Chapter 45 Biliary parasitic disease
Parasitic Infections
Parasitic infections of the biliary tract are a common cause of biliary obstruction in tropical developing countries and less frequently in developed countries. These infections are important, because they can lead to serious complications such as cholelithiasis (see Chapters 30, 35, and 39), recurrent pyogenic cholangitis (Chapter 44), cirrhosis (Chapter 70A, Chapter 70B ), pancreatitis (Chapter 53), and cholangiocarcinoma (Chapters 50A and 50B). The most common parasites of the biliary tract reported in humans are Fasciola, Opistorchis, Clonorchis, and Ascaris species. They have a wide geographic distribution, complicated life cycles, and various clinical manifestations with several diagnostic options available to detect them and multidisciplinary treatments. Millions of people are infected by these parasites, and surgical complications occur quite often.
Fascioliasis
Fascioliasis, or distomatosis, is a zoonosis caused by Fasciola hepatica or F. gigantica (Trematoda: Fasciolidae). The infection is distributed globally on all continents, but F. hepatica predominates in temperate zones, whereas F. gigantica is found in most tropical regions. In approaching a patient with suspected fascioliasis, epidemiologic (Table 45.1), clinical (Table 45.2), and imaging features (Table 45.3) can provide clues for the diagnosis before ordering a diagnostic test to confirm the infection. An algorithm for diagnosis and management is recommended and summarized in Figure 45.1.
Table 45.2 Epidemiology of Human Fascioliasis
| Geographic Area | Risk Factors | Population at Risk |
|---|---|---|
| Latin America (Andean region) Europe Africa Asia Australia | Watercress Drinking alfalfa juice Green vegetables Contaminated water Travel to endemic areas Living close to irrigation canals Eating salads | Children in endemic areas Travelers Women Vegetarians |
Table 45.3 Clinical Manifestations, Laboratory Data, and Imaging in Fascioliasis
| Clinical Picture | Imaging and Laboratory Results |
|---|---|
| Acute | |
| Prolonged fever (weeks or months) Abdominal pain (mostly upper abdomen) Hepatomegaly Weight loss Urticaria Ectopic lesions* | Eosinophilia (any cell count) Anemia Anicteric hepatitis Biliary hemorrhage or hemobilia Subscapular hematoma or hepatic rupture (seen on CT) Hepatic abscesses Tracklike lesions on CT |
| Chronic | |
| Abdominal pain in right upper quadrant Biliary colic Nausea and vomiting Recurrent or intermittent jaundice Uritcaria | Eosinophilia (sometimes) Cholestasis Hepatic abscesses Liver fibrosis and ultimately cirrhosis Necrotic granuloma (increased ALT and AST levels) Cystic tumors Cholangitis caused by Klebsiella, Escherichia coli, Enterococcus spp. Choledocolithiasis Eosinophilic cholecystitis Achalcolous cholecystitis |
CT, computed tomography; ALT, alanine aminotransferase; AST, aspartate aminotransferase
* Ectopic migration and other clinical manifestations. Acute stage: migratory nodule under the skin or peritoneal cavity, arthralgias, lymphadenopathies, hemolytic anemia, seizures, pleural effusion. Chronic stage: subcutaneous nodules and gastric nodules.
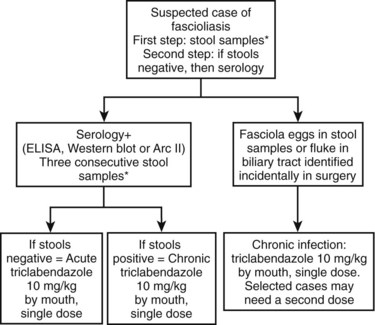
FIGURE 45.1 Summary of management and treatment of a suspected patient with fascioliasis. *Three consecutve stool samples must be examined by a sedimentation technique (Lumbreras, 1962) before ruling out the infection. Stool examination is preferred over serology because of cost effectiveness and availability. In highly suspected cases, a trial of triclabendazole is warranted. Single-dose triclabendazole 10 mg/kg has a cure rate greater than 90%. A second single dose may be used in selected cases (e.g., high intensity of infection in feces, large numbers of parasites in surgery, refractory cases). ELISA, enzyme-linked immunosorbent assay.
Epidemiology
F. hepatica infection was first documented in the Gallo-Roman period (Da Rocha et al, 2006). Today, the estimated number of human infections ranges from 2.4 million to 17 million, and 91.1 million are at risk of infection around the world (Keiser & Utzinger, 2005). In the past, fascioliasis was limited to specific and typical geographic areas, but it is now widespread throughout the world. According to the reported cases, F. hepatica transmission has increased in Europe, the Americas, and Oceania, and in Africa and Asia, where F. gigantica and F. hepatica overlap. The geographical distribution is determined by the intermediate host (Lymnaea spp.) and certain other conditions such as climate, alimentary behaviors, and poverty.
Examples of countries with estimates of the infected population include 830,000 in Egypt, 742,000 in Peru, 360,000 in Bolivia, 37,000 in Yemen, 20,000 in Ecuador, and 10,000 in Iran (Haseeb et al, 2002). Furthermore, other countries that have reported a significant number of cases in years include Argentina (Kleiman et al, 2007), Venezuela (Incani et al, 2003), Chile (Llanos et al, 2006), Ecuador (Trueba et al, 2000), Mexico (Cruz-Lopez et al, 2006), Turkey (Kaya et al, 2006; Turhan et al, 2006), Thailand (Aroonroch et al, 2006), Japan (Inoue K, et al, 2007), Korea (Lee & Kim, 2006), the United States (Fullerton et al, 2006; Graham et al, 2001), Tunisia (Khelifi et al, 2006), and Lebanon (Birjawi et al, 2002), among others. The majority of reported cases in these countries are related to complications from the infection, and the current number of cases is undoubtedly underestimated. On the other hand, globalization and migration of populations from rural areas to large cities have led to a number of cases of fascioliasis in nonendemic areas. In fact, because of clinicians’ lack of familiarity with this parasitic infection in nonendemic areas, the diagnosis can be delayed, which increases the rate of complications (Kang, 2008).
Fascioliasis is distributed globally, with the most affected area in the world being the Andean region of South America. In fact, more than half of the population may carry the infection in some selected regions, with prevalence ranging from 6% to 68% (Marcos et al, 2005b, 2007a; Parkinson et al, 2007). New evidence has shown that proximity of medium- to high-income industrialized cities to rural areas creates a potential source of infection in nonendemic areas because of the importation of contaminated vegetables to the high-consuming markets of big cities. Thus it is not uncommon to see cases of fascioliasis in nonendemic areas. Another factor that contributes significantly to the dissemination of the infection to new areas is the highly adaptable capacity of both the parasite and the lymnaeid snail hosts to challenging meteorologic conditions (e.g., 4200 meters above sea level, Andean Altiplano). Paleoparasitologic studies have shown that the introduction of F. hepatica and its snail host from Europe into the Americas has been relatively recent. Today, fascioliasis as a result of F. hepatica is the vector-borne disease with the widest latitudinal, longitudinal, and altitudinal distribution known. These key factors explain the wide geographical distribution of the disease (World Health Organization [WHO], 2007).
The epidemiologic pattern of transmission of fascioliasis can be classified as follows (Mas-Coma et al, 1999): 1) cases imported to areas where neither human nor animal fascioliasis is transmitted; 2) autochthonous, isolated, nonconstant cases of sporadic infection in areas where animal fascioliasis is present; 3) endemic fascioliasis (hypoendemic ≤1%, mesoendemic 1% to 10%, hyperendemic ≥10%); 4) epidemic fascioliasis (i) in animal endemic areas and (ii) in human endemic areas. Epidemiology is an important determinant of the initial evaluation of a patient with probable fascioliasis.
Life Cycle
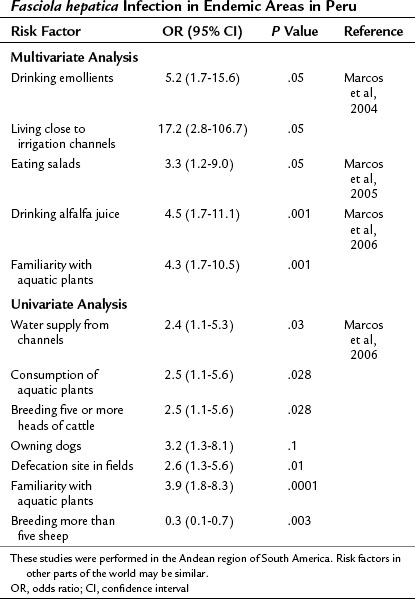
The adult F. hepatica flukes are large, flat, brown, and leaf shaped with a broad anterior portion covered with scalelike spines. Flukes measure approximately 25 to 30 mm by 10 to 15 mm, although F. gigantica can measure up to 75 mm. The adult fluke lives in the common and hepatic bile ducts of the human or animal host, and animals susceptible to becoming reservoir hosts for Fasciola species include mainly cattle, sheep, pigs, buffaloes, and donkeys, although it has been also reported in horses, dogs, goats, llamas, alpacas, dromedaries, and camels. The eggs are oval, yellowish brown, and measure approximately 130 to 150 by 60 to 90 μm (Fig. 45.2).
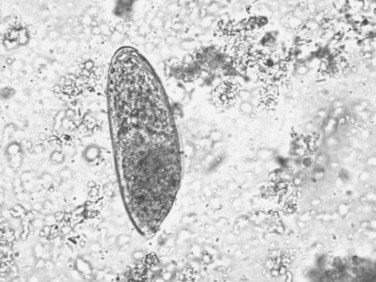
FIGURE 45.2 Adult egg of Fasciola hepatica in microscopic examination of stools by the rapid sedimentation technique.
Sometimes the larvae deviate to other locations; these are called extrahepatic forms or ectopic infections. Maturation from juvenile larvae into adult flukes takes approximately 3 to 5 months, during which time the larvae mature and migrate through the liver into the large hepatic and common bile ducts (Fig. 45.3). Mature flukes consume hepatocytes and duct epithelium and reside for years in the hepatic and common bile ducts and occasionally in the gallbladder. Adult fluke worms produce eggs within 4 months after infection (range, 3 to 18 months); these eggs traverse the sphincter of Oddi and intestine and then continue the cycle of infection. Of note, acute and chronic stages can overlap; this is commonly seen in endemic areas, and it is not unusual to find eggs in the stool samples of patients with acute infection.
Risk Factors
The main source of infection is the consumption of metacercariae-contaminated raw vegetables—such as watercress, lettuce, alfalfa juice, and mixed green salads—or contaminated water from man-made irrigation channels (Marcos et al, 2004, 2005a). Because of epidemic obesity, alimentary habits have changed; for example, higher consumption of green vegetables has led to an increase in the risk for fascioliasis, because it requires vegetables imported from endemic areas (Marcos et al, 2007b). The infection has been also seen in people who have eaten salads in luxury restaurants or hotels in endemic countries, and watercress has traditionally been the most common known source of infection. Nonetheless, a large variety of plants have been described in recent years, such as Medicago sativa (alfalfa in juice) in Peru; Taraxacum dens leonis (dandelion leaves), Valerianella olitoria (lamb’s lettuce), and Mentha viridis (spearmint) in France; green leafy Nasturtium spp. and Mentha spp. in Iran; Juncus andicola and J. ebracteatus (Juncaceae), Mimulus glabratus (Scrophulariaceae), and Nostoc species (Cianofitas) in the Bolivian Altiplano (Bjorland et al, 1995; Mas-Coma et al, 1999).
More women are affected than men, with higher prevalence rates, more severe infections, and with more reported liver or biliary complications (Marcos et al, 2006). Although, the differences are not well understood, alimentary habits, immunologic factors, and proximity to contaminated sources may play a role; for example, children are affected more than adults (Marcos et al, 2007c) likely because of alimentary habits. Reinfection is commonly seen in highly endemic areas, and the parasite can live in the biliary tract for a long time—between 9 and 13.5 years.
Epidemiologic studies have been carried out in endemic areas to measure the impact of alimentary habits on the acquisition of infection. Drinking beverages made from watercress or alfalfa leaves, called emollients, and living close to irrigation channels were found to be risk factors in a multivariate analysis in the Andean region (Marcos et al, 2004). Eating salads is the common factor among infected families, and it carries a 3.3-fold increased risk of acquiring the infection (Marcos et al, 2005b). In a logistic regression analysis, an age- and gender-matched case-control study comparing 60 infected children found that drinking alfalfa juice carries a 4.5-fold increase in the risk of acquiring fascioliasis, and familiarity with aquatic plants carries a 4.3-fold increased risk (Marcos et al, 2006). Therefore aquatic plants—watercress, alfalfa, and others—and the irrigation channels that carry the metacercariae play a key role in the transmission of fascioliasis in Andean endemic areas. Hypothetically, exportation of plants or other products could lead to transmission in nonendemic areas. Treatment of contaminated plants with high doses of potassium permanganate decreases metacercariae viability and could be used to prevent infection (Ashrafi et al, 2006), although its impact in endemic areas has not yet been tested. A summary of risk factors with studies reporting odds ratios is presented in Table 45.4.
Table 45.4 Imaging Findings in Fascioliasis
| Ultrasonography | Computed Tomography | Magnetic Resonance Imaging |
|---|---|---|
| Focal areas Multiple nodules Irregular lesions Variable or increased echogenicity Single complex mass Complex cystic mass Parasites moving in gallbladder | Multiple hepatic metastatic-like lesions Change in position, attenuation, shape in time Abscess-like lesions Low-density serpiginous tortuous tunnel-like branching Subscapular hematoma Cystic calcifications Glisson’s capsule contrast enhancement Single non–contrast-enhanced hypodense irregular mass | Homogenous hyperintense T2-weighted turbo spin-echo image Subscapular multiple hypointense areas Hypointense T1-weighted 3D gradient-echo image |
3D, three-dimensional
Clinical Manifestations
Fascioliasis has two distinct clinical phases: acute and chronic. Signs and symptoms depend on the worm burden, duration, and phase of infection. In general, the chronic infection is usually diagnosed in epidemiologic studies in endemic areas as a cause of biliary obstruction or in routine stool tests for other reasons. On the other hand, the acute infection has a more florid clinical picture that brings the patient to the emergency room. The clinical manifestations are so variable that mild right upper quadrant pain may call for a step-by-step workup that can lead to the final diagnosis of fascioliasis (Behar et al, 2009).
Acute Infection
The first acute or invasive phase lasts from 3 to 5 months and is caused by the migration of the immature larvae from the duodenum to the liver. Finally reaching the bile ducts, parasites migrate through the liver parenchyma and digest hepatic tissue, causing intense inflammation and hemorrhage proportionate to the number of worms. Migration tracks can be observed in histologic sections, and flukes sometimes die, leaving cavities filled with necrotic debris; these are eventually replaced by scar tissue. Symptoms include prolonged fever, hepatomegaly with abdominal pain, and mild eosinophilia (early infection) or hypereosinophilia (mid- or late-acute infection). Multiple hypodense lesions can be seen on CT scan, similar to metastases (MacLean & Graeme-Cook, 2002). Of note, one of the most frequent manifestations in this acute phase is hypereosinophilia, which is seen in almost all cases. If no eosinophilia is detected at the initial visit, it may be too early in the acute infection; a repeated cell blood count 3 to 5 days later will detect a significant increase in the eosinophil count. Absence of persistent eosinophilia reduces the suspicion for acute infection. In summary, acute fascioliasis is a clinical syndrome similar to acute cholecystitis with significant eosinophilia.
The acute phase presents itself with subcapsular hematomas, hepatic cysts, residual hepatic calcifications, and severe anemia. Hyperbilirubinemia is absent in the acute phase (Marcos et al, 2008b), which distinguishes it from other forms of acute hepatitis. Other manifestations are anorexia, weight loss, nausea, vomiting, cough, diarrhea, urticaria, lymphadenopathies, and arthralgias (Marcos et al, 2005c). Occasionally, the juvenile larvae reach other anatomic locations, such as the subcutaneous tissue, pancreas, eye, brain, and stomach wall, among others (Rana et al, 2007).
Chronic Infection
The chronic phase begins approximately 3 to 6 months after the consumption of the metacercariae, when the parasite reaches the bile ducts. By macroscopic examination, the liver has large, dilated, thick-walled, and calcareous bile ducts with yellowish brown bile. By microscopic examination, the bile ducts have a thickened hyperplastic wall with marked fibrosis (Haridy et al, 1999; Marcos et al, 2007c). Symptoms usually reflect biliary obstruction with colicky pain in the right upper quadrant, epigastric area, or upper abdomen (Rana et al, 2007; Maco et al, 2003; Jimenez et al, 2001). It can also be a silent, potential threat: the parasites can survive for longer than 10 years, and infection is commonly asymptomatic (Marcos et al, 2004).
Liver function tests during this phase are consistent with an extrahepatic cholestasis syndrome (Dobrucali et al, 2004) that can lead to surgery to treat the biliary obstruction (Jimenez et al, 2001). Moreover, an increase in liver enzymes can be present with minimal symptoms in endemic areas. In Egypt, it was found that patients with fascioliasis had significant liver enzyme abnormalities: elevation of alanine aminotransferase in 21.5% of the patients, aspartate aminotransferase in 21.9%, total bilirubin in 16.5%, gamma-glutamyltransferase in 80.6%, and alkaline phosphatase in 76.4% was reported. Excluding viral liver infections, F. hepatica infection is a significant cause of cholestasis in endemic areas (P < .05) (El-Shazly et al, 2005).
Imaging abnormalities were also found on ultrasound (US), including hepatomegaly, splenomegaly, periportal fibrosis, thickened gall bladder wall, dilated common bile duct, parasites in the gallbladder and common bile duct, stones in the gallbladder, stones in bile duct, cystic lesions in the liver, focal lesions in the liver, and ascites (El-Shazly et al, 2001).
Fasciola may also cause acute eosinophilic cholecystitis (Umac et al, 2006) along with pruritus and intermittent jaundice (Umac et al, 2006; Marcos et al, 2002). The parasites appear as small intrahepatic cystic lesions (Aroonroch et al, 2006) or as a large, multiloculated cyst that causes abscesses. On imaging parasites may appear very similar to echinococcosis (Maeda et al, 2008). Bacterial superinfection of Fasciola cysts is a complication of the chronic phase. Recent studies in a rat model have shown a significantly increased risk of bacterobilia in the chronic infection (Valero et al, 2006) and with gallstones (Valero et al, 2003). Even after successful treatment, abdominal pain and weight loss may still be present in approximately 2% to 4% of patients for several months (Rondelaud et al, 2006), and morbidity in these patients is significant.
Eosinophilia is absent in approximately half of the chronic cases. Upon admission to a tertiary health center, 47% of 277 complicated cases had eosinophilia (Blancas et al, 2004). A similar percentage was found in 101 chronic cases from the Andean region and other endemic areas: 48% had eosinophilia above normal levels, and only 14% had more than 1000 eosinophils/mL (Alban et al, 2002). In another study, about half of a group of 61 children in the Peruvian Altiplano with chronic fascioliasis had eosinophilia (Marcos et al, 2002). In Turkey, two (11%) of 18 subjects with fascioliasis had eosinophilia (Turhan et al, 2006).
Eosinophilia is present in a minority of cases of fascioliasis in the chronic phase. If present, it is generally mild (Gil-Gil et al, 2006). Few cases in the chronic phase have high-grade eosinophilia, in contrast with the acute phase, which presents with hypereosinophilia in almost all cases. Finally, a wide variety of other infectious agents are associated with eosinophilia, such as Strongyloides stercoralis, Ascaris lumbricoides, and hookworms or other helminths. Despite the fact that these are the most common parasitic causes of eosinophilia, they do not typically cause hepatic lesions, nor do they reach the high levels that we observe in patients with acute fascioliasis.
Another presentation of the chronic infection is hemobilia as a result of ulcerative lesions in the biliary tract caused by the adult parasite (see Chapter 105; Bahçecioglu et al, 2007). A granulomatous chronic inflammation may also be triggered by parasite ova in the liver or in other anatomic locations (Marcos & Terashima, 2007; Naresh et al, 2006). New data developed in an animal model have demonstrated a persistent immune suppression in advanced chronic infection (Gironès et al, 2007), suggesting that the infected host may be susceptible during the chronic phase to any Th2-suppression–dependent infection. Hence, a chronic immunosuppression may predispose to bacterial infection that can be life threatening.
An association exists between fascioliasis and liver fibrosis. It appears that hepatic fibrosis may evolve in some susceptible hosts, depending on the time and burden of infection. For instance, almost 50% of cattle infected chronically by fascioliasis had cirrhosis (Marcos et al, 2007c). In addition, hepatic cirrhosis has been reported in infected children (Almendras-Jaramillo et al, 1997; Marcos et al, 2005a) and adults (Heredia et al, 1984; Sanchez-Sosa et al, 2000), especially those with high-density infections. New data on the pathogenesis of hepatic involvement associated with F. hepatica infection have implicated cathepsin L1 and its collagenolytic function associated with tissue-invasion (Stack et al, 2007), with proteolytic activity leading to collagen type I expression and ultimately to hepatic fibrosis. The chronic infection can also have a negative impact on the nutrition and health of children in endemic areas.
Imaging Studies
The most common imaging findings in fascioliasis are summarized in Table 45.3.
Abdominal Ultrasound
Findings in the acute phase include focal areas of increased echogenicity, multiple nodular or irregular lesions of variable echogenicity, or a single, complex mass in the liver that resembles malignancy mimicking malignancy (Cosme et al, 2001, 2003). In frequent travelers, an abnormal liver US showing a complex cystic lesion warrants a workup for fascioliasis or other parasitic disease, such as echinococcal infection. In the chronic phase, US is even less specific, although the adult parasites can be visualized in the gallbladder (Bonniaud et al, 1984; Kabaalioglu et al, 1999; Gonzales-Carbajal et al, 2001). Overall, the detection rate is extremely low if used as the sole diagnostic tool. For instance, of 76 subjects with chronic fascioliasis evaluated by means of abdominal US, only 11 patients (14%) had visualized parasites, and in only two cases (2.6%), the parasites were spontaneously moving into the gallbladder. Therefore, the detection-rate of F. hepatica chronic infection by US is disappointingly low (Richter et al, 1999) and not specific (Turhan et al, 2006).
Computed Tomography
The most common computed tomography (CT) findings include multiple hepatic metastasis-like lesions that change in position, attenuation, and shape over time (Marcos et al, 2008). Initial lesions may be easily confused with hepatic metastases. Other findings are hepatomegaly, tract-like hypodense lesions with subcapsular location, subcapsular hematoma, and cystic calcifications (Marcos et al, 2008; Loja et al, 2003). The hepatic lesions correlate with the time of infection. Early infection is associated with contrast enhancement of the Glisson capsule as a result of inflammation stimulated as the juvenile parasite penetrates the liver capsule. This occurs in the early stage of the acute infection (first month of infection). In the intermediate stage (after the first month), multiple hypodense nodular areas (abscesslike lesions) or low-density serpiginous, tortuous, tunnel-like branching lesions that range from 2 to 10 mm are created by parasite migration through the liver and are typically visualized in the subcapsular region (MacLean & Graeme-Cook, 2002; Gonzalo-Orden et al, 2003
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree