Chapter 77 Budd-Chiari syndrome and venoocclusive disease
Overview
Budd-Chiari syndrome (BCS) is a group of disorders caused by occlusion of the major hepatic veins or the inferior vena cava (IVC) or both at or near the level of the hepatic vein ostia. Although a brief discussion of these disorders first appeared in a book by Budd in 1845, Lambron in 1842 is said to have reported the first case. In 1899, Chiari collected 10 cases and reported three personal cases and presented the first thorough clinical and pathologic description of the syndrome, including the hypothesis that the underlying mechanism is endophlebitis of the hepatic veins. The weight of evidence favors the current opinion, however, that the primary process is usually thrombotic rather than inflammatory. Since publication of the initial description, more than 8000 cases of BCS have been described in the medical literature. In recent years, the incidence has increased substantially, most likely as a result of increased awareness of BCS, improvements in diagnostic methods, and widespread use of thrombogenic agents, such as oral contraceptives (Maddrey, 1987; Valla et al, 1986). Nevertheless, BCS remains a relatively uncommon condition.
Predisposing Conditions for Budd-Chiari Syndrome
Box 77.1 lists the specific conditions that are known to predispose to the development of BCS and VOD. Over the past 50 years, a marked change has been observed in the frequency with which a known cause or predisposing condition has been identified in cases of BCS. In the classic collective review of 164 cases of BCS reported by Parker in 1959, a predisposing condition or etiology could not be identified in 70% of the patients. In recent years, the incidence of idiopathic cases of BCS has plummeted to less than 30% (Mahmoud et al, 1996; Menon et al, 2004; Mitchell et al, 1982; Murad et al, 2009; Valla, 2003; Plessier & Valla, 2008), an improvement attributed to two factors: 1) a greater awareness of BCS and 2) improved diagnostic tools for identifying the anatomic lesions and for diagnosing thrombogenic hematologic disorders.
Box 77.1 Conditions Predisposing to Budd-Chiari Syndrome and Venoocclusive Disease
Budd-Chiari Syndrome
Membranous obstruction of the inferior vena cava
Miscellaneous rare conditions (inflammatory bowel disease, hepatic torsion, lipoid nephrosis, protein-losing enteropathy)
The distinct difference has been recognized for some time between the East and West in the conditions that predispose to the development of BCS and in the anatomic pattern of BCS. Table 77.1 displays these differences. Membranous obstruction of the vena cava (MOVC) is rare in the West, but it is a frequent cause of BCS in Eastern countries such as Japan, China, and India and in South Africa. In the West, thrombosis of the major hepatic veins alone is substantially more common than thrombosis or occlusion of the IVC; whereas in India, China, and Japan, IVC occlusion is far more common than hepatic vein occlusion alone. In North America, the acute or subacute forms of BCS predominate, and chronic BCS is observed less frequently, whereas in the East, the reverse is observed. In the West, BCS is seldom found during pregnancy or the postpartum period, whereas in India, pregnancy is a major predisposing condition for BCS. The same difference is seen in the incidence of infections such as hepatic amebiasis (see Chapter 67), which are rare in the West but are reported frequently in series of BCS from India. Finally, oral contraceptives are frequently associated with BCS in the United States, where use of these agents is widespread, whereas use of birth control pills is seldom associated with BCS in Eastern countries, where women use oral contraceptives much less.
Table 77.1 Differences Between West and East in Predisposing Conditions and Anatomic Patterns of Budd-Chiari Syndrome
| Feature | West | East |
|---|---|---|
| Membranous obstruction of the IVC | Rare | Frequent |
| Hepatic vein occlusion predominates | + | − |
| IVC occlusion predominates | − | + |
| Acute or subacute BCS predominates | + | − |
| Chronic BCS predominates | − | + |
| Pregnancy/postpartum | Uncommon | Frequent |
| Infection | Rare | Common |
| Oral contraceptives | Frequent | Uncommon |
BCS, Budd-Chiari syndrome; IVC, inferior vena cava
Hematologic Disorders
Hematologic diseases that cause vascular thrombosis are the most common conditions that predispose to BCS in North America and Western Europe. Of disorders with thrombotic tendencies, polycythemia rubra vera is the most frequent, constituting 8.5% of the cases of BCS in the collected series of Parker (1959) and 10.4% of the cases in the collected series of Mitchell and colleagues (1982). In our series of 77 cases of BCS, 31% had polycythemia rubra vera, which is associated with BCS in some distinctly different ways compared with the classic disease. For one thing, it is found in young adults, rather than in middle-aged and elderly patients, and it is responsive to treatment with hydroxyurea or anagrelide, which should be started as soon as the disease is discovered and should be continued for life. In our experience, the disease runs a benign course if treated. Finally, polycythemia rubra vera associated with BCS is compatible with a long life, if it is treated with hydroxyurea.
Paroxysmal nocturnal hemoglobinuria is another hematologic disorder associated with BCS (Hartmann et al, 1980; Hoekstra et al, 2009; Liebowitz & Hartmann, 1981; Valla et al, 1987). It was responsible for 6.7% of the cases in the collected series of Mitchell and colleagues (1982) and 12% of the cases in the series of Valla and colleagues (1987). In all of the hematologic disorders associated with hepatic vein thrombosis, but particularly in paroxysmal nocturnal hemoglobinuria, thrombosis of other blood vessels outside of the liver has sometimes been observed. These cases of multiple sites of thrombosis have involved the portal, splenic, and superior mesenteric veins; pelvic veins; deep calf veins; splanchnic arteries; pulmonary artery; coronary arteries; and cerebral arteries (Peytremann et al, 1972).
As hematologic diagnosis has become progressively more sophisticated, many other thrombogenic conditions have been identified in BCS, including other myeloproliferative states—such as essential thrombocythemia, primary erythrocytosis, and myelofibrosis—and thrombophilic states, such as protein C deficiency, protein S deficiency, antithrombin III deficiency, and antiphospholipid syndrome with lupus anticoagulant or anticardiolipin antibodies or both (Bertina et al, 1994; Boughton, 1991; Dahlback, 1995; Dahlback et al, 1993; Espinosa et al, 2001; Koster et al, 1993; Mahmoud et al, 1995; Menon et al, 2004; Pelletier et al, 1994; Svensson & Dahlback, 1994; Valla, 2003; Vandenbroucke et al, 1994). Subjects with the factor V Leiden mutation, which leads to activated protein C resistance, have a 5-fold to 10-fold increase in the risk of thrombosis if they are heterozygotic and a 50-fold to 100-fold increase if they are homozygotic (Dahlback, 1995; Deltenre et al, 2001; Janssen et al, 2000). More recent evidence indicates that multiple prothrombotic factors acting concurrently are involved in a substantial percentage of patients with BCS (Denninger et al, 2000; Janssen et al, 2000). Rarely, hematologic malignancies, such as acute leukemia and lymphoma, have been associated with BCS.
In 2005, identification of the underlying cause of BCS was enhanced by the discovery of a very reliable and noninvasive marker for chronic myeloproliferative disorders. The marker is the gain-of-function mutation V617F of the JAK2 gene (Baxter et al, 2005; James et al, 2005; Jelinek et al, 2005; Jones et al, 2005; Kralovics et al, 2005; Levine et al, 2005; Steensma et al, 2005; Zhao et al, 2005). By combining identification of this marker with results of bone marrow histology and clonality assay, over 50% of the cases of BCS have been found to be due to an underlying chronic myeloproliferative disorder (Primignani et al, 2006).
It cannot be overemphasized that every patient found to have BCS should undergo a thorough hematologic evaluation. The workup that we perform is an expansion of the workup proposed by Mahmoud and Elias (1996) and others (Hirschberg et al, 2000; Valla, 2009) and is shown in Box 77.2. With these studies, it should be possible to diagnose all of the predisposing thrombogenic hematologic disorders known to be associated with BCS. If an evaluation such as this is done uniformly, it is highly likely that the incidence of idiopathic BCS will continue to decline.
Oral Contraceptives
An increased incidence of thromboembolic phenomena involving various blood vessels and organs in women taking oral contraceptives has been well established. The first case of BCS associated with use of oral contraceptives was reported by Ecker and McKittrick (1966), 5 years after these drugs became available commercially. Since then, more than 200 cases of BCS in patients taking oral contraceptive have been described (Janssen et al, 2000; Lewis et al, 1983; Maddrey, 1987; Valla et al, 1986; Zafrani et al, 1983), and the increasing overall incidence of BCS in recent years has been attributed partly to the widespread use of these agents. In the collective review reported by Mitchell and colleagues (1982), use of oral contraceptives was believed to be responsible for 9.4% of the cases of BCS during the period 1960 to 1980. Valla and associates (1986) reported that the relative risk of hepatic vein thrombosis among oral contraceptive users was close to that of stroke, myocardial infarction, and venous thromboembolism. In our series of 77 cases of BCS, 23% of patients gave a history of oral contraceptive use. The incidence is even higher if the denominator consists only of the number of women in each series. In our series, 49% of the 37 women with BCS used oral contraceptives.
Some authors have proposed that oral contraceptives are not a primary cause of BCS but contribute to thrombosis only if there is an underlying hematologic disorder (Valla et al, 1986). In our series, no hematologic disease was identified in users of oral contraceptives, but until relatively recently, our patients did not undergo the extensive hematologic workup shown in Box 77.2. The duration of usage of oral contraceptives before the diagnosis of BCS has ranged from 2 weeks to 10 years. In addition to causing BCS, oral contraceptives have been linked to other liver disorders, including VOD, portal vein thrombosis, cholestasis, hepatocellular adenoma, focal nodular hyperplasia, and possibly hepatocellular carcinoma and angiosarcoma (Zafrani et al, 1983).
Box 77.2
Screening for Hematologic Disorders in Budd-Chiari Syndrome
Complete blood count, prothrombin time, partial thromboplastin time, fibrinogen
Red blood cell mass, plasma volume
Bone marrow biopsy, cell culture, karyotype
JAK2 gene, V617F mutation in peripheral blood granulocytes
Activated protein C resistance or Factor V Leiden mutation or both
Endogenous erythroid colony assay
Flow cytometry for blood cells deficient in CD55 and CD59 (PNH)
Molecular test for G20210A prothrombin gene mutation
Anti-β2 glycoprotein-1 antibodies
PNH, paroxysmal nocturnal hemoglobinurea.
Pregnancy and Postpartum
BCS has been observed in women during pregnancy and, more commonly, during the postpartum period. The first case of BCS reported by Chiari (1899) occurred in a woman who developed the disorder after childbirth. In the collective review by Mitchell and colleagues (1982), 9.9% of the cases of BCS occurred during pregnancy or postpartum, and in a series of 105 patients with BCS observed from 1963 through 1978, Khuroo and Datta (1980) reported 16 cases (15.2%) of BCS after pregnancy; 8 of the patients died, and 7 were lost to follow-up after discharge from the hospital. The hypercoagulable state that is known to occur during pregnancy is presumed to be responsible for the association of BCS with this condition, although only 1 of our 77 cases of BCS occurred during pregnancy or postpartum.
Malignant Neoplasms
Malignant neoplasms were responsible for 13.4% of the collected cases of BCS reported by Parker (1959), 8.8% of the collected cases reported by Mitchell and colleagues (1982), and 12% of the cases reported by Powell-Jackson and colleagues (1982). Because we do not operate on patients with BCS caused by cancer, none of our 77 patients had malignant neoplasms. Occlusion of the suprahepatic IVC by invasive tumors has been the cause of BCS in many cases. The most common cancers associated with BCS are hepatocellular carcinoma, renal cell carcinoma, adrenal carcinoma, and leiomyosarcoma of the IVC. Other malignancies that rarely have caused BCS include carcinomas of the lung, pancreas, and stomach; melanoma; reticulum cell sarcoma; adrenal sarcoma; and sarcoma of the right atrium.
Infections
Infections involving the liver were believed responsible for 3% of the collected cases of BCS reviewed by Parker (1959), 9.9% of the collected cases reported by Mitchell and colleagues (1982), and none of the cases in sizable series reported in more recent years, including our own series. The most common infections associated with BCS are those caused by parasites, particularly amebic liver abscess, hydatid disease, and schistosomiasis (see Chapters 67 and 68). Syphilitic gumma of the liver accounted for 1.8% of the cases in Parker’s review but has not been reported as a cause of BCS in recent years. Aspergillosis involving the hepatic veins and IVC has been a rare cause of BCS. In India, Victor and colleagues (1994) provided evidence that filariasis can cause BCS.
Trauma
Abdominal trauma has uncommonly predisposed to the development of BCS. Trauma was responsible for 1.2% of the collected cases of BCS reported by Parker (1959) and 2.4% of the collected cases reviewed by Mitchell and colleagues (1982). Blunt and penetrating trauma have been implicated in occasional cases of BCS.
Connective Tissue Disorders
Occasional cases of BCS have been reported in association with various connective tissue and autoimmune diseases, most of which are known to have thrombotic tendencies. Included among these are Behçet disease, Sjögren syndrome, mixed connective tissue disease, sarcoidosis, and rheumatoid arthritis. Numerous cases of BCS in patients with Behçet disease, including five of our own, have been described (Bazraktar et al, 1997; Orloff & Orloff, 1999).
Membranous Obstruction of the Vena Cava
More than 600 cases of BCS resulting from MOVC have been reported from Japan (Hirooka & Kimura, 1970; Kimura et al, 1972; Okuda, 2002; Ono et al, 1983; Taneja et al, 1979; Yamamoto et al, 1968), China (Wang, 1989; Wang et al, 1989; Wu et al, 1990) and other parts of Asia, India (Khuroo & Datta, 1980), and South Africa (Semson, 1982). In the United States and Europe, MOVC is rare. A congenital cause of this condition has been proposed, but evidence strongly suggests it represents the end result of acquired thrombosis (Kage et al, 1992; Okuda 2002; Okuda et al, 1995).
MOVC usually runs a chronic course over many years, and most patients will have developed extensive hepatic fibrosis and cirrhosis and portal hypertension by the time they come to medical attention. An increased incidence of hepatocellular carcinoma has been observed in association with MOVC (Okuda, 2002; Semson, 1982). The therapeutic implications of this condition and other forms of IVC occlusion are distinctly different from those of occlusion confined to the major hepatic veins.
Causes of Venoocclusive Disease
In 1954, Bras and colleagues proposed the term venoocclusive disease to describe a serious and common liver disease in Jamaican children in which there was occlusion of the central and sublobular hepatic veins and surrounding centrilobular necrosis of the liver parenchyma. More recently, the condition has been called the sinusoidal obstruction syndrome. Shortly after his initial description, Bras and others (Bras & McLean, 1963; Brooks et al, 1970; Gore et al, 1961; Stuart & Bras, 1957) showed that VOD was due to ingestion of “bush teas” made from plants of the Crotolaria and Senecio genera, which contain well-known hepatotoxic pyrrolizidine alkaloids. It has long been known that these plants and plants of the Heliotropium genus produce liver failure in herbivorous animals and are common causes of poisoning of grazing cows and horses. Since the initial descriptions by Bras and others, VOD caused by ingestion of toxic pyrrolizidines has been observed in Israel (Ghanem & Hershko, 1981), Egypt (Safouh & Shehata, 1965), Iraq (Al-Hasany & Mohamed, 1970), Afghanistan (Mohabbat et al, 1976), India (Aikat et al, 1978; Tandon et al, 1976), Venezuela (Grases & Beker, 1972), Ecuador (Lyford et al, 1976), South Africa (Steenkamp et al, 2000; Zuckerman et al, 2002), and the United States (Abbott, 1988; Bach et al, 1989; Ridker & McDermott, 1989; Ridker et al, 1985; Stillman et al, 1977). In addition to poisoning by drinking bush teas, humans have been poisoned by eating flour milled from grain contaminated by the seeds of these plants and by taking herbal remedies.
Antineoplastic drugs have been identified as another cause of VOD. Included among these are cytosine arabinoside, thioguanine, and gemtuzumab ozogamicin (Mylotarg) used in the treatment of acute myelocytic leukemia (Cruz et al, 1983; Giles et al, 2001; Gill et al, 1982; Griner et al, 1976); carmustine used in the treatment of diffuse histiocytic lymphoma (McIntyre et al, 1981); dacarbazine used in the treatment of melanoma (Asbury et al, 1980); oxaliplatin used in treatment of metastatic colorectal adenocarcinoma to the liver (Cleary et al, 2009; Pawlik et al, 2007; Mehta et al, 2008; Kandutsch et al, 2008; Rubbia-Brandt et al, 2004); and gemtuzumab ozogamicin and mitomycin C combined with bone marrow transplantation (Kumar et al, 2003; Lazarus et al, 1982; McDonald et al, 1993; Wadleigh et al, 2003). Fatal VOD in renal transplant recipients has been attributed to azathioprine (Liano et al, 1989; Marubbio & Danielson, 1975).
Hepatic injury caused by therapeutic irradiation of malignant neoplasms in and near the liver is an important cause of VOD (Fajardo & Colby, 1980; Reed & Cox, 1966). Although radiation-induced VOD usually does not produce clinical manifestations with doses less than 3000 rad, the condition has been observed after smaller amounts of radiotherapy (Fajardo & Colby, 1980).
Currently, the most common cause of VOD in the Western Hemisphere is bone marrow transplantation (Ayash et al, 1990; Berk et al, 1979; Jones et al, 1987; Kriegshauser et al, 1988; Kumar et al, 2003; McDonald et al, 1984, 1993; Wadleigh et al, 2003; Senzolo et al, 2007; Helmz, 2006; Cacchione et al, 2008). It has been estimated that approximately one fourth of recipients of bone marrow transplants develop the disorder. It is uncertain whether VOD in these patients is caused by the marrow transplant, or if it is a result of the radiation and chemotherapy used in the pretransplant conditioning regimen. Berk and colleagues (1979) proposed that graft-versus-host disease might be responsible for VOD after marrow transplantation, but the studies of McDonald and colleagues (1984) cast doubt on this hypothesis. Finally, there have been rare reports of VOD in association with systemic lupus erythematosus (Pappas et al, 1984), familial immunodeficiency (Mellis & Bale, 1976), and the use of oral contraceptives (Alpert, 1976).
Pathology
Budd-Chiari Syndrome
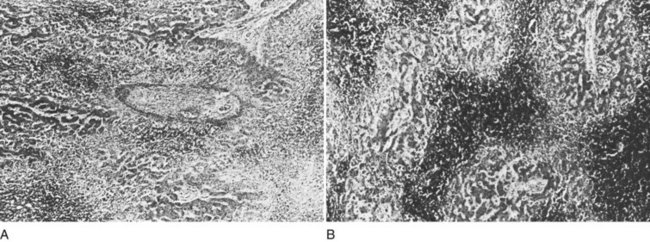
The liver receives about one fourth of the cardiac output via its dual afferent blood supply, the portal vein and hepatic artery. After perfusing the sinusoids, the blood is returned to the heart through the hepatic veins and IVC. Obstruction to the egress of blood from the liver at any point along the outflow route results in numerous serious hemodynamic and morphologic alterations. There is a marked increase in intrahepatic pressure, which is reflected by a similar increase in portal pressure. The increased intrahepatic pressure causes extravasation of plasma from the liver sinusoids and lymphatics with formation of ascites. Obstruction to the egress of blood from the liver also results in dilation of the sinusoids and intense centrilobular congestion of the hepatic parenchyma, which is greatest around the terminal hepatic venules (central veins; Fig. 77.1). Ischemia, pressure necrosis, and atrophy of the parenchymal cells in the center of the liver lobule are apparent. With persistence of the obstruction, the necrotic parenchyma is replaced by fibrous tissue and regenerating nodules of liver tissue. The end result is cirrhosis of the type associated with chronic congestive heart failure. The rapidity with which cirrhosis develops is related to the severity of outflow obstruction, but it is not unusual for cirrhosis to occur within a matter of months (Parker, 1959).
In most cases of BCS, occlusion of the hepatic veins is caused by thrombosis (Parker, 1959). The thrombus undergoes organization and ultimately is converted to fibrous tissue that permanently occludes the veins. Although recanalization of the occluded veins sometimes occurs, it rarely results in effective new outflow channels. Retrograde propagation of the thrombus into smaller hepatic veins is commonly found. Prograde propagation of the thrombus from the hepatic veins into the IVC, with partial or complete occlusion of the IVC, sometimes occurs and markedly changes the therapeutic approach and prognosis. It is important to determine by imaging studies that include angiography and pressure measurements whether the IVC has become involved in the occlusive process.
MOVC has been reported to be the most common cause of BCS in Japan (Hirooka & Kimura, 1970; Kimura et al, 1972; Okuda et al, 1995; Ono et al, 1983; Taneja et al, 1979; Yamamoto et al, 1968), India (Khuroo & Datta, 1980), China (Wang, 1989; Wang et al, 1989, 2005; Wu et al, 1990), and in the Bantu population of South Africa (Semson, 1982). The “membrane” varies from very thin to several centimeters thick and usually contains fibrous tissue, smooth muscle, and elastic tissue. The location and extent of the membrane vary considerably, and in some cases a long segment of IVC has been replaced by fibrous tissue. Occlusion of one or more of the major hepatic veins often has been associated with membranous obstruction of the IVC. Although some experienced authors have proposed a congenital cause (Hirooka & Kimura, 1970; Kimura et al, 1972; Ono et al, 1983; Semson, 1982; Taneja et al, 1979), a strong argument has been made that suggests MOVC is the end result of thrombosis of the IVC, often occurring early in life (Okuda et al, 2002). Most of the cases have run a chronic course before discovery, and when first seen by a physician, patients have had extensive hepatic fibrosis or cirrhosis with portal hypertension and all of its manifestations. The therapeutic considerations in patients with MOVC differ from those in patients with BCS caused by obstruction of the hepatic veins.
Venoocclusive Disease
VOD of the liver, more recently called sinusoidal obstruction syndrome, may mimic BCS clinically, because both conditions involve hepatic venous outflow obstruction. VOD involves the sinusoids and the central and sublobular hepatic veins within the liver, however, rather than the hepatic veins (Kumar et al, 2003; Shulman et al, 1987, 1994). The underlying process in VOD is subendothelial sclerosis of the hepatic veins and sinusoids secondary to endothelial injury caused by a toxic agent, such as a pyrrolizidine alkaloid, antineoplastic drug, radiation, or a stem cell transplant. Thrombosis of the small hepatic veins may occur after damage to the venous intima. Electron microscopic studies of liver biopsy specimens obtained from children with VOD caused by pyrrolizidine poisoning showed marked endothelial damage in the sinusoids and subterminal and terminal hepatic veins in all zones of the liver, with extravasation of erythrocytes into the space of Disse and narrowing of the lumen where the sinusoid entered the central vein (Brooks et al, 1970).
Clinical Manifestations of Budd-Chiari Syndrome
In our series of 77 patients with BCS, 12 were referred with advanced cirrhosis as a result of prolonged hepatic outflow obstruction and were not candidates for portal decompression surgery. The other 65 patients were referred at a mean 14 weeks after onset of BCS (range 4 to 78 weeks). Fifty-nine of the 65 patients (91%) were referred less than 18 weeks after onset of symptoms, relatively early in the course of BCS. The remaining 6 patients were operated on 19, 21, 23, 25, 26, and 78 weeks after onset of symptoms. In the collected series of Mitchell and colleagues (1982), which excluded cases of MOVC, two thirds had had symptoms for less than 3 months, and 83% had had symptoms for 6 months or less at the time of diagnosis. In Parker’s (1959) collected series of 133 cases, he observed that 57% had had symptoms for 3 months or less, and 71% had been symptomatic for 6 months or less.
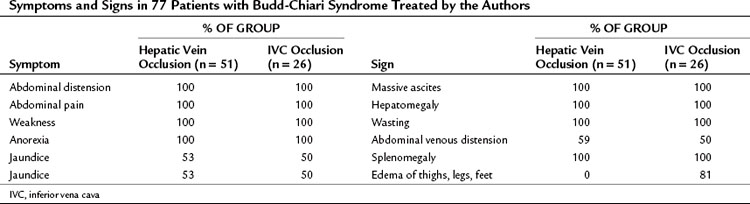
Table 77.2 presents the frequency of symptoms and signs in our series of 77 patients with BCS, 51 of whom had hepatic vein occlusion alone, and 26 of whom had IVC occlusion and hepatic vein occlusion. The only difference between the two groups—and it is an important one—was the absence of lower extremity edema in the group without IVC thrombosis and the high incidence of edema from feet to thighs (81%) that resulted from IVC occlusion.
Symptoms
Abdominal Distension
The initial symptom in all of our patients was abdominal distension secondary to ascites, which increased progressively over a few weeks. Abdominal distension caused by ascites occurs at some time in almost every patient with BCS. In the large collected series (Mitchell et al, 1982; Parker, 1959), abdominal distension was among the first symptoms experienced by most patients.
Anorexia
Anorexia was reported by all patients in our series and has been experienced by many patients in the reports of others (Mitchell et al, 1982).
Jaundice
Clinical jaundice was observed in 52% of the patients in our series but in only 28% of the patients in Parker’s collective review (1959) and in only 17% of the patients collected by Mitchell and colleagues (1982). Usually, jaundice has been mild.
Symptoms of Chronic Liver Disease
Patients with the chronic forms of BCS, such as MOVC, often have the usual symptoms of cirrhosis and portal hypertension, including upper gastrointestinal (GI) bleeding secondary to ruptured esophagogastric varices (see Chapter 75A, Chapter 75B, Chapter 75C ), hepatic encephalopathy, hepatorenal syndrome, and edema of the lower extremities (see Chapters 72 and 73). Peripheral edema is particularly prominent in patients with MOVC, and some develop varicose veins of the legs (Parker, 1959; Semson, 1982). In our series of patients who underwent surgical portal decompression for acute BCS secondary to occlusion of the hepatic veins, one patient had these symptoms.
Findings on Physical Examination (Signs)
Ascites
All 77 patients in our series had the abdominal signs of massive ascites on physical examination at the time of diagnosis. The incidence of ascites was 93% in the collective review of Parker (1959) and 83% in the series of Mitchell and colleagues (1982).
Hepatomegaly
All patients in our series and in the series of Mitchell and colleagues (1982) had marked hepatomegaly resulting from severe congestion of the liver. In chronic forms of BCS, hepatomegaly may not be as striking, but it is usually present.
Distension of Abdominal Veins
Forty-three of the 77 patients in our series (56%) and 55% of the patients in the collected series of Parker (1959) had distension of the superficial veins of the anterior abdominal wall. Abdominal venous distension is a manifestation of portal hypertension and the formation of portosystemic collaterals early in the course of BCS.
Splenomegaly
Splenomegaly, another manifestation of portal hypertension, was observed in all of the patients in our series, in 50% of those in the collected series of Mitchell and colleagues (1982), and in 30% in the collected series of Parker (1959). Enlargement and congestion of the spleen was sometimes accompanied by the hematologic manifestations of secondary hypersplenism.
Jaundice
Clinical jaundice was observed in 52% of the patients in our series but in only 28% of the patients in the collected series of Parker (1959) and in only 17% of the patients in the collected series of Mitchell and colleagues (1982). The jaundice in our patients who underwent surgical portal decompression was invariably mild; the highest serum bilirubin level was only 6.8 mg/dL.
Symptoms and Signs of Venoocclusive Disease
VOD has been observed in individuals of all ages, including infants and adults in their sixth decade; however, VOD caused by pyrrolizidine alkaloids has been seen most commonly in infants and children. The clinical manifestations depend on the disease stage at which the patient seeks medical treatment (Brooks et al, 1970; Ghanem & Hershko, 1981; Gore et al, 1961; Safouh & Shehata, 1965; Stuart & Bras, 1957). The acute stage is often preceded for 1 or 2 weeks by a febrile illness with upper respiratory symptoms, vomiting and diarrhea, or both. The patient then experiences abrupt onset of abdominal pain, weakness, anorexia, fever, and abdominal distension secondary to ascites (Kumar et al, 2003; Wadleigh et al, 2003; Senzolo et al, 2007). Jaundice is the rule, and splenomegaly with thrombocytopenia is common. Some patients develop edema of the feet and occasionally of the hands and face, and physical examination in the acute phase invariably shows hepatomegaly and ascites. Many patients have splenomegaly, some have distension of the superficial veins of the abdominal wall, and some have peripheral edema and a pleural effusion. Bone marrow transplant recipients usually develop the clinical features of VOD within 3 weeks after transplantation. Many patients have died during the acute stage from liver failure, bleeding esophageal varices, or intercurrent infection.
Patients other than bone marrow transplant recipients may be initially seen in the chronic stage of VOD with the usual clinical manifestations of cirrhosis of the liver: ascites, hepatomegaly, splenomegaly, wasting, abdominal venous distension, spider angiomata, palmar erythema, asterixis, and peripheral edema. Bleeding from esophageal varices is a major cause of death in the chronic stage, and cirrhosis of the liver has been observed 3 months after acute onset of VOD (Gore et al, 1961).
Diagnostic Studies in Budd-Chiari Syndrome
Hepatic Angiography and Pressures
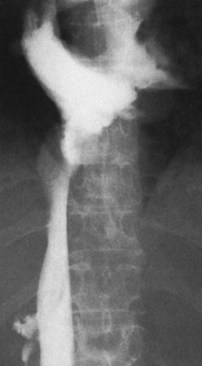
The diagnostic study of greatest value in BCS, particularly if surgical therapy is contemplated and venous pressure measurements are required, is angiographic examination of the IVC and hepatic veins with pressure measurements (Clain et al, 1967; Kreel et al, 1967; Redman, 1975; Tavill et al, 1975). This study usually is combined with hepatic and superior mesenteric arteriography and indirect portography. In BCS confined to the hepatic veins, the IVC is patent, and IVC pressure is relatively normal for subjects with ascites (Fig. 77.2). Patency of the IVC is a prerequisite for portacaval shunt (PCS) and is a crucial finding (see Chapter 76A, Chapter 76B, Chapter 76C, Chapter 76D, Chapter 76E ). In some patients, the IVC is moderately compressed in its retrohepatic course by the enlarged liver and, in particular, by a hypertrophied caudate lobe (Fig. 77.3). This finding usually is not clinically significant (Clain et al, 1967; Kreel et al, 1967; Redman, 1975; Tavill et al, 1975).
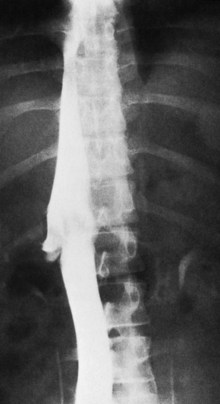
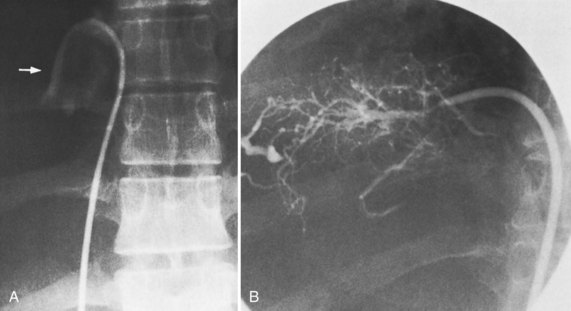
The most important angiographic finding is the demonstration by hepatic venography of occlusion or marked narrowing of the major hepatic veins. Sometimes it is not possible to find patent orifices of any of the hepatic veins, which is indirect evidence that all of the major hepatic veins are occluded. Usually it is possible, however, to enter at least one major hepatic vein and to show the presence of a thrombus or of narrowing and distortion of the vein (Fig. 77.4A). Injection of dye in the wedged position often shows a characteristic spiderweb pattern of small hepatic venous collaterals connecting to portal or systemic veins (see Fig. 77.4B). Wedged hepatic vein pressure (WHVP) usually is markedly elevated, which reflects the obstruction to hepatic venous outflow. In patients with hepatic vein occlusion alone, IVC pressure is substantially lower than WHVP.
A spiderweb pattern was observed in 48 patients (62%). WHVP was markedly elevated in all patients in whom it was measured. In the 51 patients with thrombosis confined to the hepatic veins, the IVC was patent, with relatively normal pressures for patients with ascites, ranging from 62 to 160 mm saline (Table 77.3). Many patients had moderate compression of the retrohepatic IVC by the enlarged liver, but invariably WHVP was substantially higher than IVC pressure—a crucial finding. In contrast, in the 26 patients with IVC occlusion, IVC pressure and WHVP were similar. All patients had stretched and attenuated hepatic artery branches within the liver and a patent portal vein (PV).
Table 77.3 Results of Diagnostic Studies in 77 Patients with Budd-Chiari Syndrome Treated by the Authors
| % OF GROUP | ||
|---|---|---|
| Hepatic Vein Occlusion (n = 51) | IVC Occlusion (n = 26) | |
| Angiography and Pressures | ||
| Occluded hepatic veins | 100 | 100 |
| Occluded IVC near hepatic veins | 0 | 100 |
| Spiderweb pattern of hepatic veins | 69 | 50 |
| Patent portal and splenic veins | 100 | 100 |
| Stretched hepatic arteries | 100 | 100 |
| IVC pressure much lower than WHVP | 100 | 0 |
| High WHVP | 100 | 100 |
| Percutaneous Needle Liver Biopsy (73 Patients) | ||
| Centrilobular congestion | 100 | 100 |
| Centrilobular hepatocyte loss and necrosis | 100 | 100 |
| Fibrosis | 76 | 58 |
| Cirrhosis (included also under fibrosis) | 34 | 8 |
| Hepatic Scintiscan | ||
| Decreased hepatic uptake | 100 | 100 |
| Nonhomogeneous hepatic uptake | 100 | 100 |
| Excessive extrahepatic uptake | 74 | 77 |
| Central hot spot in liver | 31 | 12 |
| Computed Tomography (64 Patients) | ||
| Absence of hepatic veins | 100 | 100 |
| Abnormal hepatic uptake of contrast | 100 | 100 |
| Occlusion of IVC | 0 | 100 |