Michael J. Morris, MD, Howard I. Scher, MD Perhaps the most controversial and problematic aspect of PSA monitoring is that it has created a new “clinical state” to characterize patients whose sole manifestation of disease recurrence after definitive local therapy is a detectable and increasing PSA value, in the absence of detectable disease according to the currently available imaging modalities. Based on the estimated probability of relapse after surgery or radiation therapy, upward of 50,000 American men fall into this category (Moul, 2000). Unfortunately, the management of these patients remains controversial because the course of the disease is highly variable and there are no completed prospective randomized studies that directly address the questions of when therapy should be initiated, should treatment should be systemic or local, and whether an intervention prolongs life. Within the clinical spectrum of men with a rising PSA value are (1) those in whom the rising PSA value reflects persistent or recurrent disease in the prostate or prostate bed, (2) those in whom the rising PSA value is a harbinger of detectable metastases, and (3) those with both local and systemic disease. Within the category of those with systemic disease are men who are at high risk of developing symptoms of or dying of their cancer. Such patients may well benefit from the early administration of systemic treatments to prevent metastases, to preserve quality of life, to delay the complications of osseous lesions, and to prolong survival. In other patients with systemic involvement the disease will follow a more indolent clinical course for which observation may be appropriate because the risk of developing metastases or symptoms, or of dying of the disease, may be very low during their life expectancy (Pound et al, 1999; Antonarakis et al, 2009). For these individuals, side effects from treatment may be worse than the anticipated morbidity of the cancer itself, exposing men to unnecessary toxicities. Some patients fall within these two extremes and warrant neither observation nor systemic treatments but rather additional therapy directed at the prostate or prostate bed. A critical issue for patients is the anxiety associated with knowing that the cancer is “progressing.” Men are diagnosed with and treated for prostate cancer earlier than ever, yet the symptoms most experience are related more to therapy than cancer. The clinical dilemma facing both the patient and the physician is how to balance the anxiety provoked by the rising PSA against the paucity of objective data regarding who would benefit from treatment and what the tangible benefits might be from such interventions. In this discussion a framework is provided to assess the prognosis of patients in the state of a rising PSA (Scher and Heller, 2000) so that a rational risk-adapted approach can be implemented. This is done with the recognition that the tools for both prognostication and treatment continue to evolve and at present must be considered works in progress. Prostate cancer presents a wide clinical spectrum from localized to metastatic and asymptomatic to symptomatic and is driven by a range of biologic factors that often change as the disease evolves. These variable disease manifestations can be segregated into patient groups, represented as easily recognizable clinical states that have shared clinical needs and treatment goals (Fig. 108–1) (Scher and Heller, 2000). Common treatment aims for patients with localized disease are eradication of the disease along with preservation of potency, continence, bladder and rectal integrity, and other quality-of-life measures. Patients with newly diagnosed metastatic disease have the treatment goal of maintaining disease control and prolonging hormonal sensitivity while minimizing the impact of hormones and other treatment strategies on quality of life and bone integrity. For patients with disease that is progressing despite androgen deprivation, the most acute issues are prolonging life and finding treatment strategies to mitigate the effects of an increasing burden of disease, such as pain, neurologic compromise, fatigue, hematologic disorders, and bone fracture. (Modified from Scher HI, Heller G. Clinical states in prostate cancer: towards a dynamic model of disease progression. Urology 2000;55:323–7.) Determining when a patient has entered the clinical state known as a “rising PSA” depends on the primary therapy the patient has received and the sensitivity of the assay used to measure his PSA level. By consensus, this clinical state does not include patients with localized disease who are being observed expectantly with “watchful waiting” or “deferred therapy.” It also does not include patients who are receiving androgen deprivation in lieu of definitive treatment. As with many issues relating to prostate cancer there is no single uniform definition for “biochemical relapse.” A patient who has undergone a prostatectomy should have no (or minimal) remaining normal, viable, PSA-producing nonmalignant prostate tissue. In contrast, patients who have received external-beam radiation therapy or brachytherapy, whether alone or in combination, have an intact, although irradiated, gland with nonmalignant prostate epithelial cells that will produce low levels of PSA that are not indicative of treatment failure. In addition, not all PSA assays have the same degree of sensitivity, so a patient’s PSA level may be reported as undetectable in one laboratory but detectable at another. A further confounder is that PSA production can vary with testosterone levels. Patients who have undergone neoadjuvant or adjuvant hormonal strategies in conjunction with their local therapy may have variable rates of treatment failure on the basis of testosterone recovery rates. In general, testosterone levels return to baseline within 3 to 6 months after cessation of short-term gonadotropin-releasing hormone analogue treatment, but the recovery can be quite prolonged in individual patients (Padula et al, 2002; Gulley et al, 2005). Age, length of androgen deprivation, and even the type of gonadotropin-releasing hormone analogue therapy used will all impact the rate of biochemical failure (Lukka et al, 2001). As noted, post-prostatectomy patients may have very low levels of PSA that can represent benign prostate tissue at the surgical margin, and as a result there is debate as to what precise level of PSA definitively represents postsurgical treatment failure. The American Urological Association (AUA) Guideline Update Panel examined 319 publications reporting on patients with biochemical relapse, revealing that 166 different definitions were applied to these various studies. The most commonly used cut point was greater than 0.2 ng/mL. The panel recommended using a cut point of greater than or equal to 0.2 ng/mL, with a second confirmatory level of greater than 0.2 ng/mL, to define surgical failure (Cookson et al, 2007), although some authors have recognized that low levels such as 0.01 to 0.07 ng/mL can also represent failure (Amling et al, 2001; Gretzer et al, 2002; Han et al, 2003). It is important to be mindful that the detection of a single abnormal PSA level does not, in and of itself, necessarily indicate that a clinically significant event has occurred, and the presence of a rising PSA level alone does not necessarily imply that a patient will develop symptoms or die of his disease. A recent analysis of 3125 patients treated at Memorial Sloan-Kettering Cancer Center (MSKCC) demonstrated that the best cut point to predict the probability of metastatic progression was greater than 0.4 ng/mL, followed by another rise (Stephenson et al, 2006). For clinical trial purposes, current consensus guidelines from the Prostate-Specific Antigen Working Group (Scher et al, 2004) recommend that a patient’s disease is considered to have progressed after a radical prostatectomy if the PSA value is greater than or equal to 0.4 ng/mL 8 weeks or more after the procedure and rises on a subsequent measurement. Eight weeks is ample time to allow PSA levels to clear, given a half-life of 2 to 3 days (Oesterling, 1991). The “date of failure” is the date that the PSA level is first detectable. Biochemical failure after radiation therapy was traditionally defined by the American Society for Therapeutic Radiation Oncology (ASTRO) as three consecutive PSA rises, optimally separated by 3 months between measurements, beginning at least 2 years after the start of radiation therapy, with the time of failure as the midpoint between the nadir and the first confirmed rise, or any rise significant enough to trigger therapy (Consensus statement, 1997). Such a definition, however, has not been wholly satisfactory even to its authors. The guidelines did not adequately account for recovering testosterone levels in patients who had received neoadjuvant or adjuvant hormones, for transient post-treatment elevations in PSA (so-called “bounces”) that do not represent disease relapse, for small serial rises in PSA that do not represent treatment failure, or for circumstances in which recurrent disease is not manifest in the form of strictly consecutive rises. In addition, bias was introduced by backdating the time of progression, given its sensitivity to the length and rigor of follow-up. To address these issues, new guidelines, termed the ASTRO Phoenix Criteria, were issued. These criteria recommend that biochemical failure be defined as a PSA rise of 2 ng/mL above the post-treatment nadir, whether or not the patient received hormonal therapy in conjunction with radiation therapy. The date at which that level was reached would be the date of relapse, and the practice of backdating would be abandoned. For patients who undergo salvage therapies such as with hormones, prostatectomy, or cryotherapy, failure would be declared not at the time of biochemical relapse but instead at the time of a positive biopsy or when salvage therapy was initiated. The consensus guidelines allowed, however, that the traditional ASTRO definitions were permissible if a patient underwent only external-beam radiation therapy or brachytherapy, provided that adequate follow-up was given and reported (Roach et al, 2006). One of the most difficult aspects of formulating a treatment plan for the patient with a rising PSA level is determining whether it represents locally persistent disease or nonlocalized systemic disease or both. The stakes of such a decision are high. Treating every patient who has undergone a prostatectomy with salvage radiation therapy exposes patients with systemic disease to unnecessary radiation therapy and the risk, albeit low, of proctitis, cystitis, and a reduced likelihood of maintaining or preserving erectile function. On the other hand, with careful patient selection, salvage radiation therapy has the potential to eliminate the disease completely. Current methods of detecting disease, whether in the prostatectomy bed, an irradiated gland, or metastatic sites such as bone or lymph nodes, are usually of very limited value. Moreover, the finding of local disease on an imaging study does not exclude a systemic component, which explains in part why radiation therapy to the prostate bed, or salvage surgery after radiation therapy, is not curative for many patients (Laufer et al, 2000; Leventis et al, 2001). By definition, the finding of overt radiographic metastases on an imaging study signifies that a patient is no longer in the clinical state of rising PSA but rather has metastatic disease (see Fig. 108–1). Nevertheless, to ensure that patients do indeed have a rising PSA level in the absence of radiographically evident metastases, a staging evaluation should be undertaken. However, bone scintigraphy and computed tomography (CT) of the abdomen and pelvis, the traditional modalities utilized, are of limited value because they are insensitive at detecting early metastatic disease. Bone scintigraphy will only detect metastatic disease that interferes with normal osteoblast/osteoclast interactions to produce abnormal bone deposition. Areas of marrow involvement that do not impact bone metabolism will remain undetected. Frequently, patients present to their clinician years before bone scintigraphy is likely to be positive. There is no single PSA value that predicts for scan positivity, although in most series PSAs will be well above 20 to 30 ng/mL before bone scintigraphy reflects metastatic disease (Cher et al, 1998). Even when bone scintigraphy is positive, tracer uptake in areas of trauma, infection, or inflammation can easily be mistaken for metastatic disease. More recently, a nomogram was developed to predict the likelihood of a positive scan. The nomogram included parameters that describe PSA kinetics—the PSA slope (odds ratio [OR], 2.71; P = .03) and PSA velocity (OR, 0.93; P = .003)—as well as the trigger PSA or current PSA value (OR, 1.022; P < .001). The concordance index for predicting the bone scan result was 0.93 (Dotan et al, 2005). Another model has been generated from the CaPSURE database, a national registry of over 13,000 patients treated in more than 40 urology practices in the United States. Of 7311 patients treated with definitive local therapy from 1990 to 2004, 1551 experienced biochemical recurrence (using the traditional ASTRO definition for postirradiation patients and a cutoff of 0.2 ng/mL for postprostatectomy patients). Of the 292 patients who had imaging studies and adequate PSA data, trigger PSA (≥5 ng/mL) and PSA doubling time (PSADT) (<10 months) predicted positive imaging results (which included both bone scintigraphy and CT) with a high concordance index (c-adj 0.84) in a multivariate model (Choueiri et al, 2008). CT is also suboptimal for the detection of metastasis not only because it has a lower limit of detection of 0.5 cm but also because the scans are nonspecific, making it difficult to distinguish scar tissue or fibrosis from tumor (Moul et al, 2001b). Magnetic resonance imaging (MRI) using an endorectal coil and a pelvic-specific acquisition methodology can reveal sites of recurrent disease in and around the prostate bed and bladder. In a preliminary study of endorectal MRI this modality correctly identified 39 of 41 patients (95%) thought to have locally recurrent disease on the basis of a positive biopsy, reduction in PSA level after radiation therapy, or serial MR images that demonstrated an increase in local disease in the fossa. Endorectal MRI may be able to identify sites of disease that would otherwise not be sampled by routine blind biopsies of the prostate bed and therefore may be useful for identifying areas suggestive of locally recurrent disease or for guiding biopsies (Sella et al, 2004). In a prospective study of men who had undergone a prostatectomy, endorectal MRI detected locally recurrent disease in all 31 men with clinical evidence of locally recurrent disease, as confirmed by biopsy. Taking together these true positives with nine true negatives found in the study, the investigators considered the sensitivity and specificity of the modality to be 100% (Tatli et al, 2006). This modality, however, may well be more appropriate for the patient who has undergone a prostatectomy rather than radiation therapy, because of treatment-related changes in the latter group. Positron emission tomography (PET) is another area actively under investigation for identifying occult metastatic or locally recurrent disease. Because fluorodeoxyglucose (FDG) is excreted through the kidneys, detection of nodal and locally recurrent disease, even in highly controlled patient populations with known progressive metastatic disease, can be challenging. In one study of 43 patients with definitively established progressive metastatic disease, patients were scanned both with FDG PET imaging as well as standard bone scintigraphy. Of the 1720 bones examined, the PET and bone scans were concordant in 1600 bones (93%) in terms of reflecting the presence or absence of metastatic disease. Of the 121 bones in which there was metastatic disease on the PET but not the bone scan, 84 (80%) ultimately became positive on the bone scan, suggesting that PET can detect disease earlier than bone scintigraphy in many patients (Meirelles et al, 2010). Schoder and colleagues (2005) used FDG-PET to examine patients with prostate cancer with a rising PSA level. Images were interpreted using a composite, though unvalidated, reference: a positive biopsy, decrease in PSA value after irradiation to the primary site, development of a detectable lesion in the primary site on follow-up conventional imaging studies, and increase in lesion size on follow-up imaging or concurrent other imaging studies within 90 days of PET. A positive FDG-PET was found to correlate with these standards in 31% of cases. Positive cases were more likely to be found in patients with higher PSA values (>9.5 ng/mL), and the probability of a positive scan correlated with PSADT (Schoder et al, 2005). In a retrospective study Chang and colleagues (2003) examined 24 patients with a rising PSA level; PET findings were correlated with pathologic evaluation from a lymph node dissection, the gold standard for disease confirmation. At the sites of pathologically proven metastases, increased FDG uptake suggestive of metastatic disease was found in 12 of 16 patients (75%) (Chang et al, 2003). Although there have been few well-controlled prospective studies of FDG-PET there are concerns about the ability of FDG to detect local and early metastatic disease, prompting investigations of newer PET modalities. Prostate cancer is associated with upregulated choline kinase activity and increased choline uptake. Choline is a component of phosphatidylcholine, which is a class of phospholipids and a major component of biologic membranes. Choline can be labeled with either carbon-11 ([11C]choline) or fluorine-18 ([18F]fluorocholine). In a prospective study, Scattoni and colleagues (2007) assessed 25 patients with PSA relapse and no evidence of local disease or bone metastases on conventional imaging who were imaged with [11C]choline-PET/CT and then underwent pelvic lymph node dissection. [11C]choline-PET/CT showed abnormal uptake in lymph nodes in 21 patients, 19 of whom had nodal metastases confirmed by histopathology. By comparison, CT or MRI revealed abnormal lymph nodes in 12 patients (Scattoni et al, 2007). Rinnab and colleagues (2007) retrospectively evaluated [11C]choline-PET/CT detection of nodal recurrence in 50 patients in the presence of a rising PSA value. The overall sensitivity of PET/CT was 95% and specificity was 40%. Fluorinated choline (FCH) is also being studied because it has the advantage of a half-life of 110 minutes versus a half-life of only 20 minutes with [11C]choline (DeGrado et al, 2001a, 2001b, 2002; Hara et al, 2002; Cimitan et al, 2006). Another PET tracer under investigation is [11C]acetate, because a high concentration of [11C]acetate in primary and metastatic lesions has been seen in prostate cancer (Oyama et al, 2002). An advantage of [11C]acetate is that it is not excreted by the kidneys, making it easier to visualize pelvic disease. In a preliminary study, uptake of [11C]acetate and [11C]choline radiotracers appeared similar (Kotzerke et al, 2003). Other preliminary studies suggest that radiolabeled acetate may detect locally recurrent disease, although all of these studies have been small and have had heterogeneous end points and populations (Sandblom et al, 2006). At this time, PET should be considered investigational, and a positive PET should not be considered definitive proof that a patient has either metastatic or locally persistent disease. ProstaScint is the only imaging agent approved by the U.S. Food and Drug Administration (FDA) to detect occult metastatic disease in patients with early prostate cancer. It also is indicated for patients with a rising PSA and a negative or equivocal standard metastatic evaluation when there is a high clinical suspicion of metastatic disease. The basis of this scan is a murine antibody, 7E11, that is combined with indium-111 to target the internal domain of prostate-specific membrane antigen (PSMA), a transmembrane type II glycoprotein found on normal prostate tissue and prostate cancers (Israeli et al, 1993, 1994). However, anti-PSMA antibodies also are taken up in the gut, liver, kidney, and other normal tissues. ProstaScint scanning is associated with a significant number of false-positive and false-negative results, in part related to these areas of uptake (Sartor and McLeod, 2001) in part owing to inflammation and even in part owing to vascular sludge (Hinkle et al, 1998). The specificity of the test may be increased if the images obtained are fused with MRI (Schettino et al, 2004). Many clinicians opt to sample palpable abnormalities in the prostate bed in a postprostatectomy patient. Indeed, even radiographic findings that are suggestive of locally recurrent or locally persistent disease may warrant pathologic confirmation. Biopsy specimens that reveal locally persistent disease may prompt a decision to choose salvage radiation therapy or systemic therapy for at least local control of palpable tumor masses. However, most patients will not have palpable lesions, and most patients will not have evidence of locally recurrent disease. The yield of blind biopsies in the prostate bed is usually low, tends not to affect clinical decision making, poorly predicts for the efficacy of salvage radiation therapy, and should not be considered a standard of care (Leventis et al, 2001; Scher et al, 2004). By contrast, performing a biopsy to prove locally persistent disease in the prostate after external-beam radiation therapy is essential before recommending that a patient receive a salvage radical prostatectomy, cryosurgery (Izawa et al, 2002), brachytherapy (Beyer 1999), or intraprostatic biologic or cytotoxic treatment. Because the risk of false-positive biopsy findings declines 2 years after the completion of radiation therapy, clinicians should wait at least that long before opting for biopsy. For the overwhelming majority of patients, biochemical relapse occurs far earlier than the development of radiographically evident findings or findings on physical examination or by biopsy. Given the limitations of these diagnostic studies, the focus of the clinician should be on using the PSA as a predictor of whether the patient will develop overt metastases, symptoms, or a high risk of death from disease. With such prognostic tools of clinical events the need for treatment can be determined and consideration can be given to what options are available and their likelihood of success in controlling the disease or, preferentially, eliminating it completely (Beekman et al, 2005). Many prognostic and predictive models have been reported. Some use determinants that reflect the disease at the time of initial diagnosis and treatment, others at the time of relapse, whereas others center on PSA kinetics after the disease has definitively relapsed biochemically. All of the models “work” in defining what they are designed to predict. Indeed, clinicians are often at a loss to decide which of several models to use in predicting a key clinical event. Multiple models now exist to predict the likelihood of a patient transitioning to the clinical state of a rising PSA value (D’Amico et al, 1998; Zelefsky et al, 1998; Moul et al, 2001a), the likelihood that a surgically removed tumor would have a specific pathologic stage according to pretreatment clinical features such as PSA or Gleason score (Partin et al, 1993, 2001; Roach, 1993, 1996), and the likelihood a patient with localized disease would achieve a given median survival (Roach et al, 2000; Vollmer and Humphrey, 2001; D’Amico et al, 2002; Sandler et al, 2000). Nomograms also have been developed that combine many of these prognostic determinants, treat them as continuous variables in multivariate models, and allow specific predictions of an outcome for a patient with given clinical features (Kattan et al, 2000). The models that have specific relevance to patients in the clinical state of a rising PSA value fall into several categories and are summarized in Table 108–1. Table 108–1 Models to Predict Clinically Significant Events in Patients with a Rising PSA Value after Definitive Local Therapy The first question the clinician evaluating a patient with a rising PSA value must address is where is the disease? In general, low pretreatment PSA levels, lower-grade tumors, low clinical or pathologic staging, late time from definitive local therapy to PSA relapse, and long PSADTs generally indicate a low likelihood of developing distant radiographically apparent metastases (Pound et al, 1999). Such patients can have durable remissions after salvage radiation therapy to the prostate bed (Moul, 2000; Leventis et al, 2001). Another modeling strategy is to examine patients who have successfully been “cured” using salvage radiation therapy and extrapolate from those data which features predict for exclusively locally recurrent disease. Features such as negative/close margins (P = .03), absence of extracapsular extension (P < .01), and presence of seminal vesicle invasion (P < .01) have been shown to predict for successful salvage radiation therapy (Katz et al, 2003). One multivariate model, derived from a retrospective examination of a multi-institutional database of patients who underwent salvage radiation therapy, showed that predictors of progression following salvage radiation therapy were Gleason score of 8 to 10 (hazard ratio [HR], 2.6; 95% CI, 1.7 to 4.1; P < .001), pre-radiotherapy PSA level greater than 2.0 ng/mL (HR, 2.3; 95% CI, 1.7 to 3.2; P < .001), negative surgical margins (HR, 1.9; 95% CI, 1.4 to 2.5; P < .001), PSADT of 10 months or less (HR, 1.7; 95% CI, 1.2 to 2.2; P = .001), and seminal vesicle invasion (HR, 1.4; 95% CI, 1.1 to 1.9; P = .02) (Stephenson et al, 2004). A subsequent report by Stephenson and colleagues (2007), which included a larger group of patients (1540) from 17 tertiary care centers who were observed over a median of 53 months, validated the accuracy of the previous model. In the updated model the same cut points yielded a clinical outcome within 10% of the original model. Based on these data a nomogram predicting the 6-year progression-free probability was constructed. The trigger PSA for radiation therapy was found to be highly predictive of progression-free probability because patients with a trigger PSA of 0.5 ng/mL or lower had nearly double the likelihood of remaining disease free than those treated at higher PSA levels (26% vs. 48%). Other predictive factors were Gleason grade, doubling time, margin status, neoadjuvant hormonal treatment, and nodal status (Stephenson et al, 2007). A second model examined 368 patients treated at multiple Mayo Clinic locations. This model again confirms that trigger PSA, doubling time, and pathologic staging are prognostic of a patient remaining disease free after salvage radiation therapy and that seminal vesicle involvement, Gleason score 8 to 10, and pre-radiotherapy PSA (treated as a continuous variable) are predictors of treatment failure (Buskirk et al, 2006). In a third model, derived from 211 patients, factors predictive of biochemical failure included a PSADT of less than 12 months (HR, 3.88; P = .032), seminal vesical invasion in the surgical specimen (HR, 3.22; P = .08), pathologic grade (HR, 1.58; P = .23), and the PSA value at the time of salvage radiotherapy (HR, 1.29; P = .04) (Ward et al, 2004).
When Does a Patient Occupy the Clinical State of a Rising Psa Value?
Prostate Cancer: A States-Based Model
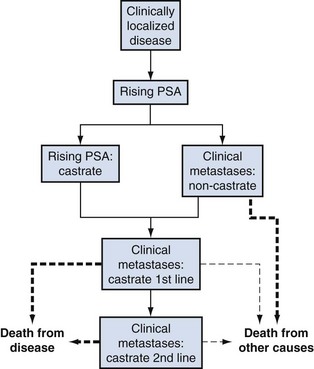
Defining the Clinical State of a Rising PSA Value
Radical Prostatectomy
Radiation Therapy
Radiographic and Other Tests to Determine Whether the Rising Psa Value Signifies Localized Disease, Metastatic Disease, or Both
Bone Scintigraphy and Computed Tomography
Magnetic Resonance Imaging
Positron Emission Therapy
Antibody-Based Imaging
Biopsies and Clinical Decision Making
Models to Predict Local Versus Systemic Recurrence and to Predict Survival
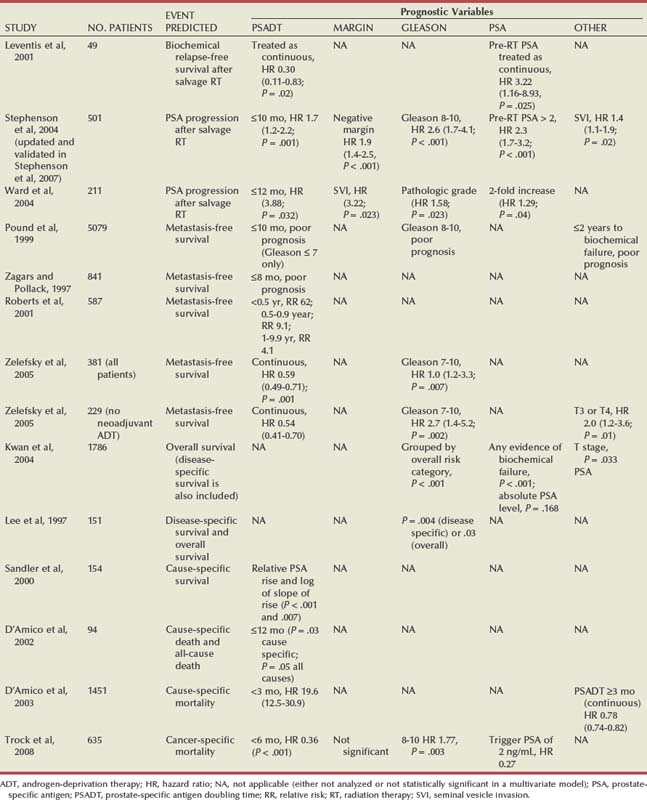
Predicting Local versus Systemic Relapse
Clinical State of the Rising PSA Value after Definitive Local Therapy: A Practical Approach
Radiographic and Other Tests to Determine Whether the Rising PSA Value Signifies Localized Disease, Metastatic Disease, or Both








