Chapter 42A Benign biliary strictures
Overview
Benign biliary strictures may affect the intrahepatic or extrahepatic bile ducts or both and may be solitary or multiple. Several causes of benign biliary strictures have been described (Table 42A.1). Iatrogenic injuries after cholecystectomy are the most commonly reported and best characterized causes, and they are emphasized in this chapter to illustrate the principles of diagnosis and management. Strictures and other injuries also may occur after common bile duct (CBD) exploration or biliary reconstruction. Other procedures in the upper abdomen may endanger the biliary tract, especially procedures involving the liver, pancreas, and stomach/duodenum. The true incidence of biliary injury after these procedures is difficult to assess, but it seems to be substantially lower than after cholecystectomy.
Table 42A.1 Causes of Benign Biliary Strictures
Bile Duct Injury at Cholecystectomy
Incidence
Because of the great frequency with which the operation is performed, cholecystectomy remains the greatest source of postoperative biliary injuries. Open cholecystectomy has long been associated with a modest incidence of biliary injuries. In a review of more than 42,000 open cholecystectomies performed in the United States in 1989, Roslyn and others (1993) documented a 0.2% incidence of biliary injuries. Strasberg and colleagues (1995) reported a 0.3% incidence of injuries in a literature review of more than 25,000 open cholecystectomies since 1980. The advent of laparoscopic cholecystectomy (see Chapter 34) has refocused attention on this issue, however, because of the significant increase in the number of injuries.
Early studies worldwide documented a marked increase in the frequency of bile duct injuries associated with the laparoscopic approach, ranging from 0.4% to 1.3% (Deziel et al, 1993; Richardson et al, 1996; Wherry et al, 1996; Adamsen et al, 1997; MacFadyen et al, 1998; Fletcher et al, 1999). In a review of nearly 125,000 laparoscopic cholecystectomies reported in the literature in the years 1991 through 1993, Strasberg and colleagues (1995) reported an overall incidence of biliary injuries of 0.85% and incidence of major injuries of 0.52%. Recent studies evaluating biliary injury rates more than a decade since the widespread introduction of the laparoscopic technique confirm a stable yet high indicence of iatrogenic injury, with the incidence ranging between 0.3% and 0.6% in larger studies (Nuzzo et al, 2005; Kaman et al, 2006; Waage & Nilsson, 2006; Tantia et al, 2008). Given the inherent difficulties in collecting data on postcholecystectomy biliary injuries, the true incidence at any one time may never be known precisely; however, the figures reported for laparoscopic cholecystectomy represent a substantial increase over historical controls reported for open cholecystectomy.
The bile duct is at increased risk of injury during laparoscopic cholecystectomy compared with open cholecystectomy for several reasons that will be discussed later. It has long been argued that surgeon inexperience is a major factor, and that with increased familiarity with the procedure, the number of injuries would decrease—the so-called learning curve effect (Southern Surgeons Club, 1991). Considerable evidence supports this view. Several authors have shown an inverse relationship between the incidence of bile duct injuries and the number of cases performed (Deziel et al, 1993; Gigot et al, 1997; Woods et al, 1994) and a decreased bile duct injury rate when performed by specialized hepatobiliary surgeons (Boddy et al, 2007). Also, large population-based reviews have documented a decline in injuries over time (Richardson et al, 1996; Russell et al, 1996). However, these trends have not been observed by all investigators. A recent report spanning more than 12 years showed that an initial decline in injuries was not sustained at the end of the study (Waage & Nilsson, 2006). Several other reports have suggested no change in the incidence of bile duct injuries over time (Adamsen et al, 1997; Fletcher et al, 1999; Wherry et al, 1996), and the number and complexity of cases referred for repair have remained static at some specialist units (Walsh et al, 1998). Reports of major bile duct injuries inflicted by surgeons with considerable experience continue to appear (Carroll et al, 1996; Gigot et al, 1997). Moreover, the role of hospital volume does not seem to affect the incidence of injuries (Waage & Nilsson, 2006; Csikesz et al, 2009). The data suggest that laparoscopic postcholecystectomy bile duct injuries will remain a significant problem for the foreseeable future.
Pathogenesis
Several factors are associated with an increased risk of bile duct injury at cholecystectomy, some of which are general and some unique to the laparoscopic approach. Ultimately, the final common pathway of most injuries is either a technical error or misinterpretation of the anatomy (Table 42A.2; Strasberg et al, 1995; Olsen, 1997). Many authors classify biliary injuries as major, such as transection of the CBD, or minor, such as biliary leak; but the line separating the two is often blurred. In general, major injuries are more serious and usually require reoperation to repair. Minor injuries are not always trivial, however, and they may require operative intervention. The classification of biliary injuries is discussed later.
Table 42A.2 Classification of Causes of Laparoscopic Biliary Injuries
Modified from Strasberg SM, et al, 1995: An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg 180:101–125.
Anatomic Variations
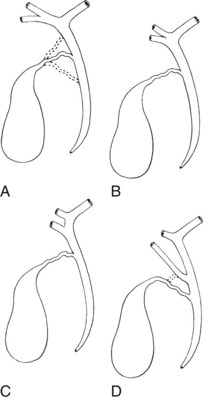
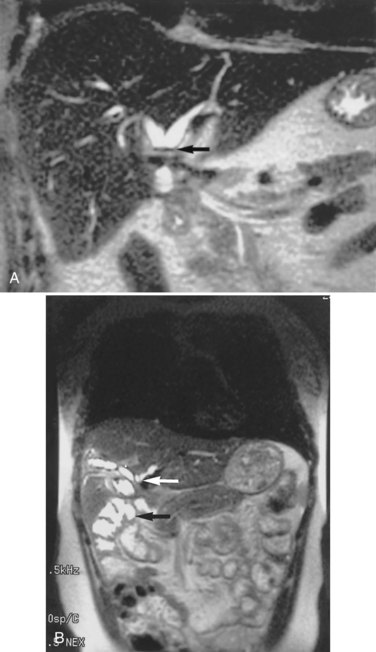
Any surgeon operating on the biliary tree must be familiar with the wide range of anatomic variations that may be encountered (see Chapter 1B). Anomalies of the cystic duct insertion into the common hepatic duct are most commonly seen (Fig. 42A.1). The cystic duct may join the common hepatic duct quite high, almost at the biliary confluence, or it may run parallel to the common hepatic duct for a long distance before joining it very low, occasionally at the level of the ampulla. In many patients, perhaps 25%, the right hepatic duct per se is absent, and the right anterior and posterior sectoral hepatic ducts join the left hepatic duct independently. In some cases, one of the right sectoral hepatic ducts, usually the anterior, may follow a prolonged extrahepatic course and enter the common hepatic duct quite low, and it may receive drainage from the cystic duct (see Fig. 42A.1D). Such unrecognized, low-entry right sectoral hepatic ducts (Bismuth type 5) are at particular risk of injury during laparoscopic cholecystectomy (Fig. 42A.2; Wherry et al, 1996).
In the future, routine use of preoperative imaging may help minimize bile duct injuries by identifying aberrant biliary anatomy. Already, some centers are examining the utility of preoperative CT cholangiography and MRI in preventing this complication (Hirano et al, 2006). Widespread adoption of this strategy will clearly be costly, but it could be justified: some imaging studies document right ductal aberrancy in the general population ranging from 4.8% to 8.4% (Kullman et al, 1996; Dusunceli et al, 2004). In some cases, the cystic duct is quite short, and misinterpretation of the anatomy can occur easily. Misinterpretation is especially likely if, on intraoperative cholangiography, the catheter has been advanced well into the CBD, and the proximal ducts are not visualized. The surgeon may mistake the common duct for the cystic duct, ligate it, and remove it with the attached gallbladder (Fig. 42A.3). This type of injury seems to be much more common after laparoscopic rather than open cholecystectomy and is well described (Davidoff et al, 1992). A related situation can occur in cases of long-standing cholelithiasis and chronic cholecystitis, resulting in obliteration of the cystic duct, which we will discuss later.
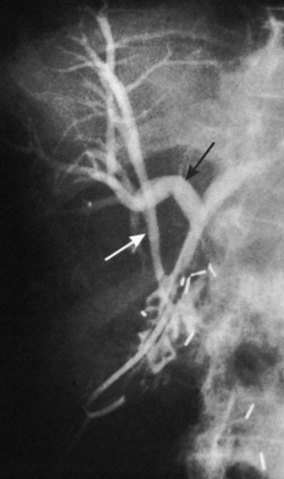
Vascular anomalies also are common, occurring in 20% of patients (see Chapter 1B). In the most common of these, the right hepatic arterial supply arises in part or in whole from the superior mesenteric artery. Such an aberrant vessel usually courses to the right of the portal vein, lateral and posterior to the CBD. During cholecystectomy, this vessel is prone to injury at the junction of the cystic and common hepatic ducts. Vascular injuries are not limited to patients with anatomic anomalies. The classic biliary injury during laparoscopic cholecystectomy, in which the surgeon mistakes the CBD for the cystic duct, is frequently associated with injury to the right hepatic artery, which will be discussed (Davidoff et al, 1992). This problem has been documented to occur in as many as 0.6% of patients after laparoscopic cholecystectomy and may not be apparent at the initial operation but may come to attention later as a hepatic artery pseudoaneurysm (Balsara et al, 1998; Nicholson et al, 1999). Rarely, concomitant vascular injury may involve both the right hepatic artery and right portal vein, which can be life threatening (Fig. 42A.4).
Ischemia
In the absence of overt injury, delayed biliary stricture formation may result from extensive periductal dissection and consequent interruption of the major ductal arterial supply (see Chapter 1B). Most of the arterial supply to the extrahepatic bile duct is provided by two small arteries that travel along the lateral duct borders in the 3 o’clock and 9 o’clock positions. Sixty percent of the blood supply runs upward from the major inferior vessels (retroduodenal, gastroduodenal), and approximately 38% runs downward from the right hepatic artery (Northover & Terblanche, 1979). Extensive circumferential dissection of the duct may disrupt this axial blood flow and has been proposed as a major mechanism of biliary stricture formation. Although there is no direct evidence to support this, it would seem reasonable not to pursue extensive dissection of the common duct during cholecystectomy. Also, damage to these vessels is likely to be a contributing, if not a central factor, in the formation of strictures attributed to electrocautery use.
Pathologic Factors
Patients with acute cholecystitis may have severe inflammation in the porta hepatis and triangle of Calot, which greatly distorts the anatomy. In addition, the gallbladder is often friable and difficult to grasp, and persistent oozing of blood often obscures the field. Some or all of these factors may conspire to preclude safe laparoscopic cholecystectomy. During the early era of laparoscopic cholecystectomy, acute cholecystitis was considered an absolute contraindication. However, as experience with laparoscopy grew, surgeons became more comfortable extending the indication to include acute cholecystitis. Several reports later documented that the laparoscopic approach not only is possible but also is safe in selected cases of acute cholecystitis (Kum et al, 1996; Wherry et al, 1996; Adamsen et al, 1997; Fletcher et al, 1999; Neri et al, 2003). As a result, laparoscopic cholecystectomy for acute cholecystitis is now widely practiced; in fact, the issue regarding timing of operation (early vs. delayed) has been settled (see Chapter 31; Casillas et al, 2008; Gurusamy et al, 2009). When laparoscopic cholecystectomy is performed for acute cholecystitis, studies have also revealed a greater potential for biliary injury (Kholdebarin et al, 2008). Fletcher and colleagues (1999) reported that complex cases that include patients with acute cholecystitis, cholangitis, and gallstone pancreatitis are associated with a dramatically increased incidence of bile duct injuries (1.7%), although acute cholecystitis alone is not an independent risk factor. Other authors have shown that acute cholecystitis increases the incidence of bile duct injuries twofold to threefold above baseline (Adamsen et al, 1997; Russell et al, 1996; Soderlund et al, 2005). In patients seen initially with acute cholecystitis, we recommend early operation by laparoscopic approach, even in patients with a history of prior open abdominal surgery. Given the increased risk of biliary injury in this setting, however, the threshold for conversion to an open procedure should always be low.
On occasion, the inflammatory reaction is so severe that even conversion to an open procedure does not clarify the anatomy. Fibrosis and inflammation within the triangle of Calot make dissection of the cystic duct particularly hazardous. In severe cases, the gallbladder neck and cystic duct may be fused to the common duct within a sheath of scar tissue. This finding should lead the surgeon away from an extensive dissection to define the cystic duct, which risks a major biliary injury. Placement of a cholecystostomy tube may be a safer approach. An alternative option is partial (subtotal) cholecystectomy with amputation of the gallbladder, well away from the cystic duct/common hepatic duct junction, and placement of a drain. This procedure is generally safe and effective, and in a review of 1100 patients, only 11.2% required operative reintervention (Soleimani et al, 2007). Moreover, centers have reported a significant decrease in the incidence of bile duct injuries since the introduction of laparoscopic subtotal cholecystectomy (Nakajima et al, 2009). In such heavily fibrosed cases, the cystic duct is nearly always obliterated, and postoperative biliary leak is infrequent.
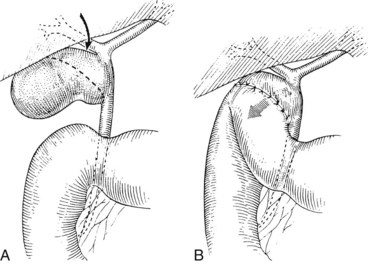
A potentially more difficult problem is the small, contracted, fibrotic gallbladder with extensive surrounding inflammation, sometimes partially embedded into the liver and obliterating the triangle of Calot such that the gallbladder neck and cystic duct are adherent to the common hepatic duct (Fig. 42A.5B). In such cases, the cystic duct is obliterated, and safe dissection in Calot’s triangle is impossible. With long-standing disease, particularly associated with large impacted gallstones in the gallbladder neck, a cholecystocholedochal fistula is possible. A common hepatic duct stricture may be present, resulting from an impacted stone and the subsequent inflammatory response (Mirizzi, 1948; see Chapter 42B). A cholecystocholedochal fistula should be suspected in any patient with gallstones, jaundice, cholangitis, and imaging studies or operative findings that suggest an inflamed and contracted gallbladder. In these cases, an attempt to perform a total cholecystectomy should be abandoned, because this would lead the surgeon directly into the common hepatic duct (Sharma et al, 1998). Rather, partial cholecystectomy with extraction of the stones allows inspection of the depths of the gallbladder and is preferable. A rush of bile into the operative field at this point is strong evidence for a cholecystocholedochal fistula, because the cystic duct is nearly always obliterated. Because a biliary stricture often lies distal to the fistula, direct closure of the defect should be avoided, and it should instead be reconstructed as a cholecystocholedochoduodenostomy (see Chapter 42B and Fig. 42A.5B).
Technical Factors
General
Early reports of laparoscopic biliary injury cited bleeding and subsequent attempts to achieve hemostasis as major contributing factors to bile duct injury (Davidoff et al, 1992; Rossi & Tsao, 1994). At laparoscopy, even a small amount of blood obscures the field and hinders dissection. If necessary, an additional port should be placed for a high-powered suctioner/irrigator to precisely define the site of bleeding. Hemorrhage must be controlled, regardless of the approach, and blind placement of clips or indiscriminate use of electrocautery must be avoided. Early demonstration of the cystic artery is desirable, and it should be divided close to the gallbladder to avoid injury to the right hepatic or common hepatic artery.
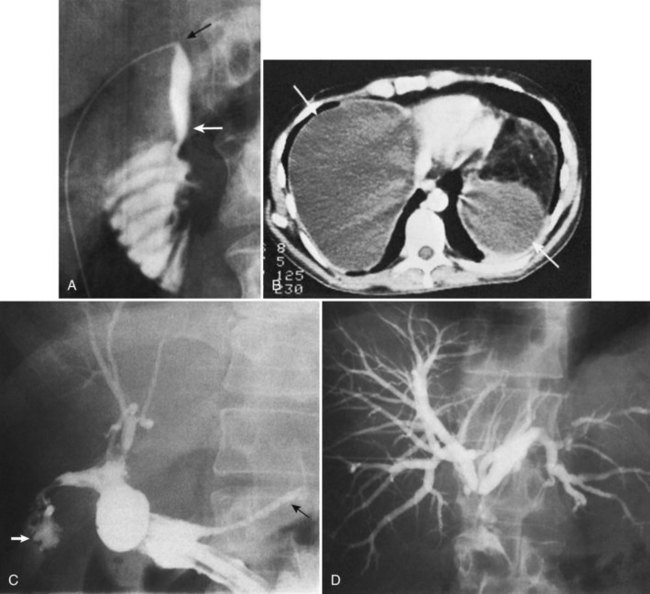
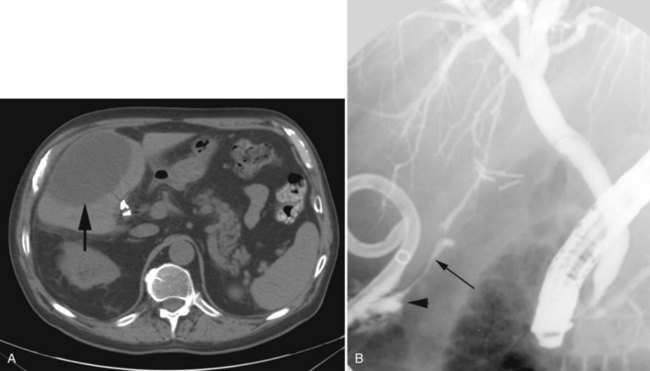
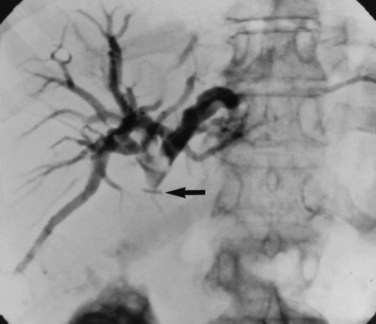
