Chapter 50A Cancer of the bile ducts
Intrahepatic cholangiocarcinoma
Overview
Intrahepatic cholangiocarcinoma is also known as peripheral cholangiocarcinoma, cholangiolar cancer, or cholangiocellular carcinoma, terms that have previously been used interchangeably. Cholangiocellular carcinoma was first used by Steiner and Higginson (1959) to describe a subtype of cholangiocarcinoma in which the glands are small and regular and resemble proliferating cholangioles with inconspicuous lumens. Current nomenclature uses the term intrahepatic cholangiocarcinoma (IHCC) to define tumors arising from biliary epithelium in intrahepatic bile ducts above the level of the left main and right main ducts (Liver Cancer Study Group of Japan, 1990). These tumors constitute 10% of primary hepatic malignancies, and although much is known about extrahepatic cholangiocarcinoma, IHCC is less well understood.
Hepatic resection for IHCC was infrequently described until recently. Foster and Berman (1977) describe only 13 cases in their summary of hepatic surgery in the United States, although they present 112 resections for hepatocellular carcinoma (HCC) and 47 cases of hepatoblastoma. This low number of resections may represent the frequency with which advanced disease was diagnosed at presentation and also reflects that recognition of IHCCs as a discrete primary liver tumor was slow to occur and that many were historically diagnosed as metastatic adenocarcinoma from unknown primary sites.
Epidemiology and Demographics
The incidence of cholangiocarcinoma is rising worldwide and is now the second most common primary cancer of the liver behind HCC (Olnes & Erlich, 2004). Approximately 5000 new cases are diagnosed annually in the United States (Vauthey & Blumgart, 1994), and more than 1000 cases are diagnosed annually in the United Kingdom (Khan et al, 2008). However, overall, cholangiocarcinoma is rare and makes up only 3% of all GI tract cancers (Lazaridis & Gores, 2005). This means that it is infrequently seen by general surgeons or gastroenterologists, and its rarity has frustrated attempts to design therapeutic trials to treat it.
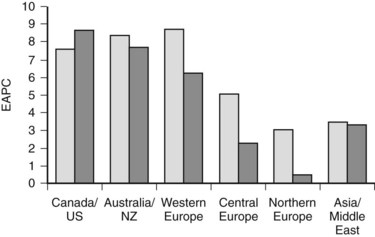
More detailed analysis of epidemiologic data shows that the incidence and mortality rates of IHCC are increasing and those of extrahepatic cholangiocarcinoma are decreasing (see Chapter 50B; Endo et al, 2008; Khan et al, 2002; Patel, 2001, 2002), suggesting that the tumors may have different etiologic factors but similar microscopic morphology. These findings must be interpreted with caution. IHCC may occur as solitary or multiple liver lesions and, in the past, many were probably diagnosed as metastatic tumors and not further investigated. In addition, analysis of the Surveillance, Epidemiology, and End Results (SEER) database has shown that more than 90% of hilar tumors were wrongly classified as IHCCs, and extrahepatic lesions were often classified as gallbladder cancers (Shaib & El-Serag, 2004). Indeed, even when these classification issues are addressed, the mortality rate of IHCC is still increasing worldwide (Fig. 50A.1; Shaib et al, 2005).
IHCC currently has an incidence of 0.85 per 100,000 population in the United States (Shaib et al, 2004; Shaib & El-Serag, 2004); the highest incidence in the world is recorded in northeast Thailand (96 per 100,000) (Khan et al, 2008). Patients most commonly are seen in the seventh decade, and IHCCs are more common in men (Khan et al, 2002; Shaib et al, 2005).
Etiology and Risk Factors
Primary Sclerosing Cholangitis
Primary sclerosing cholangitis (PSC) (see Chapter 41) is the most common risk factor for cholangiocarcinoma in the West (Farrant et al, 1991). The cumulative annual risk of cholangiocarcinoma in patients with PSC is 1.5% per year (Farrant et al, 1991), and the prevalence of cholangiocarcinoma in these patients is between 8% and 40% (Pitt et al, 1995; Rosen et al, 1991). The risk of developing cholangiocarcinoma is increased in those with associated inflammatory bowel disease, in whom the 10- and 20-year rates for cholangiocarcinoma are 14% and 31% versus 2% and 2% in patients without inflammatory bowel disease, respectively (Claessen et al, 2009).
Cholangiocarcinoma often develops 2 to 3 decades earlier in patients with PSC than in those with sporadic tumors (30 to 50 years vs. 60 to 70 years of age, respectively) (Bergquist & Broome, 2001; Farrant et al, 1991). In addition, PSC-associated tumors often present later with advanced-stage disease because of difficulties in detecting malignant change in inflammatory strictures. Surgical treatment can be difficult in the presence of chronic liver disease, and such patients are often ineligible for orthotropic liver transplantation. Consequently. they have a poor prognosis (Kaya et al, 2001). Factors suggestive of cholangiocarcinoma in patients with PSC are the sudden development of jaundice, weight loss, marked biliary dilation proximal to a dominant stricture, a sudden rise in carbohydrate antigen 19-9 (CA19-9), the presence of a hypovascular mass with late contrast enhancement on radiologic imaging, and cytologic evidence of dysplasia or malignancy obtained on brushings of the bile ducts (Harewood, 2008).
Parasitic Infections (See Chapter 45)
Chronic infection with liver flukes (Opisthorchis viverrini and Clinorchis sinensis) is closely related to increased risk of developing cholangiocarcinoma, especially in Southeast Asia (Hasweel-Elkins et al, 2008; Jang et al, 2008; Sripa & Pairojkul, 2008; Watanapa, 1996; Watanapa & Watanapa, 2002). The mechanism of carcinogenesis is unclear; however, mechanical irritation, excreted metabolic products, and the actions of proinflammatory cytokines, particularly those that stimulate the release of nitric oxide from activated white cells, may all play a role (Sripa et al, 2007).
A further parasitic hepatic infection is caused by the trematodes Fasciola hepatica and F. gigantica. These parasites are widely spread throughout Asia, Africa, the Americas, and Oceania. They migrate into the liver from the duodenum and cause hepatic fibrosis, but no evidence is available to support the theory that fascioliasis increases the risk of cholangiocarcinoma. In addition, the fibrotic pathologic changes accompanying an infection can be difficult to distinguish from carcinoma (Kim et al, 2005; Marcos et al, 2008).
Hepatolithiasis (See Chapters 39 and 44)
Recurrent pyogenic cholangiohepatitis (RPC) is characterized by recurrent episodes of ascending cholangitis, hepatolithiasis, biliary stricturing, and dilation. The syndrome is present in one fifth of the population of Southeast Asia, and up to 10% of these patients develop IHCC (Chen et al, 1993; Kubo et al, 1995; Lesurtel et al, 2002; Su et al, 1997). Patients are seen with recurrent episodes of cholangitis and, on investigation, have significant hepatolithiasis and associated inflammatory biliary strictures (Chu et al, 1997). Infection with liver flukes may also be present, but RPC appears to be a separate condition and can develop in the absence of parasitic infection (Kim et al, 2003).
Congenital Biliary Cystic Disease (See Chapter 46)
Untreated choledochal cysts carry an increased risk of developing malignant change. The incidence of cholangiocarcinoma is estimated at between 10% and 20% if the cyst is not resected by the age of 20 years (Lipsett et al, 1994; Ohtsuka et al, 2001). Correspondingly, patients who have had their cysts resected have a very low incidence of cholangiocarcinoma (Hewitt et al, 1995), although the subsequent development of cholangiocarcinoma has been recorded after cyst excision (Goto et al, 2001). The mechanism of malignant transformation is not well understood, but many patients with choledochal disease have an abnormally high union of the pancreatic and bile ducts, suggesting that biliary stasis and chronic reflux of pancreatic secretions may contribute to the development of chronic inflammation of biliary epithelium (Chapman, 1999).
Hepatic Cirrhosis and Viral Infections (See Chapter 64)
The risk of developing cholangiocarcinoma is increased in patients with cirrhosis (10.7% vs. 0.7% in the general population) (Shaib & El-Serag, 2004; Sorensen et al, 1998). In addition, the incidence of cholangiocarcinoma is increased in patients with hepatitis C viral infection (0.8% vs. 0.2% in the general population) (Donato et al, 2001; Shaib et al, 2005) and in patients with chronic hepatitis B viral infection (11.5% vs. 5.5% in the general population) (Donato et al, 2001). It has been suggested the increasing incidence of cholangiocarcinoma in Western populations is related to the increasing prevalence of chronic liver disease and chronic viral infection (Shaib et al, 2005; Shaib & El-Serag, 2004).
Human immunodeficiency virus (HIV) does not cause cirrhosis, but cholangiocarcinomas have been found in up to 0.5% of patients infected with the virus compared with 0.1% in control subjects, suggesting that HIV is also associated with an increased risk of biliary carcinogenesis (Shaib et al, 2005).
Benign Biliary Tumors (See Chapter 79B)
The development of biliary cystadenocarcinomas from biliary cystadenomas is rare; in general, it occurs if a cystadenoma is untreated for many years. Patients are first seen with cystadenocarcinomas in the sixth or seventh decades of life, although cystadenomas present at an earlier age (Buetow et al, 1995). Intrahepatic cholangiocarcinoma has also been reported to develop in patients with biliary papilliomas (Cox et al, 2005; Galluoglu et al, 2007).
Chemical Agents
Thorotrast (thorium dioxide) was used as a radiologic contrast agent between 1928 and 1950. It is an α-emitter with a biologic half-life of 400 years. It accumulates in the reticuloendothelial cells in the liver and spleen and increases the risk of developing cholangiocarcinoma by 300 times compared with the general population (Lipshutz et al, 2002; Rubel & Ishak, 1982). It is now no longer in use, although the latency period of 16 to 45 years means that patients who received this agent during childhood radiologic examinations will still occasionally come to medical attention (Massachusetts General Hospital, 1981).
A number of other agents have been implicated in the development of cholangiocarcinoma. Associations have been shown for asbestos (Szendroi et al, 1983), vinyl chloride (Wong et al, 1991), nitrosamines (Mitacek et al, 1999), the antituberculosis agent isoniazid (Lowenfels & Norman, 1978), and first-generation oral contraceptives (Yen et al, 1987).
General Risk Factors
Diabetes and obesity are associated with an increased risk of cholangiocarcinoma (Malhi & Gores, 2006; Oh et al, 2005). Similarly, surgical biliary-enteric bypass and surgical sphincteroplasty may also increase the risk (Hakamada et al, 1997). Smoking tobacco is a significant risk factor for the development of cholangiocarcinoma in patients with PSC (Bergquist et al, 1998), although the relationship is less marked in the general population (Shaib et al, 2005).
Pathogenesis
Cholangiocarcinoma develops from the malignant transformation of cholangiocytes. These cells line the intrahepatic bile ducts and canaliculi. Their physiologic functions center around the modification of bile at the canalicular surface and the detoxification of xenobiotics (Alpini et al, 2001). Normal growth and renewal of cholangiocytes is important in the maintenance of biliary mass, as well as hepatic secretory and detoxification functions, and is achieved by careful regulation of proliferation and apoptosis; however, when this process becomes uncontrolled, cholangiocarcinogenesis can occur (Wise et al, 2008).
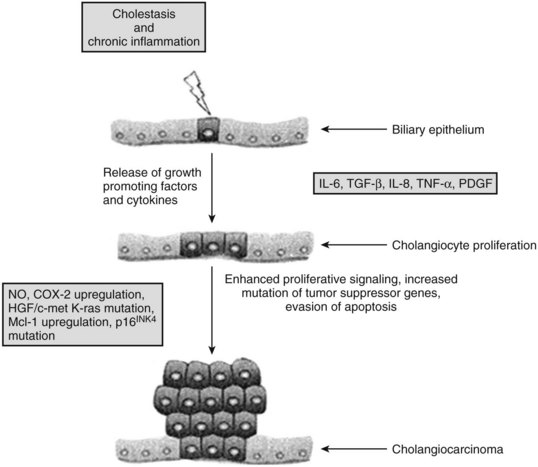
Chronic inflammation is the most common and important feature of many of the risk factors associated with malignant transformation of cholangiocytes (see Chapter 6). Chronic inflammation can result in biliary obstruction, injury to the biliary epithelium, and increased cholangiocyte turnover (Jaiswal et al, 2000). Chronic inflammation also causes DNA damage, activates local tissue repair, stimulates cellular proliferation, and results in a local environment that promotes neoplastic transformation (Fig. 50A.2) (Schottenfeld & Beebe-Dimmer, 2004). Cholangiocytes exert significant paracrine and autocrine effects and stimulate secretion of cytokines, such as interleukin-6 (IL-6), IL-8, transforming growth factor-β (TGF-β), tumor necrosis factor-α (TNF-α), and platelet-derived growth factor (PDGF) (see Chapter 10; Berthiaume & Wands, 2004).
These cytokines activate nitric oxide synthase in cholangiocytes and result in the generation of nitric oxide, which reacts with other active oxygen species such as superoxide, all of which can damage DNA and downregulate DNA repair mechanisms (Jaiswal et al, 2000). This damage can lead to expression of oncogenes and reduced expression of tumor suppressor genes. Overexpression of the epidermal growth-factor receptor (EGFR), erb-2, K-ras, BRAF, and hepatocyte growth factor c-Met have been reported in cholangiocarcinoma (Endo et al, 2002; Lai et al, 2005). The protooncogene Erbb2 is activated in cholangiocarcinoma, and tumor suppressor genes CDKN2A, P53, APC, and SMAD4 are underexpressed (Rashid, 2002; Taniai et al, 2002). Cytokines may also assist cholangiocytes in evading apoptosis. IL-6 is secreted by cholangiocytes and activates the prosurvival p38 mitogen-activated protein kinase (see Chapter 8B; Ishimura et al, 2004; Park et al, 1999).
Pathologic Subtypes and Mode of Spread
Macroscopically, IHCCs are firm, white, sclerotic tumors often with associated satellite lesions nearby. However, the variability in gross morphologic appearance has led to a number of pathologic subclassifications. Nakanuma and colleagues (1985) initially classified IHCCs into two types: mass forming and periductal. Mass-forming tumors were described as tumors with clear borders between malignant and nonmalignant tissues; periductal tumors were described as more infiltrative and extended along peri–bile duct tissues without forming a discrete nodular mass. This classification was widely adopted in Japan (Fujita, 1990) and was further modified by Ohashi and colleagues (1994), who added a specula-forming lesion present when a nodular tumor has poorly defined and irregular borders. Yamamoto and colleagues (1998) emphasized that all three morphologic types appear to have different proliferative activity and different biologic behavior. According to their research, the distinctive feature of the mass-forming type is its tendency to develop intrahepatic metastases as a result of localized vascular invasion, and the infiltrative type is distinguished by infiltrative spread via the Glisson capsule and hilar lymph node metastases. On the basis of these observations, these researchers recommended hepatectomy as the procedure of choice for the mass-forming subtype; hepatectomy with extrahepatic ductal resection and hilar lymphadenectomy is the procedure of choice for the infiltrating subtype. They also added a fourth subtype, the intraductal variant, characterized by papillary or granular growth within the lumen. They also were able to show that the frequency of lymph node metastases was higher in the mass-forming and periductal types compared with the intraductal subtype. A single report of a Western hepatobiliary unit used this classification and was unable to show any difference in overall survival or the frequency of lymph node metastases (Weber et al, 2001), although the intraductal subtype may carry a more favorable prognosis, as it does in the extrahepatic bile duct (Jarnagin et al, 2005). Although the subclassification of IHCC is intriguing and may have implications for surgical therapy, one significant weakness of this approach is that many tumors exhibit features of a number of the described subtypes (Shirabe et al, 2002).
Other less common histologic variants of IHCC have been reported, including mucin-hypersecreting lesions, which are similar to intraductal papillary mucinous neoplasms of the pancreas and are characterized by large mucin-filled cystic spaces (see Chapter 57; Chow et al, 1997; Kim et al, 2000; Suh et al, 2000) and intraductal oncocytic papillary carcinoma. These lesions have a distinctive appearance defined by the presence of oncocytes but appear to behave in a favorable manner, similar to the papillary type (Sudo et al, 2001; Wolf et al, 1992).
Intraabdominal lymph nodes are the most common site of metastatic spread for IHCCs and are present in up to 75% of cases at presentation (Shirabe et al, 2002). In addition, up to two thirds of patients may have evidence of remote organ metastases, most commonly lung and bone, at presentation (Endo et al, 2008; Shirabe et al, 2002). The common sites of lymphatic metastases are at the hepatic hilus, around the pancreas, in the retroperitoneum, around the aorta, and in the mediastinum (Nakajima et al, 1988). Nozaki and colleagues (1998) showed significant differences in lymphatic spread between left lobar and right lobar tumors. Patients with right lobar tumors always had lymph node metastases in the hepatoduodenal ligament, whereas in patients with left lobar tumors, 50% of nodal metastases were found distant from the hepatoduodenal ligament in the cardia and around the lesser curvature of the stomach. Furthermore, no lymph node metastases were present in the hepatoduodenal ligament in these patients.
Clinical Presentation
In contrast to extrahepatic cholangiocarcinoma, which usually presents with jaundice and symptoms of biliary obstruction, IHCC often presents as asymptomatic hepatic masses detected during physical examination or on cross-sectional imaging. In patients with symptoms, abdominal pain is most common (Martin & Jarnagin, 2003). A significant proportion of patients may present with nonspecific constitutional symptoms, such as weight loss and decreased energy and appetite (Weber et al, 2001). Jaundice can be present in centrally placed lesions that compress or invade the biliary confluence, although extensive replacement of hepatic parenchyma by tumor, along with portal vein compromise, intrabiliary tumor invasion, or mucobilia, can also cause symptoms of biliary obstruction. An increase in liver enzymes, most commonly alkaline phosphatase or γ-glutamyl transferase, may be the only presenting feature to prompt further investigations, including physical examination and cross-sectional imaging. Roayaie and colleagues (1998) have shown that jaundice as a presenting symptom is predictive of unresectable disease because of significant involvement of inflow structures bilaterally or because of massive parenchymal replacement.
Diagnosis and Evaluation
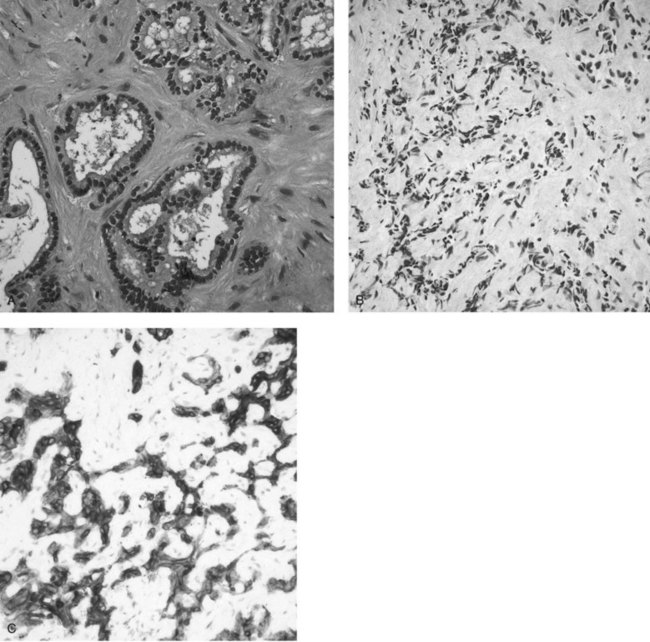
In clinical practice, the diagnosis of IHCC is established with the typical finding of a hypovascular mass present on cross-sectional imaging (see Chapters 16 and 17). GI metastases are excluded by performing upper and lower GI endoscopies without the finding of a primary tumor and in the absence of other primary malignancies (pancreas, kidney, lung) on a staging computed tomography (CT) scan of the chest, abdomen, and pelvis. Women should have had a recent mammogram and gynecologic examination. Routine tumor biopsy is often unnecessary, particularly in patients who will undergo resection, and it is not recommended because of the small risk of tumor dissemination (Metcalfe et al, 2004; Ohlsson et al, 1994). In general, biopsy is indicated only to establish the presence of irresectable disease. Final pathologic confirmation of cholangiocarcinoma is defined by a specimen showing an adenocarcinoma that stains positively for CK-7 and CK8/18 but is negative for CK-20 (Fig. 50A.3). Staging laparoscopy is also useful in the evaluation of IHCC to exclude peritoneal disease, nodal disease, or abdominal wall invasion, which may preclude an attempt at resection (D’Angelica et al, 2003).
Serum Markers for Cholangiocarcinoma
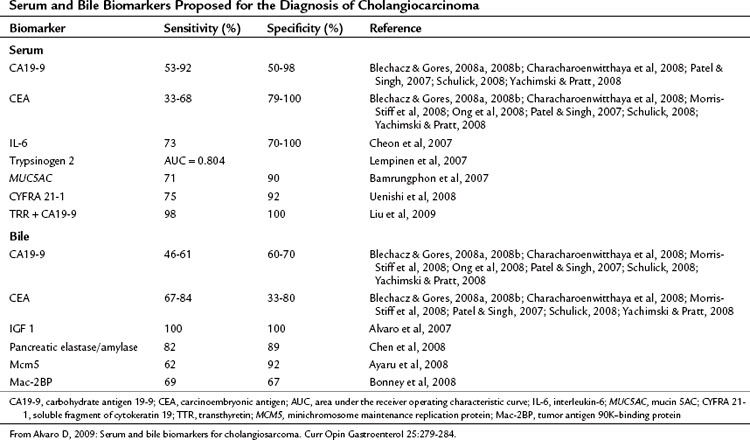
Serum tumor markers are an attractive method for diagnosing and monitoring treatment response in cholangiocarcinoma because of the ease of obtaining samples and their relatively low cost. To be effective a marker must be accurate in detecting the presence of malignancy (sensitivity) and in defining the presence of benign disease (specificity). Most of the research on tumor markers in cholangiocarcinoma has been directed at diagnosis of extrahepatic tumors, particularly in the context of PSC; however, much of this is applicable to peripheral cholangiocarcinoma. The data on tumor markers that have been applied to cholangiocarcinoma are summarized in Table 50A.1.
Carcinoembryonic antigen (CEA) is widely used because of its availability but is elevated in only one third of patients with cholangiocarcinoma (Blechacz & Gores, 2008a, 2008b
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree