Cancers of the Biliary Tree: Clinical Management
Keith D. Lillemoe
Richard D. Schulick
Andrew S. Kennedy
Joel Picus
Carcinomas of the biliary tree, including the gallbladder and bile duct, represent a significant clinical challenge. They are often asymptomatic early in their course, usually present at an advanced stage, and frequently are not amenable to curative therapy. In both gallbladder and bile duct cancers, the surgical and oncologic management has not been clearly defined, and survival even after curative resection remains poor. Furthermore, biliary obstruction may complicate both the initial and terminal management of both diseases. Biliary obstruction may cause the life-threatening complications of cholangitis and progressive liver dysfunction, and commonly causes anorexia and pruritus. Adequate decompression of the biliary tree must be obtained, even in the face of unresectable disease. This chapter focuses on the management of gallbladder and bile duct carcinoma by addressing the diagnostic, operative, adjuvant, and palliative measures in these diseases.
Gallbladder Carcinoma
Clinical Presentation
Gallbladder carcinoma is the fifth most common cancer of the gastrointestinal tract and is the most common cancer of the biliary tree (1). The clinical presentation of gallbladder carcinoma ranges from that of an incidental finding after cholecystectomy for symptomatic gallstones to a rapidly progressive disease offering little opportunity to provide treatment of therapeutic benefit. Unfortunately, many patients in the United States present with advanced disease at the time of diagnosis.
Gallbladder carcinoma is a disease of the elderly population and is three to four times more common in women than in men. Symptoms of gallbladder carcinoma are similar to those of benign gallbladder disease, including biliary colic and acute cholecystitis. Right upper quadrant abdominal pain is the most common symptom and is present in >80% of patients. The pain is often continuous rather than the colicky pain typical of gallstone disease. Nonspecific symptoms such as nausea, intolerance of fatty foods, anorexia, weight loss, fever, and chills are also common. In more advanced cases, the gallbladder malignancy obstructs the biliary system, which results in obstructive jaundice. Physical findings in these cases include right upper quadrant tenderness, a palpable mass, hepatomegaly, and ascites. Laboratory studies are generally nonspecific unless biliary tract obstruction has developed. Levels of common tumor markers such as carcinoembryonic antigen and carbohydrate antigen 19-9 may be elevated but are not reliably useful for diagnosis.
Diagnosis
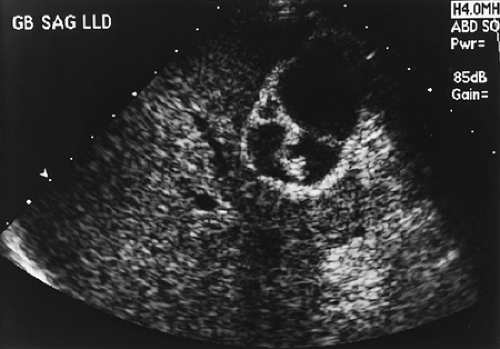
Gallbladder carcinoma is not diagnosed preoperatively in the majority of cases due to the nonspecific presentation and lack of reliable diagnostic criteria. Most patients present with symptoms suggestive of benign gallstone disease, and ultrasonography is the usual initial diagnostic procedure. Because gallstones are present in >90% of patients with gallbladder carcinoma, the ultrasonographic findings often suggest benign cholelithiasis as the cause of the patient’s symptoms. A thickening of the gallbladder or a polypoid or fungating mass protruding into the gallbladder lumen, or both, should raise suspicion of a gallbladder neoplasm (Fig. 37.1). Less subtle findings of liver invasion, lymphadenopathy, or blood vessel invasion are seen in more advanced cases.
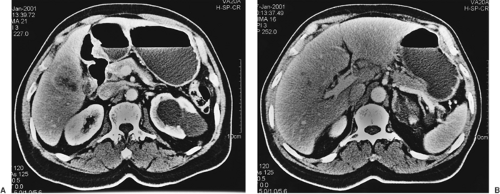
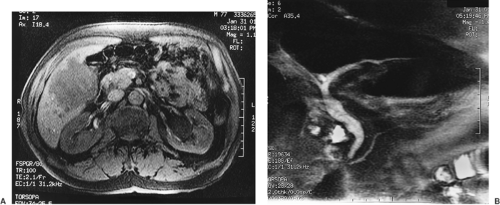
Computed tomography (CT) is more sensitive than ultrasonography in identifying a gallbladder carcinoma and better delineates a gallbladder mass (Fig. 37.2A), lymphadenopathy (Fig. 37.2B), and invasion of adjacent organs. In addition, CT can demonstrate the presence of liver metastases and ascites. The sensitivity and specificity of contrast-enhanced CT in diagnosing neoplastic lesions of the gallbladder is close to 90% (2). CT is also valuable in defining major vascular invasion of portal structures (portal vein and hepatic artery), which may indicate unresectability. Improvements in magnetic resonance imaging, including the development of magnetic resonance cholangiopancreatography (MRCP), have enabled its use as a single noninvasive imaging modality that allows complete assessment of the hepatic parenchyma (Fig. 37.3A), biliary tree (Fig. 37.3B), vasculature, and lymph nodes. Endoscopic ultrasonography has been reported to be useful for diagnosis and staging of gallbladder cancer (3). This technique has been found to be particularly useful in distinguishing early- and advanced-stage tumors by demonstrating tumor invasion and lymph node metastasis. Finally, positron emission tomography with fluorine-18–labeled fluorodeoxyglucose (FDG–PET) scanning has been shown to demonstrate uptake in patients with gallbladder cancer assisting in both diagnosis and staging (4).
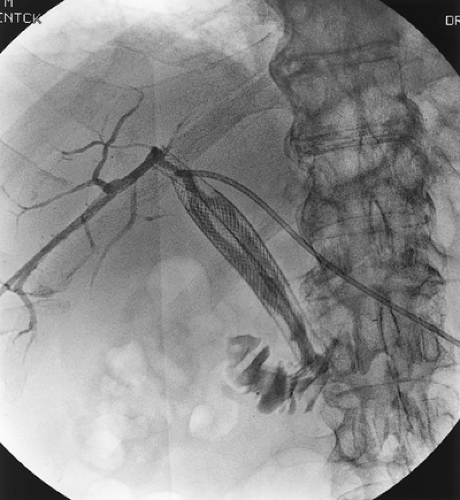
Cholangiography has generally been used for patients with gallbladder carcinoma and obstructive jaundice. Either endoscopic retrograde cholangiography or percutaneous transhepatic cholangiography (PTC) can be useful in identifying the area of obstruction. The typical finding in a patient with gallbladder carcinoma is a long stricture involving the common hepatic duct (Fig. 37.4). Although biliary stents can be placed by either the endoscopic or percutaneous route, percutaneous catheters are a more reliable means to relieve the biliary obstruction and may be helpful in operative management. As stated previously, MRCP techniques can provide similar, high-quality imaging
of the biliary tree, and their use has eliminated the need for invasive cholangiography in many patients.
of the biliary tree, and their use has eliminated the need for invasive cholangiography in many patients.
Management
The management of gallbladder carcinoma depends largely on the mode and the stage of presentation. The disease generally presents in one of three ways: (i) as an incidental finding during or after cholecystectomy for suspected benign disease, (ii) as a suspected or confirmed lesion that appears resectable based on preoperative evaluation, or (iii) as an advanced intra-abdominal malignancy. Each of these specific presentations generally requires a different management strategy. In general, an aggressive attitude favoring surgical resection is appropriate because it offers the only chance for cure. The appropriate extent of surgical resection for each stage of disease is controversial and highly debated. Alfred Blalock wrote in 1924, “in malignancy of the gallbladder, when a diagnosis can be made without exploration, no operation should be performed, inasmuch as it only shortens the patient’s life” (5). Although the ability to diagnose gallbladder carcinoma has improved over the last 75 years, Blalock’s point still remains valid for many patients, and palliation is often the primary goal of treatment.