The observation of bile draining from the gallbladder fossa always suggests the possibility of a biliary injury, perhaps to an aberrant right sectoral hepatic duct. It is tempting to ascribe such a finding to drainage from a subvesical duct of Lushka, a slender, 1- to 2-mm in diameter duct that passes from the right lobe in the gallbladder fossa to join the right hepatic or common hepatic duct (McMahon et al, 1995). In this situation, a major bile duct injury must be considered the leading diagnosis until disproved, and the surgeon is compelled to convert to an open procedure or to place a drain and investigate the cause postoperatively. Regarding the duct of Lushka, although some surgeons question its existence, several detailed anatomic and radiologic imaging studies have reliably identified its structure and course (Kocabiyik et al, 2009). Most patients with bile leaks attributable to a subvesical duct experience a delayed onset of symptoms and are usually seen in the first or second week following the procedure (Fig. 42A.6). In a review of nearly 10,000 patients, the incidence of bile leakage was 0.7%, of which 52% were leaks from subvesical ducts (Spanos & Syrakos, 2006).
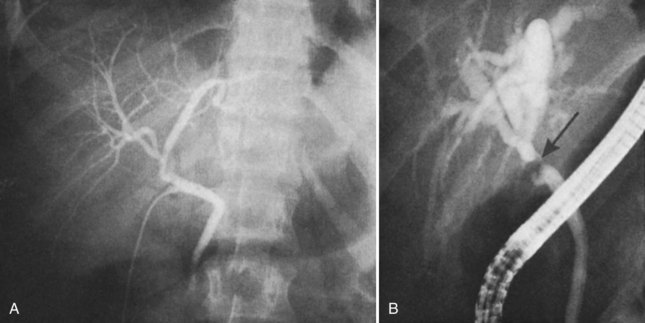
The utility of routine cystic duct cholangiography is controversial (see Chapter 21), particularly with respect to reducing the incidence of biliary injury at laparoscopic cholecystectomy, which will be discussed later. Occasionally, operative cholangiography reveals a very small CBD with a filling defect, suggesting a small stone. In this situation, simple cholecystectomy without exploration of the common duct is probably safer than operative pursuit of a tiny stone in a small duct. Postoperative endoscopic cholangiography with stone extraction is nearly always possible if subsequently indicated. In general, exploration of a small caliber, otherwise normal-appearing common duct is frequently unrewarding, potentially dangerous, and more likely to result in postoperative symptoms than if such a stone were left in situ. Biliary strictures are more than just a theoretical concern after exploration of a small bile duct (Fig. 42A.7). Rarely, bile duct stones retained after cholecystectomy that are not removable by endoscopic means require reoperation (Fig. 42A.8; see Chapters 35 and 36). Under these circumstances, laparoscopic removal is usually possible via a transcholedochotomy approach (see Chapter 34). If this approach fails, an open exploration can often be avoided by placing a T-tube into the CBD for subsequent operative retrieval by intervential radiology techniques, which are successful in more than 95% of cases (Burhenne, 1980). When indicated at cholecystectomy or after failed attempts by other methods, open exploration of the CBD should be performed through a supraduodenal choledochotomy with careful exposure of a sufficient length of duct but without excessive dissection (see Chapters 29 and 35). Exploration must be gentle and performed only with soft bougies, Fogarty-type balloon catheters, or a choledochoscope (rigid or flexible). Metal bougies such as Bake’s dilators or stone forceps should be used with caution and should never be forced through the papilla of Vater into the duodenum; otherwise, secondary postinflammatory papillary stenosis may occur, or a false passage into the duodenum or pancreas may be created. After bile duct exploration, the choledochotomy should be closed with care, because this is also a potential source of biliary stricture (see Chapters 29 and 35).
Transduodenal sphincteroplasty probably is best reserved for patients with stones impacted in the distal bile duct (see Chapters 29 and 35). Stenosis or stricture of the distal CBD may occur after surgical or endoscopic sphincteroplasty or sphincterotomy. Side-to-side choledochoduodenostomy is an option that should be considered in patients with multiple stones, primary stasis stones, or distal obstruction by stricture or papillary stenosis. The results are generally good, provided that the CBD is dilated to at least 15 mm in diameter, and a stoma of at least 2 cm can be created (Schein & Gliedman, 1981). Choledochoduodenostomy performed to a decompressed duct is more likely to result in stricture formation and cholangitis (see Chapter 29; Johnson & Stevens, 1969).
Laparoscopic-Specific Factors
Several technical factors specific to laparoscopic cholecystectomy predispose to biliary injury (see Chapter 34). Mistaking the common duct for the cystic duct is a frequent technical error (Davidoff et al, 1992; Gigot et al, 1997; Olsen, 1997). In the most common sequence of events, a long length of the common duct is excised up to the proximal common hepatic duct, which is either occluded or left to drain bile into the peritoneal cavity. This devastating injury—referred to as the “classic” laparoscopic injury, rarely seen at open cholecystectomy—also may be associated with damage to the right hepatic artery (Davidoff et al, 1992; Strasberg et al, 1995). Less severe injuries also may occur, such as lacerations of the duct wall or near-complete transections. Proper exposure is essential to avoid injuries arising from such anatomic misinterpretations. Maximum cephalad traction on the gallbladder fundus is necessary to expose the cystic duct and triangle of Calot. Concomitant lateral traction on the infundibulum is equally important, however, to open the angle between the cystic and common hepatic ducts. If lateral traction is inadequate, Calot’s triangle is not opened sufficiently, and the course of the common duct is placed directly in line with that of the cystic duct (Fig. 42A.9; Rossi & Tsao, 1994; McMahon et al, 1995). The common duct may be damaged during dissection of the cystic duct because of its proximity, or because it is misidentified. Before any structures are divided, Calot’s triangle must be cleared of all fatty and areolar tissue. The peritoneal reflection along the posterolateral aspect of the gallbladder should be opened to fully expose the junction of the gallbladder neck and cystic duct. At the completion of the dissection, only the cystic artery and cystic duct should be seen entering the gallbladder, and the bottom of the gallbladder fossa should be visible. The use of a 30-degree laparoscope has been advocated to improve visualization of this area (Hunter, 1991), and the resulting anatomically satisfying final view has been described by Strasberg (1995) as the “critical view of safety.” The dissection should be maintained close to the gallbladder wall, and the cystic duct should be divided as close as possible to its junction with the gallbladder.
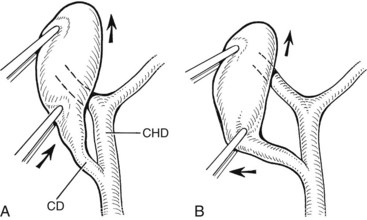
FIGURE 42A.9 Area of the triangle of Calot as it appears during laparoscopic cholecystectomy. A, Upward traction on the gallbladder, with insufficient lateral traction on the infundibulum, fails to open Calot’s triangle adequately. This causes the cystic duct (CD) and common hepatic duct (CHD) to become aligned within the same plane. Injury to the CHD can occur during dissection of the CD because of its proximity or as a result of mistaking the CHD for the CD. B, Proper lateral traction on the infundibulum opens Calot’s triangle, allowing safer dissection of the CD. In some cases, obliteration of Calot’s triangle by dense fibrosis prevents adequate exposure of this area, even with adequate traction (see Fig. 42A.5).
Routine intraoperative cholangiography has been advocated by many to minimize the incidence of laparoscopic biliary injuries (Way, 1992; Moossa et al, 1992; Asbun et al, 1993), but it remains controversial. In a meta-analysis encompassing more than 327,000 laparoscopic cholecystectomies, Ludwig and colleagues (2002) noted contribution of intraoperative cholangiography to incidence and outcome of CBD injuries during laparoscopic cholecystectomy and found injury rates to be halved in a routine cholangiogram group (0.21%) compared with a selective group (0.43%). A valid concern regarding the conclusions from these studies is that surgeons who use selective cholangiography do so appropriately in situations of inflammation or unclear anatomy inherently associated with increased risk of bile duct injuries. Thus, the injury rate under these circumstances can be artificially elevated, because the denominator—that is, the number cholecystectomies done without cholangiogram—is not typically included in the overall analysis. In a large review of cholecystectomies from Western Australia, Fletcher and colleagues (1999) reported that intraoperative cholangiography reduced the incidence of biliary injury twofold overall and eightfold in complex cases (acute cholecystitis, cholangitis, pancreatitis). In addition, some authors noted a strong inverse correlation between biliary injury and nonperformance of cholangiography (Torkington et al, 1999). Cholangiography is clearly not preventive if the injury occurs after a normal study, or if the study serves only to identify an injury that already has been inflicted (Andren-Sandberg et al, 1985).
Other groups, however, have touted the lack of statistical benefit with the routine use of intraoperative cholangiography. A survey analysis of more than 184 Italian hospitals accounting for more than 56,000 operations demonstrated no significant difference between bile duct injury rate when cholangiogram was used routinely or selectively (Nuzzo et al, 2005). In contrast, several other reports have suggested that routine cholangiography may not reduce the incidence of biliary injury but may minimize injury severity and allow immediate recognition and definitive repair (Gigot et al, 1997; Woods et al, 1994; Carroll et al, 1996; Russell et al, 1996); this depends on proper interpretation of the study. In a review of 177 biliary injuries, Olsen (1997) found that only two of 32 cholangiographies were correctly interpreted, despite evidence of impending bile duct transection in most. The most common error was accepting an incomplete study as normal, without opacification of the right and left hepatic ducts. In a similar study, Stewart and Way (2003) showed that in their series of 252 bile duct injuries, cholangiograms were obtained in 43 patients, but only nine were correctly interpreted intraoperatively. They concluded that these injuries stemmed from a misperception that was often so compelling that adjunctive cholangiographic data was likewise misperceived. Failure to opacify the proximal bile ducts in their entirety should prompt the surgeon to strongly consider open conversion. Laparoscopic ultrasound has been described as an alternative to cholangiography for delineation of the cystic duct and identification of common duct calculi (Tomonaga et al, 1999). Recent studies demonstrate the efficacy of the technique as an alternative to cholangiography (Machi et al, 2009; Hublet et al, 2009), but it is not widely used.
Bile leakage occurs with greater frequency after laparoscopic cholecystectomy than after open cholecystectomy, which may be related to the use of clips rather than ties or suture ligatures (McMahon et al, 1995). Clips are less secure than sutures and may become dislodged if improperly placed or if manipulated after placement (Nelson et al, 1992; Fig. 42A.10). Reports of clip migration leading to complications such as choledocholithiasis and pancreatitis have also been reported (Raoul et al, 1992; Cetta et al, 1997; Dolay et al, 2007). Also, incomplete occlusion may occur if clips are placed across a thickened, rigid cystic duct or a cystic duct containing stones or if other tissues are included. When placing clips, it is important that the tips meet to ensure adequate occlusion. If both tips cannot be visualized as the clip is being applied, damage to adjacent structures may occur. If the clip does not fit across the entire width of the cystic duct, the surgeon must consider the possibility that the structure about to be divided is not the cystic duct but the common duct. If the anatomy is clear, and the cystic duct is simply thickened, placement of a ligature may be more appropriate (Michalowski et al, 1998). Also, using electrocautery in areas adjacent to clips should be avoided, because this may result in conduction of thermal energy to the adjacent common duct and lead to delayed biliary structure.
Location and Classification
The ease of management, operative risk, and outcome of biliary injuries vary considerably and depend on the type of injury and its location (Chapman et al, 1995). It has long been recognized that strictures involving the CBD or distal common hepatic duct are easier to repair than are more proximal injuries. The anatomic classification of Bismuth has been widely adopted in recognition of this fact (Fig. 42A.11; Bismuth & Lazorthes, 1981). In this classification scheme, five stricture types are recognized, reflecting the location with respect to the hepatic duct confluence (types 1 through 4) or the involvement of an aberrant right sectoral hepatic duct with or without a concomitant hepatic duct stricture (type 5; Table 42A.3).
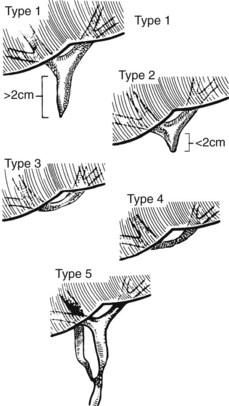
FIGURE 42A.11 Classification of bile duct strictures based on location with respect to the hepatic duct confluence (see Table 42A.3).
(From Bismuth H, 1982: Postoperative strictures of the bile duct. In Blumgart LH [ed]: The Biliary Tract: Clinical Surgery International. Edinburgh, Churchill Livingstone, pp 209-218.)
Table 42A.3 Bismuth Classification of Biliary Stricture
The Bismuth classification is useful for localization and for prognosis after repair, but it does not encompass the entire spectrum of injuries that are possible. No provision is made for biliary leaks or major ductal injuries without stricture. Even within the group of patients with biliary leaks, there are varying degrees of severity. In an effort to fill this gap, Strasberg and colleagues (1995) proposed a comprehensive classification system that incorporates Bismuth’s scheme but is much broader in scope (Fig. 42A.12). Injuries are classified as type A to type E, with the latter representing biliary strictures and further subdivided as E1 to E5 according to the Bismuth classification. Type A injuries are bile leaks from minor ducts still in continuity with the CBD. This encompasses the most common causes of biliary leaks seen after laparoscopic cholecystectomy—leakage from the cystic duct and from a subvesical duct of Luschka (see Figs 42A.6 and 42A.10). Type B injuries involve occlusion of part of the biliary tree, which for practical purposes almost always is an aberrant right sectoral hepatic duct. When this duct is transected without ligation, the injury is termed type C, reflecting the differences in presentation and management between the two. A lateral injury to an extrahepatic bile duct is termed type D, which is similar to type A injuries in that the extrahepatic biliary tree remains in continuity but is classified separately to underscore the greater severity and potential need for major reconstruction (Fig. 42A.13).
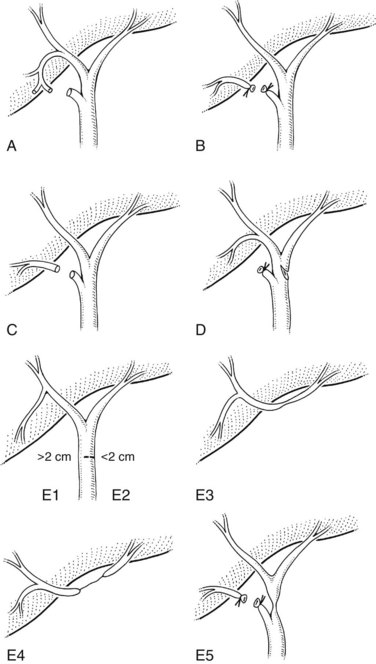
FIGURE 42A.12 Classification of laparoscopic biliary injuries according to Strasberg and colleagues (1995). Injuries are stratified from type A to type E. Type E injuries are subdivided further into E1 to E5 according to the Bismuth classification system.
The incidence of each type of biliary injury is difficult to ascertain with certainty. Most literature reports are derived from tertiary referral centers and are biased toward more severe injuries. Most surgical series focus on management of type E biliary strictures, whereas endoscopic and radiologic reports are concerned mainly with the treatment of biliary leaks. Type A injuries may be the most common, but many are managed successfully without referral and are underrepresented in reports from major centers (Strasberg et al, 1995).
Clinical Presentation
Most patients with postcholecystectomy biliary injuries, if not diagnosed at operation, come to medical attention early in the postoperative period. After open cholecystectomy, only about 10% of injuries are suspected after the first week, but nearly 70% are diagnosed within the first 6 months after operation (Pitt et al, 1982). By contrast, injuries after laparoscopic cholecystectomy seem to be recognized earlier (Davidoff et al, 1992; Lillemoe et al, 1997). This probably reflects differences in the pattern of injuries between the two approaches combined with a heightened awareness of the potential for injury at laparoscopy (Strasberg et al, 1995).
The clinical presentation depends on the type of injury; conversely, the type of injury may be inferred based on the clinical picture at presentation. Patients with significant bile leaks—types A, C, and D—generally present within the first week after operation, but some leaks may not become apparent for several weeks, and few are diagnosed intraoperatively (Strasberg et al, 1995). Most patients have abdominal pain coupled with fever or other signs of sepsis, which is most common, or bile leakage from an incision. A few patients have none these signs and symptoms but rather have nonspecific complaints of weakness, fatigue, or anorexia. Elevated alkaline phosphatase levels are characteristic, as is mild hyperbilirubinemia, but markedly elevated serum bilirubin levels (>3 mg/dL) are uncommon (vanSonnenberg et al, 1993; Brooks et al, 1993).
Major injuries to the common duct (type E injuries) are more likely to be discovered intraoperatively, although most remain unrecognized until after operation. Similar to bile leaks, these injuries are diagnosed more often within the first few postoperative weeks, although patients with a slowly evolving stricture may not come to attention for several months (Strasberg et al, 1995), which is distinctly uncommon for patients with bile leaks. Most patients with these injuries present with jaundice often coupled with pain and occasionally sepsis. Jaundice is not always present early in the course of the illness. In some patients, the stricture may evolve slowly or cause only partial obstruction. Such patients may have nonspecific complaints, pruritus, or derangements in liver function tests (LFTs), any or all of which should prompt an investigation. In addition, patients with an isolated right sectoral hepatic duct injury (type B) or an internal biliary fistula may be seen initially with a history of unexplained fevers, pain, or general debilitation.
Pathologic Consequences
Fibrosis
Biliary obstruction is associated with the formation of high local concentrations of bile salts at the canalicular membrane, and these initiate pathologic changes in the liver (Schaffner et al, 1971). Bile thrombi form within dilated centrilobular bile canaliculi, and secondary changes develop in adjacent hepatocytes. A complex cascade of molecular and cellular events ensues, collectively termed fibrogenesis (Friedman, 2008), which ultimately leads to the deposition of collagen and other extracellular matrix proteins and eventually to fibrosis and scarring around bile ducts and ductules (Maher & McGuire, 1990; Jarnagin et al, 1994; Friedman, 1997; see Chapter 6). As this process progresses, mechanical interference with bile flow develops in these intrahepatic biliary radicles and perpetuates cholestasis.
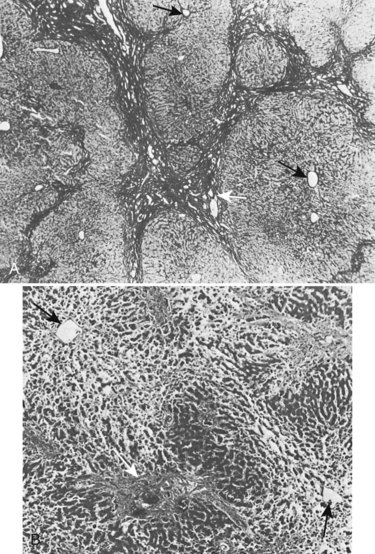
Fibrosis is accompanied by liver cell hyperplasia (Weinbren et al, 1985); this is not true in secondary biliary cirrhosis, because the lobular structure of the liver is usually well preserved (Fig. 42A.14) and the marked fibrosis that occurs in advanced cases only rarely progresses to true cirrhosis. This knowledge is important in planning therapy because many of these pathologic changes are reversible (Duffield et al, 2005). A histologic return of normal liver parenchyma is seen after relief of obstruction in both animal and human models (Sikora et al, 2008; Kirkland et al, 2009), which correlates to the return to near-normal liver function after relief of biliary obstruction (Blumgart, 1978). Fibrosis also may develop in the extrahepatic ducts proximal to the stricture, which is especially likely after biliary intubation. Upward retraction of the ducts is accompanied by a sequence of mucosal atrophy, squamous metaplasia, inflammatory infiltration, and further fibrosis in the subepithelial layers of the ducts.
It usually takes several years for liver fibrosis and portal hypertension (see Chapter 70A, Chapter 70B ) to become evident, although it may manifest 2 years after the development of the stricture. Major stigmata of hepatocellular dysfunction, such as spider angiomata and encephalopathy, are uncommon in benign biliary obstruction and should raise the possibility of preexisting parenchymal disease (e.g., chronic hepatitis, alcoholic liver disease). If liver biopsy reveals significant primary hepatocellular disease in association with a benign stricture, the prognosis is especially poor.
Atrophy
The distribution of liver mass is regulated by a poorly understood balance of bile flow, portal venous flow, and hepatic venous flow. Segmental or lobar atrophy may result from portal venous obstruction or bile duct occlusion in the affected area (see Chapter 5). Unilobar atrophy is associated with hypertrophy of the contralateral lobe and may present diagnostic and operative difficulties. Liver lobe atrophy and compensatory hypertrophy is frequently found in benign strictures and may be associated with asymmetric involvement of lobar or sectoral hepatic ducts, interference with portal venous blood supply, or decreased portal perfusion owing to secondary fibrosis. In benign strictures, the dilated ducts within the atrophic segments often are filled with infected bile and debris, and even though drainage of an atrophic segment would not be effective in relieving jaundice, cholangitis may continue unabated unless satisfactory drainage of the atrophic and hypertrophic segments is achieved.
The presence of significant atrophy and compensatory hypertrophy greatly influences the approach to repair. The most common situation is gross hypertrophy of the left lobe accompanied by right lobe atrophy (Czerniak et al, 1986). Anastomosis in the region of the hilum is made difficult by the rotational deformity and anatomic distortion imposed by this condition (see Chapters 5 and 29). A thoracoabdominal approach to such strictures provides more direct exposure and access for repair by allowing rotation of the liver to the left (Bismuth & Lazorthes, 1981). Recent reports verify that the presence of atrophy and contralateral hypertrophy are associated with significantly longer reconstructive operations, higher intraoperative blood loss, and greater blood transfusion requirements (Pottakkat et al, 2009). Similar problems may occur in bile duct strictures after right hepatic resection.
Portal Hypertension (See Chapter 70A, Chapter 70B )
It is estimated that approximately 15% to 20% of patients with benign biliary stricture have concomitant portal hypertension (Blumgart & Kelley, 1984; Chapman et al, 1995). Patients with biliary strictures may develop portal hypertension as a result of secondary hepatic fibrosis or direct damage to the portal vein. Alternatively, portal hypertension may be due to preexisting liver disease. It is important that these patients undergo further workup, including a liver biopsy, to exclude underlying chronic parenchymal disease. Iatrogenic biliary injuries are often the subject of medicolegal proceedings, and precise documentation of all injuries is essential to provide an accurate assessment of the cause of symptoms and prognosis. The outcome of patients with biliary strictures and portal hypertension is much worse than for patients without portal hypertension, with an in-hospital mortality rate of 25% to 40% (Blumgart & Kelley, 1984; Chapman et al, 1995). It has been suggested, however, that adequate biliary drainage may be followed by some resolution of fibrosis and perhaps a reduction in portal pressure (Blumgart, 1978).
Management
Radiologic Investigations (See Chapter 13, Chapter 15, Chapter 16, Chapter 17, Chapter 17 )
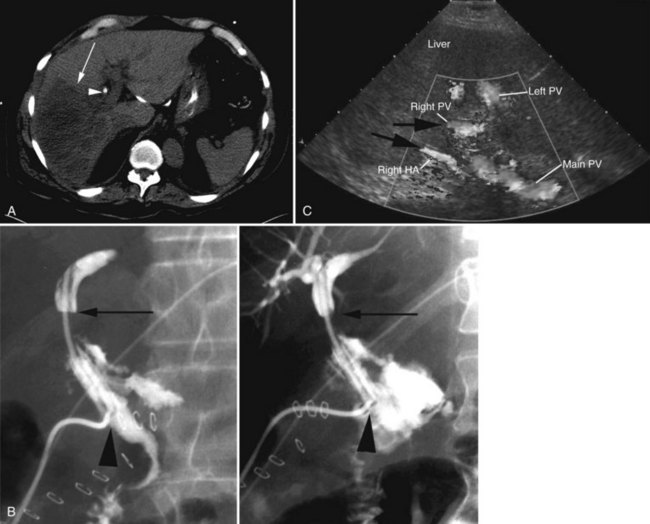
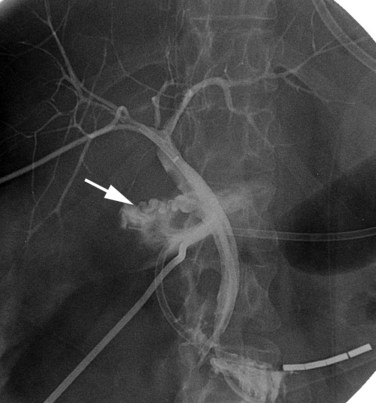
In patients with biliary strictures, complete delineation of the level and extent of injury is necessary. All branches of the right and left intrahepatic biliary tree must be outlined, particularly in cases of high bile duct stricture and recurrent stricture after previous reconstruction. Displaying the hepatic duct confluence (if intact) and the left ductal system and its branches is especially important in selecting the appropriate reconstruction. Percutaneous transhepatic cholangiography (PTC) is much more likely than endoscopic retrograde cholangiopancreatography (ERCP) to provide this information, and PTC remains the standard investigation in this setting (Fig. 42A.15). Drainage catheters should be left in place following PTC if a complex injury is identified on cholangiogram, because palpation of the catheter intraoperatively often helps guide identification of ductal structures later during definitive repair. The risk of cholangitis can be reduced with prophylactic antibiotics.
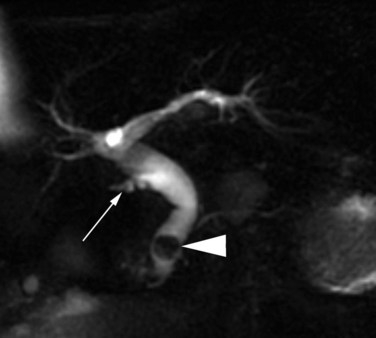
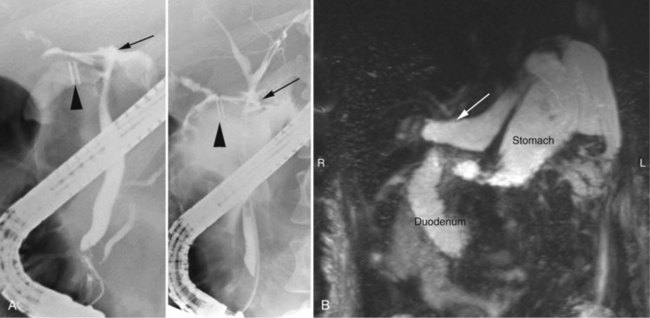
Magnetic resonance cholangiopancreatography (MRCP) has emerged as a valuable tool in evaluating proximal bile duct injuries (Coakley et al, 1998). This noninvasive modality provides striking images of the biliary tree and yields anatomic information in a single study that was previously obtainable only with CT and PTC (Fig. 42A.16). Multiple studies have now confirmed the high accuracy and detailed information provided by MRCP (Bujanda et al, 2003; Jarnagin et al, 1994; Ragozzino et al, 2004). However, in some cases involving severely distorted anatomy as a result of atrophy, hypertrophy, or dense scarring, preoperative PTC and catheter placement should still be strongly considered, not only for additional cholangiographic information or intraoperative guidance but also because catheters can be easily exchanged for soft tubing to stent across worrisome small caliber anastomoses.
ERCP is seldom of value in the precise diagnosis of complete proximal bile duct strictures because there is often discontinuity of the CBD preventing visualization of the intrahepatic ductal system. ERCP may be more helpful for incomplete strictures (stenoses) and is appropriate for patients with a history of sphincteric damage at previous common duct exploration, or if there is suspicion of papillary stenosis or other periampullary pathology. ERCP also has a role in the diagnosis and treatment of patients with bile leakage from the cystic duct stump or from a laceration of the common duct (Brooks et al, 1993
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree