Radionuclide
Half-life (days)
Emitted energy spectra (weighted average MeV)
Half-value layer of lead (mm)
125I (Iodine)
59.4
0.028
0.025
103Pd (Palladium)
17.0
0.021
0.008
131Cs (Caesium)
9.7
0.030
0.022
192Ir (Iridium)
73.8
0.38
2.5
Iridium-192 (192Ir) emits a spectrum of photons of different energies. It is used in the form of an iridium/platinum alloy wire enclosed by a thin platinum sheath. Its half-life is relatively long (73.8 days), which is convenient for the transport and storage of sources. This is the most common radioisotope employed in HDR afterloading units.
Permanent LDR seed implants are made from radioisotopes with relatively short half-lives which emit photons of energy low enough that the patient does not pose a significant risk of irradiating others nearby. A typical example of an Iodine-125 seed is the model 6711 seed (GE Healthcare/Oncura). It contains a silver wire with the silver iodide of 125I adsorbed onto its surface. This is encapsulated within a 0.05 mm thick titanium cylinder that is welded at both ends. The silver wire has the advantage of being radiographically distinctive. Seeds may be inserted individually using a Mick applicator or preloaded into a needle. Figure 43.1 shows a central strand of model 6711 seeds spaced regularly along a braided carrier. Before insertion, the strand of seeds is cut to the required length and loaded into a needle.


Fig. 43.1
RapidStrand containing Model 6711 seeds (Courtesy of GE Healthcare/Oncura, NY, USA)
103Pd seeds contain graphite-pellets plated with 103Pd and lead radiographic markers sealed within a titanium capsule. It was hoped that the shorter half-life of 103Pd might offer a radiobiological advantage over 125I, but this has not been shown to be clinically significant in trials [4, 5]. 131Cs is a newer radioisotope that is used by some practitioners in permanent seed implantation [6, 7].
The Development of Modern LDR Prostate Brachytherapy
Permanent prostate implantation was explored at the Memorial-Sloan Kettering Cancer Center in the 1970s [8]. They performed bilateral pelvic lymphadenectomies and implanted 125I seeds within the prostate using an open retropubic approach in a series of patients, several of whom had pelvic lymph node metastases. This technique was abandoned due to poor results and excessive morbidity. In the 1980s, the modern non-surgical technique of seed implantation was developed. Holm described a technique for guiding transperineal prostate implantation using transrectal ultrasound [9]. 125I or 103Pd seeds were implanted within the prostate using a transperineal approach with a perineal template, under transrectal ultrasound guidance – a technique later modified in Seattle by Blasko, Ragde and Grimm [10].
Clinical Evaluation of Patients
The clinical assessment establishes whether the patient is a suitable candidate for prostate brachytherapy and confirms their disease stage and risk group. Relevant features in the medical history include anticoagulant use, diuretic therapy, diabetes, inflammatory bowel disease, connective tissue disorders, previous diagnosis of pelvic cancer (especially bladder or rectum), prior pelvic radiotherapy and radiation sensitivity syndromes such as ataxia telangiectasia. Important urologic history would include prior transurethral or open resection of the prostate, urethral surgery, procedures for benign prostatic hyperplasia such as transurethral needle ablation or microwave therapy, medications for urinary symptoms and prior erectile dysfunction. Prior pelvic surgery or the presence of a total hip replacement would be relevant features of the surgical history. Information about the patient’s baseline urinary, erectile and bowel function may be obtained from self-administered validated questionnaires such as the International Prostate Symptom Score (IPSS), the International Index of Erectile Function (IIEF) and the EORTC QLQ/PR-25 questionnaire [11–13]. During the outpatient consultation, the clinical T stage is evaluated by digital rectal examination. Uroflowmetry may be carried out in the outpatient clinic to assess the Qmax (maximum urinary flow rate) and voided volume. The post-void residual volume may be measured by transabdominal bladder ultrasound and should be less than 200 cm3 for patients being considered for brachytherapy [14]. Transrectal ultrasound may be performed in the clinic to visualise the prostate gland. This can demonstrate median lobe hyperplasia, an existing TURP defect, eccentric BPH (which may displace the urethra from the midline) or the presence of intra-prostatic calcification (which creates artefact on the TRUS imaging used to guide seed implantation).
The authors consider undertaking cystoscopy and bladder neck resection in patients with small prostates (<40 cm3) who have obstructive urinary symptoms or signs, together with median lobe hyperplasia, to attempt to reduce urinary obstruction in the long term. Figures 43.2 and 43.3 show the bladder neck at cystoscopy before and after resection.
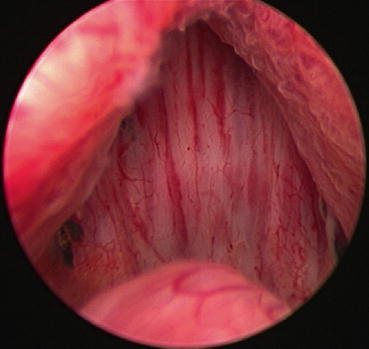
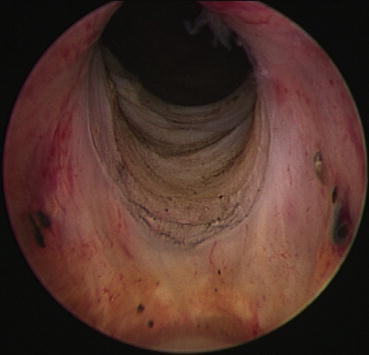

Fig. 43.2
View of the bladder neck and lateral lobes of prostate at cystoscopy

Fig. 43.3
View of resected bladder neck at cystoscopy
The maximal cross-section of the prostate gland should be carefully compared to the anterior-posterior space between the pubic arch and the ultrasound probe to avoid the phenomenon of pubic arch interference during the eventual seed implant. This occurs when the pubic arch blocks the passage of the introducing needles into the anterior and antero-lateral prostate [15]. This tends to affect large prostates (>60 cm3) but may also depend on pelvic anatomy, patient position and implant technique.
Selection of Patients for Prostate Brachytherapy
The diagnosis of prostate cancer has increased rapidly with the advent of widespread PSA screening of men in Western countries. The majority of these men are asymptomatic and have organ-confined disease which may be managed with active surveillance, radical prostatectomy, external beam radiotherapy or brachytherapy. Several guidelines for the selection of patients for prostate brachytherapy are in use, but there is no universal consensus [16–18]. The selection criteria may be classified into general, functional, tumour biology and technical criteria.
Absolute Contraindications to TRUS-Guided Prostate LDR Brachytherapy
According to the updated American Brachytherapy Society (ABS) guidelines for TRUS-guided permanent prostate brachytherapy [14], absolute contraindications to brachytherapy include:
Limited life expectancy (less than 10 years)
Unacceptably poor performance status
Unacceptable operative risks
Distant metastases
Absence of rectum such that TRUS-guidance is precluded
Large TURP defects which preclude seed placement and acceptable dosimetry
Ataxia telangiectasia
General Criteria
Co-morbid conditions that pose unacceptable surgical or anaesthetic risks should be considered. Active prostatitis and active inflammatory bowel disease may be considered relative contraindications [19], although there are small series of patients with a history of inflammatory bowel disease who have tolerated brachytherapy well [19–21]. Patients who have received prior pelvic radiotherapy need to be carefully assessed for symptoms and signs of late radiation toxicity to the pelvic organs, and may require evaluation with sigmoidoscopy and cystoscopy. The dose previously delivered to the prostate, rectum and bladder should be estimated from the details of their previous radiation therapy. Advanced age is not considered a contraindication to brachytherapy. Obesity may not be a contraindication provided the patient has an acceptable performance status and life expectancy, and can be supported by the operating theatre apparatus.
Functional Criteria
The presence of pre-treatment obstructive urinary symptoms is predictive of the development of acute urinary retention post-operatively [22]. This may be indicated by an International Prostate Symptom Score (IPSS) greater than 15 or a post-void residual volume of greater than 200 cm3. Patients with an IPSS of 8 or less have a low risk of developing acute retention and prolonged urethritis post-operatively [14]. Pre-implant IPSS correlates with the duration of post-implant obstructive symptoms but is not a predictor of long-term urinary quality of life [23, 24]. The pre-implant maximum urine flow rate, Qmax, and the presence of prostate median lobe hyperplasia are both predictive of post-implant acute urinary retention [12, 25, 26]. It is recommended that patients selected for brachytherapy should have Qmax greater than 15 ml/s [17].
Tumour Biology Criteria
The biological criteria of pre-treatment PSA, prostate biopsy Gleason score and clinical T stage are used to stratify patients with localised prostate adenocarcinoma into low-, intermediate- and high-risk groups. Several risk classifications exist, each based on the same three risk factors.
Patients presenting with high-risk disease or more than one intermediate-risk factors should be staged with an isotope bone scan and CT or MRI of the abdomen and pelvis [14]. The ABS favours the National Comprehensive Cancer Network (NCCN) classification (Table 43.2).
Table 43.2
NCCN risk group classification
Low risk | PSA < 10 ng/ml and T1c-T2a and Gleason score 6 |
Intermediate risk | PSA 10–20 ng/ml or T2b-T2c or Gleason score 7 |
High risk | PSA > 20 ng/ml or T3a or Gleason score 8–10 |
The risk of subclinical extracapsular extension (ECE) is reflected by the risk group classifications. Patients in the low risk group may be suitable for treatment with LDR seed implantation alone (monotherapy). Some practitioners select patients from the intermediate risk group for monotherapy and others use a combination of EBRT and brachytherapy. The ABS recommend combined EBRT and LDR implantation in patients with initial PSA >20 ng/ml or Gleason score 8–10 and T2b-T2c disease. The authors consider seminal vesicle invasion to be a contraindication to LDR brachytherapy. Such cases may be considered for neoadjuvant and adjuvant ADT with EBRT, with or without an HDR brachytherapy boost.
Technical Criteria
Prior TURP used to be considered a contraindication to LDR brachytherapy, due to rates of urinary incontinence as high as 17 % in early series in the 1990s, but this is no longer the case. With careful pre-operative assessment of the TUR defect, incontinence among selected patients treated with brachytherapy after TURP may be avoided. The seed implant should be delayed until 2–4 months after TURP to allow healing [14].
Pubic arch interference with large prostates (>60 cm3 in volume) may be overcome by a period of 3–4 months of ADT, which may downsize the prostate volume by about 30 %. For more modest-sized glands, placing the patient in an exaggerated lithotomy position and re-aligning the TRUS probe in a horizontal rather than angled-down position may overcome pubic arch interference.
Selection of Patients for HDR Prostate Brachytherapy
The GEC/ESTRO-EAU selection criteria for patients for HDR brachytherapy are: T1b-T3b disease with any Gleason score and any initial PSA, with no evidence of distant metastases [18]. Their exclusion criteria include: a prostate volume greater than 60 cm3, a TURP within the last 6 months, disease infiltrating the external sphincter of the bladder neck, significant urinary obstructive symptoms and a rectum-prostate distance less than 5 mm on TRUS. The ABS also consider offering HDR brachytherapy to selected patients with T4 disease.
LDR Brachytherapy: Monotherapy and in Combination with External Beam Radiotherapy and Hormone Therapy
Low-Risk Localised Prostate Cancer
Prostate LDR brachytherapy alone (monotherapy) is recommended for low-risk disease (see Table 43.2 for risk group definitions). Published series demonstrate that excellent long-term clinical outcomes can be obtained in this patient group provided adequate dosimetric parameters are achieved [14, 27–29]. ADT may be useful for downsizing the prostate gland [30–33]. The authors consider combining ADT with LDR brachytherapy in patients with a high burden of disease (i.e. all biopsies cores involved with Gleason 3 + 3 disease) due to the increased risk of occult extracapsular extension and metastasis.
Intermediate-Risk Localised Prostate Cancer
Patients in the intermediate-risk group are more likely to harbour adverse pathological features such as extracapsular extension, seminal vesicle invasion and lymph node metastasis than patients in the low-risk group. Pathological series have demonstrated that extracapsular extension of prostate cancer rarely extends beyond 5 mm in patients with clinically organ-confined disease [34, 35]. This region of potential extracapsular extension is covered by most good quality brachytherapy implants due to the typical margin of 5 mm added around the prostate to generate the Planning Target Volume and the fact that the radiation dose distribution extending several millimetres beyond the prescription isodose is often adequate to treat microscopic disease [36–38].
Patients with low-volume disease, a predominant Gleason score 3 pattern and a single adverse factor for intermediate-risk disease may be treated with monotherapy [14, 39]. Among the remaining patients in the intermediate-risk group, the optimum therapeutic strategy is controversial. A pattern-of-care study among brachytherapists with a combined experience of more than 10,000 patients identified additional factors such as proportion of positive biopsy cores and the presence of perineural invasion which practitioners consider with T-stage, initial PSA and Gleason score when trying to decide whether to treat intermediate-risk cases with monotherapy or in combination with EBRT and ADT [39].
High-Risk Localised Prostate Cancer
Patients with high-risk disease have a substantial chance of subclinical ECE beyond the range of a permanent prostate brachytherapy implant. This was reflected in the poor results of monotherapy for this group in early brachytherapy series [29]. It has become standard practice to combine an LDR brachytherapy implant with EBRT for this reason. Retrospective series suggest that escalating radiation dose by combining EBRT and a LDR brachytherapy implant improves local control of prostate cancer and metastasis-free survival.
Large randomised prospective trials of EBRT dose escalation for high-risk locally advanced prostate cancer with 3-dimensional conformal radiotherapy (3D-CRT) and, more recently, intensity modulated radiotherapy (IMRT), have confirmed improvements in disease control [40–42]. Combining EBRT with LDR brachytherapy aims to deliver greater dose escalation to the prostate and any surrounding subclinical ECE.
The use of neoadjuvant and adjuvant ADT with primary EBRT is supported by randomised prospective evidence [43, 44]. Although the use of ADT with combined EBRT and LDR brachytherapy for high-risk disease is popular, the evidence that it produces improved clinical outcomes is limited [17]. Delivering a greater biologically effective dose in patients with Gleason score 8–10 disease was shown to improve overall and metastasis-free survival in a large multi-institutional series [45].
Permanent LDR Prostate Brachytherapy Technique
Permanent seed implantation may be performed as a daycase or with an overnight stay. The technique comprises of a volume study, treatment planning and the implant procedure itself. Written informed consent should be obtained at the time of the implant and it should be documented whether prior pelvic EBRT has been given. The three main techniques for LDR prostate brachytherapy are: the two-stage Seattle technique (employing stranded seeds); the one-stage real-time technique using intra-operative dosimetry (as popularised by Stock and Stone: Proseed); and more recently, the 4D-Brachytherapy technique, which utilises stranded seeds in the prostate gland peripherally and loose seeds centrally, in a real-time technique with intra-operative dosimetry [46].
Volume Study
The volume study is a series of transverse TRUS images of the prostate which is used to plan the implant. Some centres perform this under anaesthetic; others in the outpatient setting with compliant patients who are able to remain still during the procedure. The rectum and sigmoid colon is emptied either by bowel preparation or an enema pre-operatively. The patient is placed in the dorsal lithotomy position and the perineum is infiltrated with local anaesthetic. Appropriate antibiotic cover for the implant procedure is recommended. A urinary catheter is inserted and then instilled with aerated gel to improve its visibility on the TRUS, so that it may be contoured on the treatment planning computer as a surrogate for the urethra. A biplanar TRUS probe is carefully positioned within the patient’s rectum. Care must be taken to obtain ultrasound images of the best quality because they will form the basis of the treatment plan.
The TRUS is mounted securely on a stepper unit which allows the probe to be advanced and retracted along its cranio-caudal axis in precise increments, while the rotation of the probe along this axis is also monitored (Fig. 43.4).


Fig. 43.4
Template and TRUS probe mounted on a stepper unit
This positional data is digitised, transferred to a treatment planning system and reconstructed in 3D. The TRUS probe is positioned so that the prostate is in the middle of the TRUS display. The urethra is usually in the central vertical row and the posterior prostate border along the lowest horizontal row of the template grid (Fig. 43.5). Axial images of the prostate gland are acquired at 5 mm intervals from the prostate base to the apex. The outline of the prostate on each axial image is contoured on the treatment planning system. The prostate is also visualised in the longitudinal plane to check the length and shape of the gland.
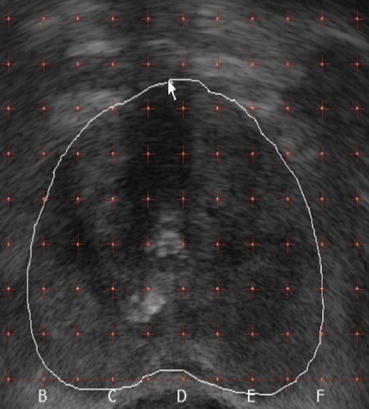

Fig. 43.5
Template grid superimposed upon axial TRUS image of prostate
One-Stage and Two-Stage Techniques
The practitioner must implant a seed arrangement that meets accepted dose constraints for the prostate and adjacent normal organs to achieve good clinical results for each patient [47]. Techniques for LDR prostate brachytherapy implantation are broadly divided into two-stage and one-stage techniques, according to the number of attendances required for treatment planning and delivery.
Seattle Technique
At centres using a two-stage technique, the patient attends for the volume study and the seed implantation is performed on a later occasion [48]. The treatment planning is completed in the meantime and the required seeds are ordered. It can be difficult to accurately replicate the patient position from the volume study and internal pelvic organ motion may cause some variation.
Loose Seed Technique
Single-stage techniques, where dosimetry is calculated just before or during the implant procedure, are increasingly popular due to the flexibility of planning and need for just one anaesthetic. They were popularised by Stock and Stone [49]. The ABS subcategorises intraoperative planning techniques into: intraoperative preplanning, interactive planning and dynamic dose calculation.
Intraoperative preplanning involves performing a TRUS in the operating room and importing the 3-dimensional ultrasound data into the treatment planning system on a computer in the room. The target volume and organs at risk are contoured on the treatment planning system and the prostate is implanted according to plan generated.
Interactive planning involves real-time feedback of needle positions to the treatment planning system via image guidance so that the plan can be recalculated after each needle position is adjusted. Dynamic dose calculation uses feedback of 3-dimensional seed position to continuously recalculate the dose distribution throughout the procedure. Being able to re-plan the treatment intraoperatively gives a further opportunity to optimise the treatment plan compared to preplanned techniques.
Hybrid Techniques
Two of the authors have developed a versatile real-time one-stage technique (“4D-Brachytherapy”) which avoids seed migration by using a combination of preloaded stranded seeds around the periphery of the prostate and loose seeds loaded with a Mick applicator [46]. An institutional nomogram has been developed from over 1,000 brachytherapy cases to enable the appropriate combination of stranded and loose seeds to be ordered based on the measurements of the prostate from a TRUS in the outpatient clinic. The technique has shortened the time taken for planning and implantation to around 40 min. Changing from the 2-stage Seattle technique to 4D-Brachytherapy has resulted in a significant improvement in prostate D90 and V100 doses [46]. Acute urinary morbidity and erectile function have also improved significantly (with potency rates 2 years post-implant improving from 61.7 to 83.3 % and use of phosphodiesterase-5 inhibitor medication falling from 53.3 to 31.7 %).
Target Volume Definitions in Prostate Brachytherapy
The concepts of Target volumes and Organs at risk were formalised by ICRU Report 50 [50], however brachytherapy practitioners had been using varying definitions of these volumes for prostate brachytherapy. The recommendations of the PROBATE group of GEC ESTRO sought to standardise the definitions, as shown in Table 43.3 [51].
Table 43.3
Volume definitions based on ICRU Report 50 and the GEC ESTRO recommendations
Gross tumour volume (GTV) | Gross palpable, visible or radiological extent of tumour |
T1: there is no GTV | |
T3: includes extracapsular extension or seminal vesicle disease | |
Clinical target volume (CTV) | GTV + margin including subclinical disease at a particular probability |
T1-2: Visible contour of prostate + 3 mm margin in all directions, constrained by the anterior rectal wall and the bladder neck | |
T3: Visible contour of prostate and extracapsular extension + 3 mm margin, as above | |
Planning target volume (PTV) | CTV + margin for organ motion and daily setup error |
With real-time 3D dosimetry and TRUS image guidance, no further margin is required, so PTV = CTV |
The Gross Tumour Volume (GTV) is the gross palpable, visible or radiological extent of tumour growth. Usually prostate cancer is not visible on transrectal ultrasound and, by definition, T1c tumours are be impalpable. In these situations, there will not be a GTV to be visualised.
The clinical concept of the Clinical Target Volume (CTV) includes the GTV as well as adjacent tissue, which has a substantial chance of containing significant subclinical disease. The whole CTV must receive a tumoricidal dose to achieve eradication of local disease. Since prostate adenocarcinoma is often a multifocal disease, the CTV is considered to include the whole prostate gland. For organ-confined disease (T1-T2), the CTV will be the visible prostate capsule with an added margin of 3 mm in all directions, limited by the bladder neck superiorly and the anterior rectal wall posteriorly. For T3 disease, the CTV will contain the gross disease, including any visible extracapsular extension, with a margin of 3 mm in all directions.
The geometric concept of the Planning Target Volume (PTV) contains the CTV with a margin to account for uncertainties in the position of the CTV due internal organ motion and setup errors. Since implanted seeds or applicators are placed within the CTV under image guidance and move with the prostate gland these uncertainties and the margin from CTV to PTV are minimal in prostate brachytherapy.
Organs at risk can be outlined during the planning stage, to enable the treatment planning computer to estimate the dose they will receive and ensure that their dose constraints are met. The rectum surrounds the TRUS probe and its outer or inner wall may be delineated to estimate its dosimetry. The prostatic urethra is very difficult to visualise, so the surface of the urinary catheter is delineated as a surrogate. Instilling aerated gel into the urethra or urinary catheter, if present, may facilitate visualising them on the transrectal ultrasound intraoperatively. Some centres have delineated the penile bulb and neurovascular bundles, seeking to minimise the dose they receive to try to improve erectile function. However, the dose received by the penile bulb may not correlate with postimplant erectile function [52].
Treatment Planning
The treatment planning system reconstructs the 3-dimensional arrangement of seeds within the prostate and calculates the dose distribution and the dose delivered to the target volume and adjacent organs at risk. TRUS is considered the standard imaging for the treatment plan and during the implant but planning may be performed on a CT or MRI dataset. Pre-planning with CT has been shown to produce consistently greater prostate target volumes than with TRUS and could theoretically lead to unnecessary implantation of the urogenital diaphragm and penile urethra [53].
The treatment plan combines the transverse images of the prostate with the numbered needle positions according to the template. The number of seeds in each needle and their activity are also denoted. The radiation distribution is represented visually by coloured isodose lines which run through points receiving equal dose levels, usually expressed as a percentage of the prescribed dose. In a brachytherapy treatment plan, the dosimetrist will aim to enclose the CTV so that at least 95 % of it receives the prescribed dose, while meeting volumetric dose constraints for the target and the adjacent organs at risk (such as the rectum and prostatic urethra). The GEC ESTRO recommendations for dosimetric criteria, which have been correlated with good dosimetry are shown in Table 43.4.
Table 43.4
GEC ESTRO recommendations for dosimetric parameters
Structure | Parameter | Constraint |
|---|---|---|
CTV | V100 (percentage of CTV receiving the prescription dose) | ≥95 % |
V150 (percentage of CTV receiving 150 % of the prescription dose) | ≤50 % | |
D90 (dose which covers 90 % of the volume of the CTV) | >145 Gy | |
Rectum | D2cc (the minimum dose to the most exposed 2 cc of rectum, as a percentage of the prescription dose) | ≤145 Gy |
D0.1cc (the minimum dose to the most exposed 0.1 cc of rectum, as a percentage of the prescription dose) | <200 Gy | |
Prostatic urethra | D10 | <150 % |
D30 | <130 % |
Dose Prescription
The recommendations of GEC ESTRO and the American Association of Physicists in Medicine (AAPM) task group 137 are that the standard prescribed dose for 125I monotherapy is 145 Gy and for 103Pd, 125 Gy [51, 54]. For combination therapy, the ABS recommends 41.4–50.4 Gy EBRT in 1.8–2.0 Gy daily fractions and an LDR brachytherapy dose of 108–110 Gy with 125I or 90–100 Gy with 103Pd [14].
Implant Procedure
The seeds are implanted via the transperineal approach and template guidance with the patient in the lithotomy position. If a two-stage technique is employed, the patient should be matched closely to their position during the pre-planning volume study. The TRUS should have a high-resolution biplanar 5–12 Hz ultrasound probe and be equipped with software to generate an electronic grid, which is calibrated to coincide with that of the template (see Fig. 43.5).
The insertion of the implant needles into the prostate is guided by the template and determined by the pre-plan in the two-stage approach, or by interactive planning in the one-stage approach. Methods of seed implantation include using a Mick applicator to insert loose seeds individually [55], afterloading applicators and using preloaded needles [56].
Post-implant Dosimetry
The quality of the implant is assessed by dosimetric analysis post-implant. This is typically obtained from a CT scan performed 4–6 weeks post-implant, although some centres have experience using MRI image fusion. Some practitioners favour a post-implant CT on day 0 or 1, before the urinary catheter is removed, because it enables a more accurate estimation of the urethral dose (using the contour of the catheter as a surrogate for the urethral mucosa) [57]. This is more convenient for the patient but may underestimate dose delivered to the prostate due to postoperative oedema. The target volume, individual seeds and organs at risk are contoured on the postoperative CT scan and the dosimetry is re-calculated. The outer wall of the rectum is easily visualised for contouring on the CT, however the presence of a catheter or the instillation of aerated gel is required to visualise the prostatic urethra. The post-implant contour of the prostate capsule is delineated for the CTV and then a 3 mm margin is added [51].
Several studies have suggested that parameters based on the dosimetry calculated from this post-operative CT scan are related to clinical outcomes. A study of patients with localised prostate adenocarcinoma treated with 125I implants found a dose response of 4-year freedom from biochemical failure for the dose received by 90 % of the prostate, the D90, at a level of 140 Gy [58]. In a study of over 700 patients treated with LDR implants, a cut-off of prostate D90 of 90 % of the prescribed dose was found to predict 4-year PSA-relapse free survival [59].
Radiation Protection
Patients are mildly radioactive after LDR brachytherapy implantation but the low energy of the photons emitted by 125I and 103Pd seeds mean that the dose rate at the skin surface does not pose a risk to the general public. The UK Royal College of Radiologists recommend that patients should be advised to avoid close contact with children and pregnant women in the first 2 months post-implant and that condoms should be used for the first five ejaculations. The brachytherapy centre should be contacted if pelvic surgery, post-mortem examination or cremation are required within 20 months of the implant. The centre provides each patient with a laminated card containing essential details about the implant that they must carry with them for the first 20 months post-implant [60].
Complications and Toxicities of LDR Brachytherapy
Immediate side effects may include discomfort and bleeding at the perineal needle holes, haematuria and haematospermia.
Seed Migration
Cystoscopy may be required to remove misplaced seeds or blood clots from the bladder but is not performed routinely if bladder irrigation is clear and there is no radiological evidence of seeds within the bladder. Techniques employing loose seeds have a rate of seed migration as high as 15 %. Examples of seed migration to the chest, abdomen and pelvis have been documented, including a case of a non-smoker developing a limited stage small-cell lung cancer [61–64].
Urinary Toxicity
Irritative and obstructive urinary symptoms are the most common acute side effects of prostate LDR brachytherapy. Patients may be offered a 2–3 month course of an α-blocker post-implant, prophylactically. In many cases, exacerbation of urinary symptoms resolves within 2–3 months of the implant. The authors published a study of 100 patients treated with LDR brachytherapy, which found that acute urinary symptoms peaked at 6 weeks post-implant and that a statistically significant decrease of IPSS score persisted until 9 months post-implant [12]. Seven patients in this study developed acute urinary retention, but the mean IPSS score 2 years post-implant was improved. In one study of 712 consecutive patients treated with LDR brachytherapy, resolution of IPSS (defined as the post-implant IPSS returning to within 2 points of the pre-implant score) occurred in 85.3 % of patients [65]. A questionnaire study of 174 patients with over 5 years follow up after LDR brachytherapy reported good or acceptable quality life due to urinary symptoms (an IPSS quality of life score of 0–4) among 98 % of respondents [66].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