Surgical Resection
The role of surgery for gallbladder carcinoma depends on the mode of tumor presentation and the extent of disease. The discovery of gallbladder carcinoma during or after laparoscopic cholecystectomy is a common scenario, as many patients present with signs and symptoms similar to those of benign gallstone disease. If preoperative studies suggest gallbladder carcinoma, laparoscopic cholecystectomy should be avoided. Should gallbladder carcinoma be found at the time of laparoscopic cholecystectomy, biopsy of the gallbladder mass should be avoided, and conversion to an open laparotomy should be considered unless liver metastases or carcinomatosis is identified. Tumor dissemination is the major concern related to laparoscopic cholecystectomy. Diffuse peritoneal tumor spread associated with disruption of the gallbladder wall and bile spillage as well as cancer recurrence at trochar sites have been well documented (6,7).
If gallbladder cancer is diagnosed on pathologic examination after laparoscopic cholecystectomy, further therapy is dependent on the pathologic findings. Patients with T1 N0 M0 (stage IA) gallbladder carcinoma, with disease confined to the lamina propria or muscle layer, generally do not require more than a simple cholecystectomy as long as the cystic duct margin is negative. Furthermore, T1 tumors have not yet invaded the subserosal layer, which contains the lymphatics, and therefore lymphadenectomy is not required. Most surgeons believe that the morbidity and mortality of an extended resection are not justified for disease at this early stage as the 5-year survival for stage IA disease in most series exceeds 85% (8,9,10,11,12). In contrast, most centers advocate a more extensive resection for T2, which invades the muscle layer of the gallbladder wall (stage IB) (8,13,14,15,16,17). Table 37.1 summarizes the results of a series of studies on the surgical resection of T1 N0 M0 (stage IA) gallbladder cancer.
Patients with disease of higher T stage have a significant chance of having lymph node metastasis. A series at the Memorial Sloan-Kettering Cancer Center reported the incidence of lymph node involvement with T2 lesions
to be 33% (14). In the same series, the incidence of lymph node involvement with T3 lesions was 58% and with T4 lesions was 69%. The management of T2 and T3 lesions is generally accepted to include extended or radical cholecystectomy consisting of en bloc resection of the gallbladder and wedge excision of liver segments IV and V with at least a 3- to 4-cm margin of normal parenchyma. Some centers advocate more radical liver resections, including extended lobectomies for the bulkier lesions and for cases in which differentiation of tumor from inflammation is difficult at the second operation. Regional lymphadenectomy, including resection of all choledochal, periportal, hilar, and high pancreatic lymph nodes, should be performed. Bile duct resection and reconstruction may be necessary depending on the location of the tumor with respect to the junction of the cystic duct and common duct, and to facilitate lymph node dissection. Most groups also recommend resection of all laparoscopic trochar sites.
to be 33% (14). In the same series, the incidence of lymph node involvement with T3 lesions was 58% and with T4 lesions was 69%. The management of T2 and T3 lesions is generally accepted to include extended or radical cholecystectomy consisting of en bloc resection of the gallbladder and wedge excision of liver segments IV and V with at least a 3- to 4-cm margin of normal parenchyma. Some centers advocate more radical liver resections, including extended lobectomies for the bulkier lesions and for cases in which differentiation of tumor from inflammation is difficult at the second operation. Regional lymphadenectomy, including resection of all choledochal, periportal, hilar, and high pancreatic lymph nodes, should be performed. Bile duct resection and reconstruction may be necessary depending on the location of the tumor with respect to the junction of the cystic duct and common duct, and to facilitate lymph node dissection. Most groups also recommend resection of all laparoscopic trochar sites.
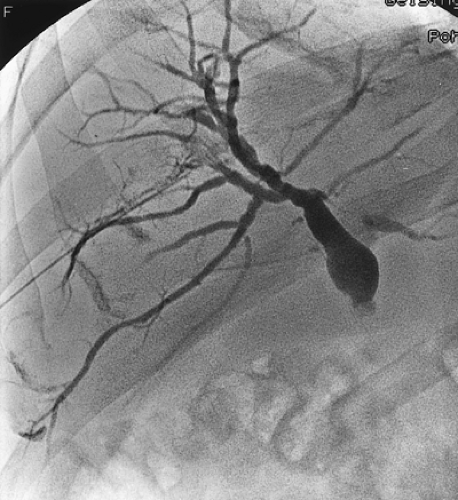 FIGURE 37.4. Percutaneous transhepatic cholangiogram of a gallbladder carcinoma that obstructs the common hepatic duct. |
In patients in whom the gallbladder cancer is detected preoperatively, ample evidence supports more radical excision for T2 and T3 tumors. Patients with T2 lesions undergoing simple cholecystectomy have had reported 5-year survivals of 36% to 40% (15,18), whereas patients with T2 lesions undergoing wide resection and lymphadenectomy have had reported 5-year survivals of 83% to 100% (13,15,18). Proponents of wide resection and lymphadenectomy for T3 lesions point out that distinguishing T2 from T3 tumors at the time of surgery is often difficult and that resection of the liver allows the best chance for tumor clearance.
With T4 lesions, the enthusiasm to perform wide resection and lymphadenectomy is somewhat more tempered. Conventional clinical judgment is that the prognosis is poor in these patients regardless of treatment, and the morbidity of an extensive operation is not justified. The group at Memorial Sloan-Kettering Cancer Center, however, has demonstrated long-term survival in patients with T4 N0 M0 lesions who underwent wide resection usually including at least liver segments 4b and 5, and in may cases extended right hepatectomy (segments 4,5,6,7, and 8) lymphadenectomy (14). In this series, 27 patients who underwent resection for T4 disease were described. The 5-year survival rate in this group of patients was 28%, and five patients actually survived beyond 5 years. These results suggest that resection may be justified, especially if no gross nodal involvement is apparent at the time of operation.
The results of a number of series studying radical resection for gallbladder carcinoma are shown in Table 37.2. These series demonstrate that radical resections for gallbladder carcinoma can be performed with low mortality rates in the range of 0% to 4% (12,13,14,15,16,17,18,19,20). They also demonstrate that radical resections for gallbladder carcinoma can lead to long-term survival. Representative of modern Western results, a review at Memorial Sloan-Kettering Cancer Center examining 410 patients who
presented over a 14-year period found that only 102 patients were able to undergo potentially curative resection (14). Fifty-one patients had inoperable disease, 135 patients underwent noncurative cholecystectomy, and 92 patients underwent exploration and biopsy only. Median survival for patients undergoing resection was 26 months, and 5-year survival was 38%. Patients who did not undergo resection had a mean survival of 5.4 months and a 5-year survival of only 4%. In patients undergoing resection, the factors that most influenced survival based on multivariate analysis included T stage and N stage. Patients with T2 tumors had a more favorable outcome than those with T3 or T4 tumors, but advanced T stage did not preclude long-term survival. Patients with nodal metastasis had a poor outcome. Of 36 patients with node-positive disease after tumor resection for curative intent, only 2 survived more than 5 years, and both eventually died of disease.
presented over a 14-year period found that only 102 patients were able to undergo potentially curative resection (14). Fifty-one patients had inoperable disease, 135 patients underwent noncurative cholecystectomy, and 92 patients underwent exploration and biopsy only. Median survival for patients undergoing resection was 26 months, and 5-year survival was 38%. Patients who did not undergo resection had a mean survival of 5.4 months and a 5-year survival of only 4%. In patients undergoing resection, the factors that most influenced survival based on multivariate analysis included T stage and N stage. Patients with T2 tumors had a more favorable outcome than those with T3 or T4 tumors, but advanced T stage did not preclude long-term survival. Patients with nodal metastasis had a poor outcome. Of 36 patients with node-positive disease after tumor resection for curative intent, only 2 survived more than 5 years, and both eventually died of disease.
Table 37.1 Results of surgical resection of T1 N0 M0 (stage IA) gallbladder cancer | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
This study also analyzed results with respect to long-term survival after curative resection involving two operations versus one; that is, results among patients who underwent an inadequate first operation (cholecystectomy only) and then had a second definitive procedure. The long-term survival after curative resection was no different for patients who had one operation and for those who had two. These data indicate that reoperative radical surgery in carefully selected patients is associated with the same long-term outcome as primary radical surgery. This finding was confirmed by a recent report from The Johns Hopkins Hospital (17).
Table 37.2 Results of radical surgical resection for locally advanced gallbladder cancer | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Palliation
The major indication for palliation of gallbladder carcinoma is biliary obstruction caused either by direct extension of the tumor into the extrahepatic biliary tree or by compression produced by lymph node metastasis. If unresectable local disease is found at the time of laparotomy, a biliary bypass (hepaticojejunostomy) can be performed to alleviate extrahepatic biliary obstruction. If, however, disseminated disease is found at laparotomy (or laparoscopy) or if the patient is found to have unresectable disease based on preoperative evaluation, palliative biliary drainage can be performed by either percutaneous or endoscopic stent placement. Metallic expandable Wallstents (Boston Scientific Corp., Natick, MA) placed by either percutaneous or endoscopic techniques can provide permanent internal palliation of biliary obstruction in patients with life expectancy limited to a few months (Fig. 37.5).
Adjuvant Therapy
The role of adjuvant chemotherapy and radiotherapy for gallbladder carcinoma has been poorly defined because the available literature is derived from small, single-institution experiences in which heterogeneous treatment methods were used. The small percentage of patients with gallbladder carcinoma undergoing curative surgery, the failure to accrue patients under protocol, and the incomplete reporting of technical treatment data, histology, and tumor extent in these studies contribute to this problem. Finally, these reports are often strongly biased by patient selection, which makes interpretation even more difficult. The rationale for the use of adjuvant chemoradiation for gallbladder carcinoma is that minimal tumor-free margins are often achieved even after radical surgery. Therefore, radiotherapy is added to control microscopic residual deposits of carcinoma in the tumor bed and regional lymph nodes. Chemotherapy is added as both a radiation sensitizer as well as for potential systemic effects.
Approaches to delivering radiotherapy to the gallbladder fossa have varied from standard external beam radiotherapy using multiple-field arrangements and low daily fractions, to intraoperative external beam radiotherapy and brachytherapy (21,22,23,24). Typically, external beam radiation for gallbladder cancer treats the tumor bed, with a 2- to 3-cm margin around the primary tumor and the regional lymph node drainage basin. Often, this treatment incorporates the porta hepatis, a portion of the liver, celiac axis, regional periaortic nodes, and pancreaticoduodenal nodes. The typical delivered dose of 45 Gy is unlikely to control gross disease, so approaches using brachytherapy and intraoperative radiotherapy (IORT) have been attempted (25,26,27). Due to intolerance of the liver, kidneys, spinal cord, and C loop of the duodenum, doses above 54 Gy are prohibited. IORT or brachytherapy can exclude these structures from the high-dose region and enable delivery of doses above 50 Gy. Unfortunately, the data on these techniques are limited.
A few recent reports are available describing experience with adjuvant therapy for resected gallbladder cancer. Czito et al. (28) reported a single-institution retrospective experience of primary adenocarcinoma of the gallbladder. Twenty-two patients with primary and nonmetastatic gallbladder cancer were treated with radiation therapy after surgical resection over a 23-year period. The median radiation dose was 45 Gy. Eighteen patients received concurrent 5-fluorouracil (5-FU) chemotherapy. Median follow-up was 1.7 years in all patients and 3.9 years in survivors. The 5-year actuarial overall survival, disease-free survival, metastases-free survival, and local-regional control of all 22 patients were 37%, 33%, 36%, and 59%, respectively. Median survival for all patients was 1.9 years. The conclusion of the authors was that 5-FU concurrent with radiotherapy in the adjuvant setting was helpful in nonmetastatic gallbladder carcinoma patients. Houry et al. (29) performed a meta-analysis of all publications on radiotherapy for gallbladder carcinoma between 1974 and 2000. The best benefit was obtained when only microscopic residual disease was found. Higher doses of radiation were recommended especially delivered as an intraoperative “boost” (15 Gy). Postoperative adjuvant external radiotherapy (45 to 50 Gy) was found to slightly improve survival time.
Itoh et al. (30) described the adjuvant treatment of 18 patients with gallbladder carcinoma using radiotherapy alone. The 5-year survival was 56% with multivariate analysis, confirming that the best results were found in patients with R0 (76%) versus R2 (0%) and that radiotherapy had a positive effect on survival.
Neoadjuvant Radiotherapy
Due to the locally advanced stage of many gallbladder cancers at presentation, some investigators have attempted to downsize these tumors with neoadjuvant therapy in hopes of enabling surgical resection. Aretxabala et al. (31) have reported on the use of preoperative external beam radiotherapy with chemotherapy in a phase II trial. Twenty-seven eligible patients unexpectedly found to have localized gallbladder cancer were enrolled to receive chemoradiation before definitive tumor bed and regional node resection. They received 45 Gy of external beam radiation at 1.8 Gy per fraction concurrent with two cycles of short-term continuous 5-FU infusion (days 1 through 5 and 28 through 32). Eighteen patients accepted preoperative treatment, and 15 patients completed reoperation. Surgical resection was possible in 13 cases, and three patients were found to have biopsy-proven residual disease. The median follow-up was 4 months. Seven patients were alive at last follow-up, with local failure proven in only one patient, who succumbed to disease. Uno et al. (32) attempted to downstage disease in 22 patients with unresectable gallbladder adenocarcinoma with preoperative external beam radiotherapy. Only five patients eventually underwent resection for cure; five others underwent palliative bypass; and in 12 patients, tumors remained unresectable or demonstrated metastases. Patients responding to radiotherapy had a significantly longer survival (p = .0008) than did patients who did not respond. Overall survival was 36% at 1 year and 14% at 2 years.
Czito et al. used radiation with concurrent eniluracil in a phase I study of gallbladder, cholangiocarcinoma, and pancreatic tumors. After 45 Gy, a field reduction was made and a 5.4-Gy boost completed (33). Oral eniluracil/5-FU mimics a continuous infusion of 5-FU via a safe oral preparation. Eniluracil inactivates dihydropyrimidine dehydrogenase (DPD), enabling sustained plasma levels of 5-FU. Patients were considered for surgery 4 weeks after chemoradiation. A total of 13 patients were enrolled with encouraging downstaging found at surgery, and one patient had a pathologic complete response. The authors concluded that this regimen was safe and potentially effective in the neoadjuvant setting and that the maximum tolerated dose was not achieved.
Palliative Therapy
The reports describing chemotherapy and/or radiotherapy in patients undergoing biopsy only for unresectable gallbladder cancer are highly biased by patient selection, include small numbers of heterogeneously treated patients, and have an extended period of patient accrual. Most series suggest a survival of >2 months after biopsy only (24) and up to 6 months after palliative surgery with bypass (34). In multiple series (21,23,24,26,35,36,37,38), the addition of palliative chemotherapy and/or radiotherapy after biopsy only has led to an increase in median survival to approximately 4 months (range, 1 to 20 months). Palliative radiotherapy after surgical bypass has yielded median survival times of >8 months (range, 1 to 15 months) in patients fit to undergo this combination (23,36).
In addition to modestly improving survival times in these treated patients, radiation for advanced-stage gallbladder carcinoma has some effect in palliating pain, pruritus, jaundice, early satiety, and other locoregional symptoms. The reports cited have suggested a 50% to 90% improvement in symptoms with radiotherapy. Balanced against this are the time, expense, and potential complications involved in this therapy. Fortunately, few complications have been reported with doses of <54 Gy using radiotherapy alone, although the acute
treatment side effects that often accompany upper abdominal radiotherapy, such as nausea, vomiting, weight loss, and diarrhea, may be underreported.
treatment side effects that often accompany upper abdominal radiotherapy, such as nausea, vomiting, weight loss, and diarrhea, may be underreported.
The major cytotoxic chemotherapy in use for the palliative treatment of gallbladder cancer remains 5-FU. In the last several years, several modifications of 5-FU–based therapy have become available, or have been in development. Most notable among these are the development of oral formulations, such as capecitabine. Such treatments allow less frequent visits, and may allow better preservation of quality of life. Whether such treatments will lead to equivalent tumor suppression as the parent compound may never be known, given the complexity of conducting phase III trials with such an uncommon tumor.
Other drugs that have some activity in this disease include drugs such as cisplatin and doxorubicin. Again, published literature concerning these drugs is limited and is closer to anecdotal in nature. Also concerning these groups of drugs is their propensity for increased toxicity, which has limited their widespread use in this group of patients. In this particular setting, the use of combination therapy has not been shown to dramatically increase clinical responses.
A newer analog of cisplatin is oxaliplatin. This compound has a broader spectrum of action, as it shows substantial activity in other gastrointestinal malignancies, including colon cancer, where cisplatin is inactive. In addition, it has less nephrotoxicity, allowing a wider use. It preserves a high level of synergy with 5-FU–based chemotherapies. Several trials have been reported with the activity of this drug given in conjunction with either 5-FU or capecitabine.
Some of the newer chemotherapy compounds that have begun to show activity in closely related tumors are entering trials for the treatment of biliary tract tumors. One of the first of these drugs is gemcitabine. The original U.S. Food and Drug Administration (FDA) approval for this drug was for the treatment of pancreatic cancer, based on both some degree of clinical benefit and some improvement in quality of life, as compared to 5-FU–based therapy (39). Given the similarity of this cancer to biliary tract cancers, phase II trials in bilary tract cancers were performed using gemcitabine as a single agent, as well as in combination with capecitabine. A response rate of 36% was seen in one small trial using gemcitabine as a single agent (40). In a more recent trial, gemcitabine was combined with capecitabine; 45 patients were treated. The response rate was 31%, with a median progression-free survival of 2 months. The investigators suggested that enough activity had been seen to warrant a phase III trial (41). Targeted agents have begun their trial in bilary tract tumors. The epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor erlotinib was studied in a group of 42 patients with bilary tract cancers. EGFR expression was found in 29 of 36 patients in whom immunohistochemistry was performed. Three of 40 patients with evaluable disease had objective tumor response (42).
Cholangiocarcinoma
Cholangiocarcinoma is a rare cancer that arises from the biliary epithelium and occurs at a frequency of 2 per 100,000 people in the United States and in Western Europe. It represents approximately 3% of gastrointestinal malignancies, but there are considerable worldwide differences in incidence (43). Cholangiocarcinoma can occur anywhere along the biliary ductal system. The most pragmatic way to classify these tumors is by dividing them into intrahepatic, perihilar, and distal lesions, based on their likely surgical management if they are resectable. At tertiary referral centers, the majority of patients with cholangiocarcinoma have perihilar tumors (40% to 60%), followed by distal tumors (30% to 40%), and the least common intrahepatic tumors (approximately 10%) (44,45). Perihilar cholangiocarcinoma is managed by excision of extrahepatic biliary tree and typically extended right hepatectomy or sometimes extended left hepatectomy. Distal cholangiocarcinoma is managed by pancreaticoduodenectomy and intrahepatic cholangiocarcinoma by partial hepatectomy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree