Central Hepatectomy
Jonathan B. Koea
Introduction
Central hepatectomy is the only anatomical liver resection that utilizes hepatic venous anatomy rather than the anatomy of hepatic inflow structures as the basis for segmental resection. The procedure removes hepatic parenchyma drained by the middle hepatic vein (Couinaud segments IV, V, and VIII) while preserving inflow to segments II, III, VI, and VII. Resection of the caudate lobe (segment I) can be undertaken in conjunction with the procedure if indicated (see Chapter 22). It is not commonly performed and central resections constitute between 4% and 6% of segmental resections reported from high volume hepatobiliary units. Central hepatectomy is technically more challenging than an extended right hepatectomy, but the additional parenchyma that is preserved translates into a lower risk of postoperative hepatic failure.
The procedure was first described in 1972 by McBride and Wallace. Pack and Miller described middle hepatic lobectomy for cancer in 1961, although this only removed parenchyma between the main interlobar fissure (Cantlie’s line) and the umbilical fissure and consequently represents resection of segment IV only. Central hepatectomy has been variously named middle lobectomy, central bisegmentectomy, mesohepatectomy (meso: Greek, translates to middle or central), lobectomie médiane totale, hépatectomie or lobectomie médiale, or mixed right intermediate hepatectomy. The Brisbane nomenclature does not assign a single term for the procedure since it comprises a combination of a left medial sectionectomy (segment IV resection) and a right anterior sectionectomy (segments V and VIII resection) although a central bisectionectomy is probably the correct nomenclature. Currently the terms central hepatectomy or mesohepatectomy are used most commonly.
 INDICATIONS
INDICATIONSCentral hepatectomy is indicated for the resection of tumors located in segments IV, V, and VIII. The tumor must be separate from the left and right hepatic veins such that it can be dissected free of these structures and removed with an adequate surgical margin. Consequently, central hepatectomy can be utilized in the management of the following tumors, if suitably located.
Symptomatic benign liver tumors (adenoma, focal nodular hyperplasia, hemangioma) and asymptomatic adenoma ≥5 cm in diameter
Primary malignant tumors of the liver (hepatocellular carcinoma, intrahepatic cholangiocarcinoma)
Gallbladder carcinoma
Hilar cholangiocarcinoma
Metastatic tumors, most commonly colorectal cancer, neuroendocrine carcinoma, melanoma, genitourinary tumors. Generally these patients will have only liver metastatic disease
 CONTRAINDICATIONS
CONTRAINDICATIONSCentral hepatectomy cannot be applied to tumors that have spread beyond segments IV, V, and VIII. In particular, tumors that involve either the right or left hepatic veins or the right or left inflow pedicles will require a more extensive resection than central hepatectomy in order to obtain adequate surgical margins.
Central hepatectomy is also contraindicated in the following patients.
Patients with comorbidities that render them unfit for general anesthesia.
Patients with normal hepatic parenchyma in whom a central hepatectomy will leave less than 20% hepatic volume since these patients are at significant risk of postoperative liver failure.
Patients with cirrhosis in whom a central hepatectomy will leave less than 40% of hepatic volume as these patients will be at significant risk of postoperative liver failure.
Anatomical Considerations
The safe performance of central hepatectomy requires that segments IV, V, and VIII can be isolated and removed while maintaining the vascular inflow and venous and biliary drainage to the remaining five hepatic segments. Several anatomical variations must be recognized preoperatively as they may make safe performance of the procedure impossible or technically difficult.
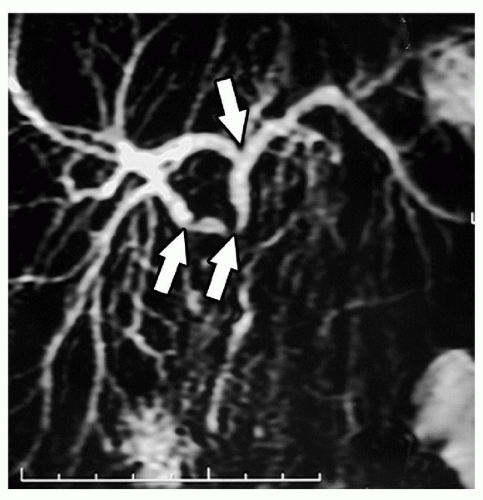
The inflow to segment IV is generally constant and is from two discrete branches that arise from the right side of the left main pedicle within the umbilical fissure. Rarely, the main left portal vein arises from the right anterior sectoral pedicle and traverses segment IV supplying it as well as segments II and III (Fig. 21.1). The presence of this variation makes the procedure impossible to perform since the blood supply to segments II and III cannot be preserved, when segment IV is resected.
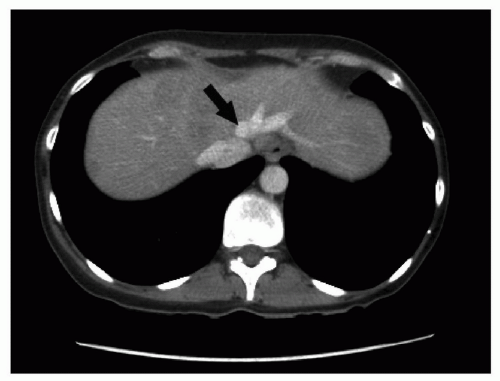
The right posterior sectoral bile duct drains into the left bile duct in 1% of patients (Fig. 21.2). The presence of this variant makes the procedure technically difficult but the posterior sectoral duct generally joins the left main duct close to the hilus and can be isolated and protected, or reconstructed using a Roux-en-Y hepaticojejunostomy.
The right anterior sectoral duct draining segments V and VIII can arise from the left main pedicle (Fig. 21.3). This does not prevent the procedure from being performed but means that the anterior sectoral branch must be clearly defined and controlled without compromising the main left pedicle.
The presence of a long common venous trunk formed by the union of the left and middle hepatic veins (Fig. 21.4). This does not prevent the performance of central hepatectomy but emphasizes that the middle hepatic vein must be carefully controlled and isolated without compromising the left hepatic vein.
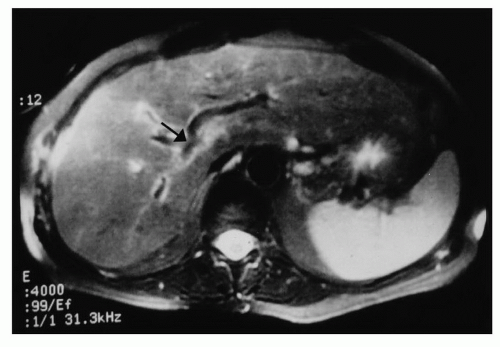 Figure 21.1 Magnetic resonance scan showing the left hepatic inflow arising from the right inflow pedicle within segment IV (arrow). |
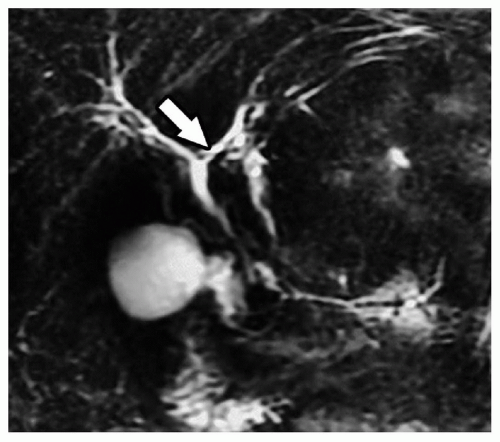 Figure 21.2 Coronal magnetic resonance cholangiogram showing the right posterior sectoral duct joining the proximal left main hepatic duct (arrow). |
 PREOPERATIVE PLANNING
PREOPERATIVE PLANNINGBefore surgery a comprehensive assessment of the patient must be carried out. This evaluation should define the patient’s overall state of fitness for general anesthesia, the presence of any comorbidities, the functional status of the liver and establish the diagnosis, clinical stage and precise location of the tumor.
Patient Assessment
A thorough history and physical examination should be performed with specific emphasis on defining any underlying cardiovascular, pulmonary, renal, or neurologic disease. If signs of major organ dysfunction or comorbidity are present then these should be investigated appropriately and referred to the relevant specialty for detailed assessment. Measurement of relevant tumor markers such as α-fetoprotein, carcinoembryonic antigen (CEA) and CA19-9 should also be undertaken at this stage.
Hepatic Function
A physical examination should be conducted looking for stigmata of chronic liver diseases (palmar erythema, flap, gynecomastia, spider nevi, ascites, and edema) and liver function should be formally assessed with measurement of platelet count, prothrombin time or INR, albumin, total protein, bilirubin, alkaline phosphatase, aspartate transaminase, and gamma glutamyl transferase. Cross-sectional imaging should be reviewed for signs of portal hypertension (portal vein dilation, varices, splenomegaly, and ascites) and cirrhosis (ascites, small irregular liver outline). Functional hepatic assessment with indocyanine green retention can also be performed.
Radiologic Imaging
Most patients who present with hepatic tumors undergo cross-sectional imaging with various modalities. In general, patients are often assessed initially with transabdominal ultrasound and then undergo imaging with computed tomography (CT scan) and/or magnetic resonance imaging (MRI) scans. Staging with computed tomography/positron emission scans (CT/PET) is now also accepted as standard of care for many tumor types, in those centers where it is available. A thorough review of all imaging modalities in a multidisciplinary meeting will generally clarify potential diagnoses based on tumor imaging characteristics and the precise anatomical location. Further review of
imaging will assist with staging, the presence of any important anatomical variations, and the expected size and volume of the liver remnant following resection. In addition, discussion in a multidisciplinary context will allow consideration to be given to treatment related issues such as the potential role of neoadjuvant chemotherapy, radiotherapy or the intraoperative placement of catheters for postoperative regional chemotherapy.
imaging will assist with staging, the presence of any important anatomical variations, and the expected size and volume of the liver remnant following resection. In addition, discussion in a multidisciplinary context will allow consideration to be given to treatment related issues such as the potential role of neoadjuvant chemotherapy, radiotherapy or the intraoperative placement of catheters for postoperative regional chemotherapy.
 SURGICAL TECHNIQUE
SURGICAL TECHNIQUEAnesthesia and Postoperative Analgesia
Patients are managed with general anesthesia and usually require placement of a central venous line, peripheral venous lines, an arterial line, and a urinary catheter. Postoperative analgesia is provided by thoracic epidural analgesia or a single dose of intrathecal morphine depending on the assessment of the anesthetist. All patients are managed intraoperatively with sequential calf compression devices. Subcutaneous administration of unfractionated heparin is begun in the postoperative period when the INR is less than 1.5 and is continued until discharge.
Positioning
Patients are positioned supine with both arms at right angles from the body taking due care to protect against traction injuries with adequate padding. The urinary catheter and electrocardiogram leads should be kept clear of the sides of the operating table to permit attachment of a subcostal retraction system. The abdomen should be prepped and draped between the nipples and suprapubic area. Upper and lower body warming systems should be applied. If a staging laparoscopy is planned it can be performed at this stage.
Technique
Exposure
The abdomen is opened through a right subcostal incision some two finger breadths below the costal margin with a midline extension to the xiphisternum. This provides excellent exposure for central liver tumors. The ligamentum teres should be divided and secured with a ligature (Fig. 21.5). This ligature should be left long as it is a useful mechanism for securing the left side of the liver and opening the parenchyma between segments II/III and IV. The falciform ligament should be divided back to the level of the vena cava. The left (Fig. 21.6) and right coronary ligaments (Fig. 21.7) should be taken down.
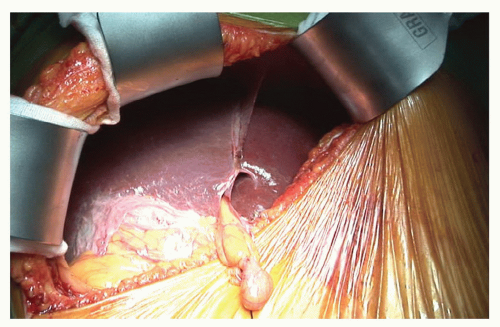 Figure 21.5 The abdomen has been opened and the liver exposed via a right subcostal incision. A subcostal retractor has been deployed and the ligamentum teres has been secured with a silk tie. |
Initial Assessment and Intraoperative Ultrasound
Intraoperative assessment should first focus on detection of metastatic spread to peritoneum, regional lymph nodes, or the remainder of the liver in operations for malignant disease where these findings may preclude resection. An assessment should also be made of liver quality noting signs of steatosis or cirrhosis. If portal hypertension is suspected, portal pressures can be measured directly, although this is usually recognized before operation and is best performed in the radiology suite.
An intraoperative ultrasound should be performed. This should verify the position of the tumor within segments IV, V, and VIII and the presence of standard inflow and hepatic venous anatomy. In addition, in operations for malignant disease, a thorough assessment of hepatic parenchyma should be made to locate any intrahepatic metastatic lesions that have not been detected with preoperative imaging investigations.
Control of Segment IV Inflow
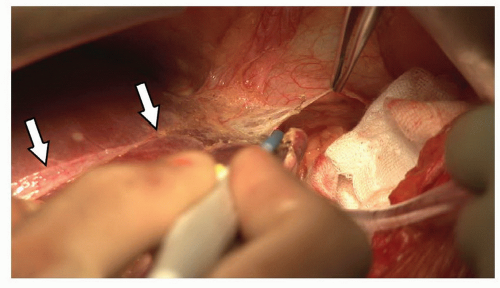
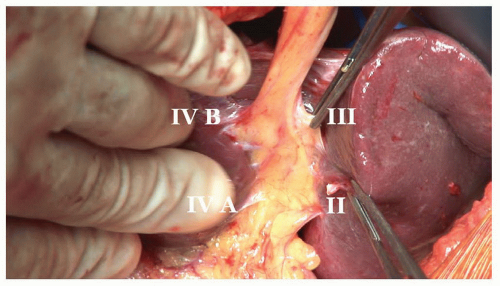
The inflow to segment IV is approached in the umbilical fissure. The anatomy of the fissure is constant (Fig. 21.8). When viewed from the caudal perspective, the left hepatic artery, left bile duct and left portal vein run within the umbilical fissure contained within Glisson’s sheath. Branches to the caudate lobe (segment I), segment II and segment III arise sequentially from the left side of the sheath. Branches to segment IVA and IVB arise from the right side of the sheath. Each segmental pedicle contains the respective branch of the hepatic artery, portal vein and bile duct contained within a connective tissue sheath.
The segment IV pedicles can be isolated by gentle dissection within the right side of the umbilical fissure. Segment IVB is approached first using a right angle forceps to develop the plane between Glisson’s capsule and parenchyma until the pedicle is encircled (Fig. 21.9). Occasionally a small amount of parenchyma needs to be divided within the line of the falciform ligament to expose the anterior aspect of the IVB pedicle to assist with safe mobilization. If necessary this can be undertaken with intermittent mass
inflow control (Pringle maneuver). Once the pedicle has been encircled a vascular tape can be passed around it and the pedicle then divided with a linear stapler or between clamps and suture ligated with a 4-0 non-absorbable suture.
inflow control (Pringle maneuver). Once the pedicle has been encircled a vascular tape can be passed around it and the pedicle then divided with a linear stapler or between clamps and suture ligated with a 4-0 non-absorbable suture.
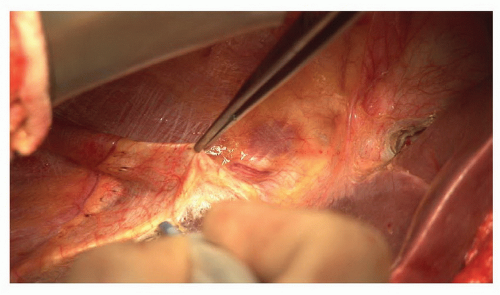 Figure 21.7 The right coronary ligament is divided separating the right lobe of the liver from the diaphragm and exposing the bare area. |
Further dissection within the right side of the fissure will expose the segment IVA pedicle which can be controlled in the same manner and this should be followed by a significant color change in segment IV (Fig. 21.10). Significant branches to segment IV can arise from the posterior aspect of the left pedicle and should be sought if parts of segment IV appear to be vascularized. However, great care must be taken not to damage or occlude the pedicles to segments I, II, and III which arise in the left side of the fissure.
Control of Segments V and VIII Inflow
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree