Essentials of Diagnosis
- Cardiovascular disease (CVD) is highly prevalent among patients with chronic kidney disease (CKD) and is the most common cause of death in this population.
- The manifestations of CVD in CKD are variable and include left ventricular hypertrophy, ischemic heart disease, heart failure, and peripheral vascular disease.
- Traditional and nontraditional (or “uremia-related”) cardiac risk factors are common in CKD.
- Clinicians should maintain a high index of suspicion for the presence of CVD in patients with CKD, even when the presentation is atypical.
- An aggressive approach to diagnosis and treatment of CVD is recommended in patients with CKD.
General Considerations
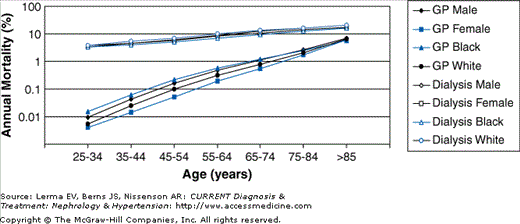
CVD is highly prevalent among patients with CKD and is the most common cause of death in this population. Importantly, patients with impaired kidney function are more likely to die than to progress to end-stage renal disease (ESRD) requiring renal replacement therapy and those who do reach dialysis have a staggering mortality rate of about 20% per year. In dialysis patients of all ages, the mortality rate from CVD far exceeds that observed in the general population (Figure 19–1). Dialysis has the greatest impact on younger patients, whose mortality rate from CVD is more than 100 times greater than that of their counterparts with normal kidney function.
The burden of CVD begins to accumulate long before patients reach ESRD. For example, left ventricular (LV) hypertrophy (LVH) increases in prevalence with declining renal function and is present in 75% of patients beginning dialysis treatment. Ischemic heart disease (IHD) and heart failure also develop early, and are present in 40% and 35% of incident dialysis patients, respectively. Recent publications have demonstrated a profound impact of a reduced glomerular filtration rate (GFR) below 60 mL/minute on cardiovascular event rates.
In recent years both the National Kidney Foundation (NKF) and the American Heart Association (AHA) have recommended that patients with CKD be placed in the highest risk group for the development of CVD. However, while recognition of their high-risk status has improved, evidence from clinical trials evaluating the benefit of interventions aimed at reducing CVD risk in the CKD population is still largely lacking.
Recommendations by the NKF for the evaluation and treatment of CVD in dialysis patients were published in April 2005. The key to these guidelines is the recommendation that an aggressive approach to diagnosis and treatment is warranted in patients with ESRD due to the high risk of CVD in this patient group.
The spectrum of CVD in CKD is wide, and includes abnormalities of the heart and blood vessels, such as LVH, congestive heart failure (CHF), valvular heart disease, pericarditis, cardiac arrhythmias, IHD, and peripheral vascular disease (PVD). Those disorders that are most common and account for the majority of the morbidity and mortality in patients with CKD will be the focus of this chapter: LVH, IHD, heart failure, and PVD. In this chapter, the term CKD is used generically to refer to patients with all degrees of kidney dysfunction, including those on dialysis. Comments pertaining to a particular subgroup of patients (for example, predialysis patients or ESRD patients) are specified as such.
Pathogenesis
Traditional and nontraditional risk factors have been discussed in the literature as contributors to the high rate of CVD in CKD (Table 19–1). Certainly, traditional cardiac risk factors are highly prevalent in these patients. These include advanced age, diabetes mellitus, hypertension, low high-density lipoprotein (HDL), and LVH. However, the complexity of the relationship between some traditional cardiovascular risk factors and overall mortality in ESRD must be appreciated. For example, a “reverse epidemiology” or “U-shaped” mortality curve has been observed whereby ESRD patients with low cholesterol and low blood pressure paradoxically have an increased mortality. This increase in mortality is hypothesized to reflect underlying malnutrition and/or inflammation (in the case of low cholesterol) and advanced cardiomyopathy (in the case of low blood pressure). Thus, the benefits of strategies traditionally used to combat CVD in the general population, such as lowering of cholesterol and blood pressure, may be questioned in the setting of kidney disease.
Traditional risk factors | Nontraditional factors |
|---|---|
Older age | Albuminuria |
Male gender | Homocysteine |
Hypertension | Lipoprotein (a) and apo (a) isoforms |
Higher LDL cholesterol | Lipoprotein remnants |
Lower HDL cholesterol | Anemia |
Diabetes | Abnormal calcium/phosphate metabolism |
Smoking | Extracellular fluid volume overload |
Physical inactivity | Electrolyte imbalance |
Menopause | Oxidative stress |
Family history of cardiovascular disease | Inflammation (C-reactive protein) |
Left ventricular hypertrophy | Malnutrition |
Thrombogenic factors | |
Sleep disturbances | |
Altered nitric oxide/endothelin balance |
Furthermore, there is accumulating evidence that a reduced GFR per se is an independent risk factor for CVD. It may be that lower GFR is associated with, or marks for, nontraditional or “uremic” risk factors. These nontraditional risk factors, outlined in Table 19–1, include anemia, abnormalities of calcium and phosphate metabolism, inflammation, prothrombotic factors, oxidative stress, and perhaps also hyperhomocysteinemia, and elevated levels of lipoprotein (a). However, despite strong associations between these factors and CVD in epidemiologic studies, a causal relationship has not been proven, nor have interventional studies been conducted that demonstrate changes in outcomes with treatment of these abnormalities.
In patients with residual kidney function (ie, those not yet on dialysis), the issue of proteinuria and cardiovascular risk merits specific comment. First, microalbuminuria is associated with an increased risk of cardiovascular events in both diabetic and nondiabetic subjects, even in the absence of renal insufficiency. While patients with microalbuminuria often have an increased prevalence of traditional cardiac risk factors, it is also thought that microalbuminuria may be a marker of generalized endothelial dysfunction or inflammation. Second, individuals with the nephrotic syndrome are known to have an increased risk of myocardial infarction (MI). Among the potential explanations for this are that hyperlipidemia, hypercoagulability, and hypertension are common in the nephrotic syndrome and these may contribute to the increased risk of IHD. In addition, recent publications describe clear associations between proteinuria and increased rates of CVD at all levels of kidney dysfunction.
Given the documented burden of CVD in CKD, the association with adverse outcomes in patients where the two coexist, and the biologically plausible explanations as to the impact of traditional and nontraditional risk factors in CKD populations, it is important for the practicing clinician to understand the complexity of this area, what is known and where gaps in our knowledge base exist.
The work performed by the LV in each cardiac cycle is equal to the product of the ventricular pressure and stroke volume. In kidney disease, the work of the LV is increased due to both pressure and volume overload. Hypertension, arteriosclerosis, and aortic stenosis contribute to pressure overload, while volume overload occurs as a result of factors such as anemia, increased extracellular fluid volume, and the presence of arteriovenous fistulas. These and potentially other factors, such as hyperparathyroidism and the “uremic milieu,” contribute to the development of LVH, an independent risk factor for mortality in all CKD patients, independent of dialysis status.
The LVH that occurs intitially as an adaptive response to physiologic stimuli (pressure or volume) eventually becomes maladaptive. At the cellular level, the metabolically active myocytes begin to experience an energy deficit, partly due to ischemia, resulting in cell death. In addition, cardiac fibroblasts proliferate, expanding the extracellular matrix of the myocardium and causing myocardial fibrosis. From a functional point of view, the hypertrophied ventricle becomes stiff, impairing relaxation and resulting primarily in diastolic dysfunction, at least initially. These changes may partially explain the high prevalence of heart failure in this patient group.
Heart failure is common in CKD and is present in about one-third of incident dialysis patients. Patients may have systolic dysfunction, diastolic dysfunction, or both. The pathogenesis of heart failure is multifactorial with contributions from LVH, IHD, valvular heart disease, and other abnormalities specific to the uremic state such as chronic extracellular fluid volume expansion, disturbances in divalent ion metabolism, anemia, and the presence of arteriovenous (AV) fistulas.
There are two general types of arterial vascular disease in CKD: Atherosclerosis and arteriosclerosis. Atherosclerosis is a disease of the intima and is characterized by plaques and vessel occlusion. The most common vessels affected are the medium-sized arteries, such as the coronary, femoral, and carotid arteries. There are many contributors to atherosclerosis, including the high prevalence of cardiac risk factors such as advanced age, dyslipidemia, hypertension, and the metabolic syndrome.
Patients with CKD also have a high prevalence of arteriosclerosis, or stiffening of the arteries; this may occur in the presence or absence of significant atherosclerosis. Although it normally occurs with aging, the process appears to be accelerated in kidney disease. The intima and media of the large, elastic arteries such as the aorta and the common carotid are affected in particular. The vessel wall is remodeled and becomes thickened and stiff, reducing compliance. These stiff vessels contribute to hemodynamic changes, including an increase in systolic blood pressure, a decrease in diastolic blood pressure, and as a result, a widened pulse pressure and increased pulse wave velocity. The raised systolic blood pressure increases LV afterload and contributes to the development of LVH, while the reduced diastolic pressure compromises coronary artery perfusion and contributes to myocardial ischemia. Recent data from both dialysis and predialysis patients have confirmed the association among an elevated pulse pressure, a clinical manifestation of arterial stiffness, and adverse outcomes.
A contributor to the arterial stiffening in kidney disease that has recently been given some attention is calcification of the intimal and medial layers of these vessels. It has been observed that this calcification is a key feature of the arterial disease in CKD, especially in ESRD. The calcification is more extensive and is observed much earlier in ESRD than in the general population. Abnormalities of bone mineral metabolism, including elevations in serum phosphorus, calcium, and calcium × phosphorus product, among other factors, are intimately involved in promoting vascular calcification.
This unique contribution of specific factors to arterial calcification and measured arterial stiffness, in conjunction with anemia (also common in CKD), may explain in part the high prevalence of CVD in CKD patients.
Clinical Findings
LVH may be asymptomatic or patients may present with diastolic dysfunction, which is discussed in further detail in the next section.
On physical examination, hypertension is common. Particular attention should be paid to the pulse pressure as a surrogate measure of arterial stiffness, where “normal values” are <40–60 mm Hg. Precordial palpation may reveal a left ventricular heave and a sustained and diffuse cardiac apical impulse. On auscultation, a fourth heart sound may be heard.
Due to the lack of sensitivity of symptoms and physical examination findings, echocardiography is usually used for diagnosis and clinical follow-up of patients with LVH.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree