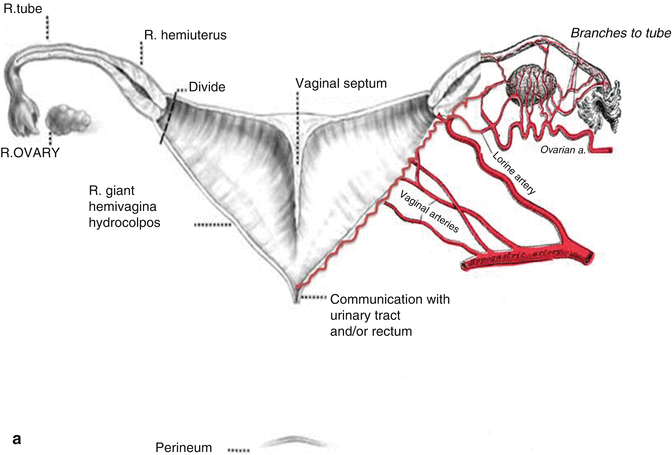
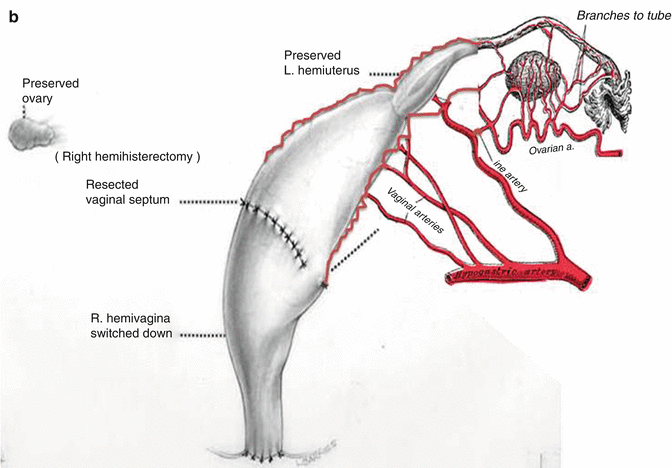

Fig. 16.45
Anatomic characteristics of a case that will benefit from a vaginal switch maneuver


Fig. 16.46




Schematic representation of the basic principles of a vaginal switch maneuver. (a) One hemiuterus will be amputated. The blood supply of that hemivagina is sacrificed being careful to preserve the blood supply of the other hemivagina and ovaries. The vaginal septum is resected and both large hemivaginas are tubularized together. (b) What used to be the dome of one hemivagina is pulled down to create the introitus
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








