Introduction
Antibody incompatibility has been a central issue in renal transplantation from the beginning; over 50 years ago, Hume and colleagues at the Brigham Hospital described early attempts to transplant kidneys into the thigh [1]. In reporting their experience, they described the transplantation of a kidney from a blood-group-B donor into a 28-year-old, blood-group-O female recipient. After initial function, urine output decreased, and the organ was removed after 7 days. Histology showed infarction of the kidney. This would appear to be the first recorded case of blood-group-incompatible transplantation, and it suggests the development of hyperacute rejection. Subsequently, seminal work in the 1960s by Terasaki and colleagues determined the importance of human leukocyte antigen (HLA) antibodies in transplantation [2] and the need for a negative crossmatch prior to implantation. With the recognition that allografts were doomed to fail in the presence of significant circulating antibody in the recipient against donor antigens, antibody-incompatible transplantation (AIT) was rarely performed until the 1980s, when several groups began to attempt antibody removal in order to avoid hyperacute rejection [3–4]. By the last decade of the 20th century, a mismatch between organ supply and demand generated more interest in AIT; results improved and larger series were reported [5–6], so that currently many large-volume transplant centers have an antibody-incompatible program.
There is little doubt that antibody incompatibility is a major problem in the 21st century. Although estimates vary, some 6000 patients with an antibody-incompatible donor remain on the deceased-donor list in the USA [7]; between 15 and 30% of those presenting for transplantation have an antibody-incompatible donor [8–10], and many will remain on the waiting list, since incompatibility against their living donor may also indicate difficulty in obtaining a deceased-donor organ, particularly with significant HLA sensitization.
What has also become clear is that outcomes after HLA-incompatible transplantation (HLAi) are significantly different from those following blood-group-incompatible transplantation (ABOi); the latter is often more easily performed, and has excellent graft and patient survival rates. For this reason, this chapter considers each of these categories separately, and in addition looks at alternatives to AIT, such as paired-exchange schemes. The chapter ends with a summary of how we might approach transplantation in an individual patient with antibody incompatibility.
Blood-group-incompatible transplantation
Hemagglutinins
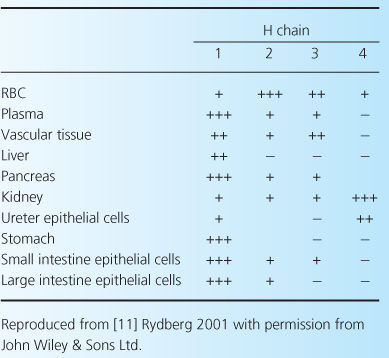
Blood-group antigens were initially described early in the 20th century by the Austrian biologist Karl Landsteiner, who characterized hemagglutinins present on red blood cells and the corresponding presence (or lack) of antibody in the serum, as shown in Table 4.1. Thus, individuals with antibody against a particular agglutinin would be expected to react to blood containing that antigen. However, for the purposes of transplantation, the issue is more complex. First, although these antigens are expressed on red cells, they are not expressed equally on all other tissues, as shown in Table 4.2. It is clear that ABOi will potentially be more difficult in kidney or pancreas recipients—since there is a high level of expression in these organs—but easier in heart and liver recipients. Indeed, cardiac ABOi has been well-established for some years. Second, as described later, hemagglutinin antibody titers can vary, and this may affect the ease of transplantation.
Table 4.1 Blood-group antigens and antibodies.
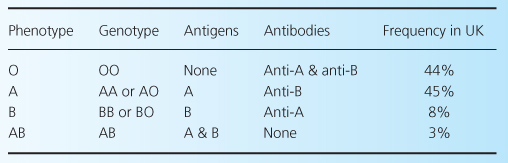
Table 4.2 Hemagglutinin core-chain H distribution in various human tissues. After [11].
The hemagglutinin molecule is derived by the action of glycosyl transferase on a core chain, substance H, which is a glycoprotein [11]. Group-A individuals (who are subdivided into A1 and A2) have N-acetylgalactosamine added to the core chain, while group-B have D-galactose added. No terminal residues are added to the core chain in group-O individuals. There are four types of H core chain, but it is noteworthy that although in group A1 glycosyltransferase acts on all four, in group B and in group A2 only types 1 and 2 are used. Furthermore, as shown in Table 4.2, core-chain expression varies in different tissues, and in the kidney is mainly type 4, with low expression of types 1 and 2. For this reason, groups A2 and B are said to be less antigenic than A1.
Early experience of ABOi therefore focused on A2 donors, with a report of 50 such cases resulting in a 2-year graft-survival rate of 94%, using OKT3 or antithymocyte globulin (ATG) but without plasma exchange or splenectomy [12].
Hemagglutinin antibody
Isohemagglutinin antibody titers are conventionally expressed in dilutions (negative, neat, 1 in 2, etc.). A variety of methods exist to determine the presence of antibodies; a gel and tube hemagglutination are the most common. There is, however, the potential for considerable variation in technique in this method, both between and within laboratories. A recent study from three of the most experienced centers undertaking ABOi in Europe showed a median variation of three titer steps when the same samples were sent to all three laboratories [13]. This was reduced to a median difference of one titer step when the gel technique using Diamed cards was adopted at all institutions, and indeed this is the technique used at Guy’s Hospital. Direct and indirect agglutination is performed, on the basis that this allows assessment of both immunoglobulin G and M (IgG and IgM) antibodies, but currently the exact significance of IgM antibodies is unknown. Clearly, inaccurate assessment of titer levels could have dire clinical consequences. It is uncertain whether the use of donor red cells or blood from volunteers with the same blood group is important.
Newer techniques, such as the use of flow cytometry, have been advocated in an attempt to standardize results, but these have not yet been widely adopted [14]. Nevertheless, it is vital for centers to ensure that they are obtaining consistent and reliable results when assessing hemagglutinin titers.
Principles of ABOi transplantation
There are two fundamental principles of ABOi transplantation: first to remove the hemagglutinin antibody, and second to prevent it recurring. The first is relatively straightforward, while the second is a matter of some debate. Each will be discussed in turn.
Antibody removal prior to transplantation
There are currently essentially three methods of removing antibody prior to transplantation: plasma exchange (or variations on this technique), nonspecific immunoabsorption, and antigen-specific immunoabsorption.
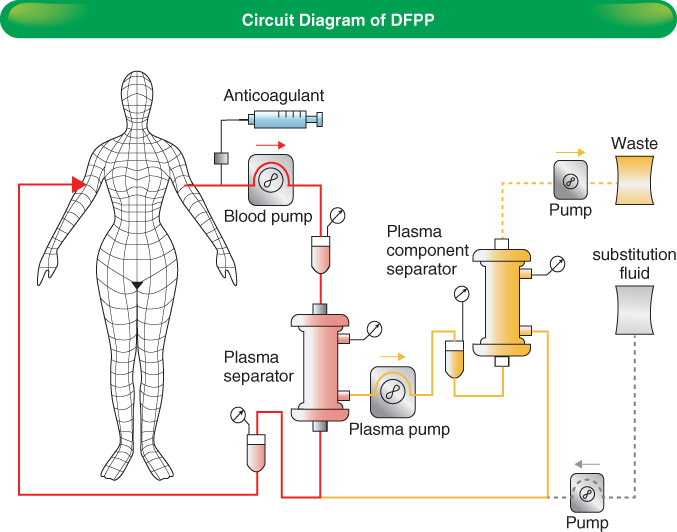
Plasma exchange, or plasmapheresis, involves the separation of the patient’s blood into a cellular component and plasma, after which the plasma is replaced with albumin or fresh-frozen plasma (FFP) and the components are recombined and returned to the body. This results in the removal of plasma proteins, including hemagglutinins, but as a consequence beneficial proteins, such as clotting factors, may be lost. After several sessions of plasma exchange, it is common for clotting to be significantly deranged, with a prolonged INR and low plasma fibrinogen. Clearly the risks of surgery in this situation are dramatically increased. A refinement of plasma exchange, double-filtration plasmapheresis (DFPP—Figure 4.1), involves two separations and the use of a microfilter that removes high-molecular weight proteins, thus allowing preservation of a significant component of plasma proteins. Hypoalbuminemia and coagulopathy are less likely, but will still occur in prolonged courses of therapy. Larger plasma volumes can be processed due to the decreased fluid loss. Correction of coagulopathy prior to surgery may be done with FFP (which should be either group AB or the donor’s blood group, to avoid introduction of hemagglutinins), cryoprecipitate, or fibrinogen, according to local protocol. At Guy’s Hospital, fibrinogen is given if the plasma level is below 1 g/l on the day of surgery.
Immunoabsorption requires the use of a plasma separator, followed by passage of plasma through an absorbent column. Nonspecific immunoabsorption has previously been carried out using protein-A columns, but currently the most frequently used column is TheraSorb. The former is derived from staphylococcal proteins which bind human immunoglobulin, while the latter uses sheep-derived polyclonal antihuman antibody bound to a sepharose matrix [15]. The TheraSorb system allows reduction in both IgG (approximately 70% per treatment) and IgM (50% per treatment) antibody, and will remove both HLA antibody and hemagglutinins [16]. It appears to be more effective at removing antibody across all IgG subclasses when compared with the protein-A column. [17] Due to the lack of complete specificity, albumin is reduced by around 20%. The significant advantage of the TheraSorb column is the fact that it may be reused for up to 10 treatments, making it currently relatively cost-effective, as well the fact that it can be used in combined ABOi and HLAi transplantation, as described later.
Specific immunoabsorption is performed using the Glycorex column; this consists of a carbohydrate moiety bound to a sepharose matrix, which will absorb anti-A, anti-B, or both anti-A and anti-B isohemagglutinins [18], with a median reduction of 81% for IgG and 56% for IgM [19]. The column appears to be highly specific, with minimal effect on other proteins (including HLA antibody) and in particular coagulation factors; there is evidence that antibodies to some pneumococcal and hemophilus antigens are reduced, possibly due to a crossreactive effect, while antitetanus and antidiphtheria antibodies are unaffected [19]. Although there is significant patient variability, a reduction by approximately one or two dilutions may be achieved after each treatment. The significant difficulty with this column is the fact that it is only licensed for single use, making it a relatively expensive treatment. However, it can be incorporated into a tailored protocol, as described later.
The aim of antibody removal is to ensure that titers are sufficiently low to enable transplantation to take place without the occurrence of hyperacute rejection. Perhaps surprisingly, there is only anecdotal evidence regarding the appropriate levels immediately prior to transplantation, and indeed given the variation in reported levels from the same sample in different laboratories, it is difficult to be certain about absolute parameters. However, most centers would accept titer levels of 1 in 8 for A1 donors and 1 in 16 for A2. For group-B donors, some centers would accept 1 in 16, while others would require 1 in 8; at Guy’s Hospital we use the latter. It should be noted that autoantibody can cause spuriously high results, and we have reported a technique to allow accurate assessment to take place despite apparently high levels of anti-A titers [20].
Recently, a new specific immunoabsorption column has appeared on the market, the Adsopak column. This contains a synthetic oligosaccharide bound to an agarose matrix, and will selectively remove anti-A or anti-B antibody. The advantage is that the column can be reused, making it relatively cost-effective. However, so far data on efficacy are limited.
Typically, two and a half to three plasma volumes are processed in one session of immunoabsorption. It should be noted that rebound of antibodies after either DFPP or immunoabsorption is common; within 12 hours, this may be due to release of tissue-bound antibody and antibody from the lymphatic and interstitial compartments, while a later rise (after 7 days) would be related to increased antibody production [15]. For these reasons, it is important to measure antibody titers on the day of transplantation, and to consider strategies for antibody suppression.
Antibody suppression
Splenectomy and rituximab
In the 1980s and 1990s, most ABOi was carried out in Japan, due to the lack of deceased donors there, and the protocols used in these cases most commonly involved splenectomy. The rationale was that removal of the spleen resulted in debulking of the B-cell population and thus reduced subsequent antibody production. The results from these series were very good when compared with compatible transplants from the same era, with graft survival rates of 84% at 1 year [21]. In theory, concomitant splenectomy carries increased risks due to the additional surgical procedure and the subsequent risk of infection from encapsulated bacteria, although in practice this does not seem to have been a major problem in Japan [22].
Splenectomy was replaced as a therapeutic strategy when Tyden, in his landmark paper [18], described four ABOi carried out using rituximab as a “chemical splenectomy.” Rituximab is an anti-CD20 chimeric mouse/human antibody that results in rapid depletion of CD20-positive B cells via antibody-dependent cell-mediated cytotoxicity. B-cell counts fall effectively to zero within 2 weeks of administration, and take 12 months to recover after a single dose [23]. It should be noted that B cells in the peripheral blood account for only a small proportion of the total B-cell population, and rituximab has been shown to reduce B cells in both lymph nodes [23] and the spleen [24]. Current regimens for ABOi include a single dose of rituximab given 1 month prior to transplantation. There is no evidence that this results in any short-term reduction in antibody, and indeed one would not expect this to be the case, since long-lived plasma-cell clones would continue to produce antibody for months, or possibly years. It may be, however, that B-cell elimination has significant effects due to the actions of B-cells as antigen-presenting cells and in cytokine production [25]. The conventional dose of rituximab used is 375 mg/m2, but some have experimented with lower doses with good results [26]. The Johns Hopkins group has dispensed with rituximab altogether, and recently reported outcomes in 28 patients [27]. However, they used both pre- and post-transplant plasmapheresis and intravenous immunoglobulin (IVIG), and there were five clinical and three subclinical cases of antibody-mediated rejection (AMR). Another center has achieved excellent results in a study of 37 patients, although two had AMR [28]. A recent study suggested that when compared with splenectomy, the use of rituximab resulted in lower rates of acute AMR after ABOi (although this was not statistically significant) [29]. At present, there is no convincing evidence that rituximab can be omitted routinely, although we have performed ABOi without it in selected cases, as described later.
IVIG
IVIG, which consists of pooled immunoglobulin from healthy volunteers, has formed an integral part of many ABOi regimens. The Swedish group originally described using a small dose (0.5 g/kg) on the day prior to transplantation [30], and many other centers now use this approach. The effects of IVIG are incompletely understood but may be due to a combination of an anti-idiotypic effect, downregulation of antibody production (due to an increase in circulating antibody, which is particularly important after antibody removal), interference with complement and cytokine production, and effects on the activation of T and B cells [31]. In the UK, it is expensive and difficult to source, and we have removed it from our protocol with excellent results, as described later.
Post-transplant antibody removal
Modern ABOi regimens have typically included routine post-transplant antibody removal using immunoabsorption or plasma exchange for the first week [27,30]. However, more recently the group in Freiburg developed a protocol which calls for post-transplant removal only if titers rise [32]. Thresholds for intervention were 1 in 8 in the first week and 1 in 16 in the second week, and 7 of 22 patients required post-transplant intervention.
In our own protocol at Guy’s Hospital, we have abandoned routine postoperative antibody removal (after the first nine patients in the program). We intervene if there is a rise of two titer dilutions or more postoperatively, or a rise associated with biopsy-proven rejection. In 67 patients, we have only carried out “on-demand” postoperative removal on four occasions, with a good response in each case.
Accommodation
“Accommodation” has been defined as the absence of graft dysfunction in the presence of circulating antidonor antibody—clearly this could be a hemagglutinin or an HLA antibody. After ABOi, hemagglutinin titers usually remain low, but the antibody is still detectable, despite normal graft function. Given the fact that titers fall immediately post-transplantation and remain low despite—in most cases—no further treatment, it seems likely that at least part of this fall simply represents binding of antibody to the allograft with subsequent depletion of the circulating pool. Additional evidence for this comes from the fact that the majority of ABOi recipients will have positive C4d staining on biopsy, without graft dysfunction [33–35], implying that complement activation occurs as a result of antibody binding to the endothelial cell. However, it may also be the case that downregulation of antibody production occurs in the longer term, and there is additional evidence of downregulation of blood-group antigen expression on the endothelial cell surface after ABOi [36]. Although data are limited, it would seem that initial concerns that circulating antibody could lead to the development of transplant glomerulopathy and impaired graft function are not borne out [35]. In summary, accommodation does seem to occur in ABOi, but the mechanisms are poorly understood, and further research is clearly needed.
Outcomes after ABOi
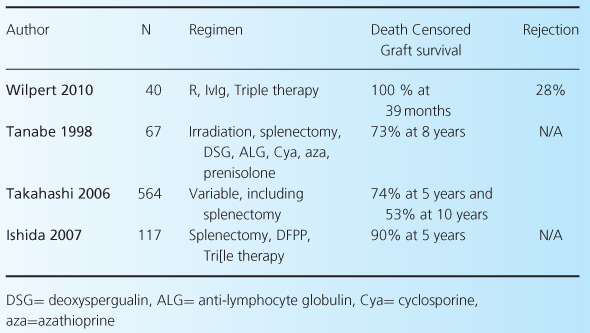
As noted earlier, there are a number of different protocols currently being used in ABOi, including standard- and reduced-dose rituximab and IVIG, as well as plasmapheresis or immunoabsorption pre- and possibly postoperatively. For this reason, accurate comparisons of outcomes after ABOi are difficult. However, it is fair to say that there is good evidence that short-term outcomes are excellent, and broadly comparable with blood-group-compatible transplants. Table 4.3 shows results from all studies with over 30 patients reporting outcomes over the last 10 years [22,27,28,37–40], and includes our own data [41]. What is clear from these data is that outcomes after ABOi in the short term are excellent, although they mostly come from small, single-center studies. Longer-term studies are less common (see Table 4.4) but also show excellent outcomes [5,42]. These include a large series from Japan [21].
Table 4.3 Short-term outcomes of ABOi.
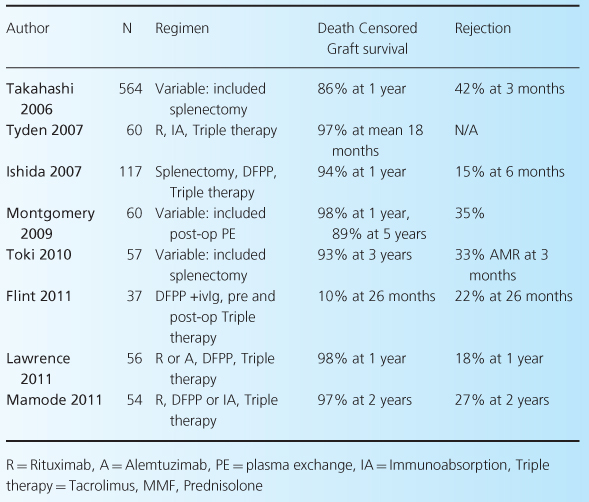
Table 4.4 Long-term outcomes of ABOi.
A key question is whether all patients undergoing ABOi are likely to have the same outcomes or whether baseline hemagglutinnin titers will affect success rates. In our own experience, we have never failed to reduce titers adequately, despite baseline titers of 1 in 1024 in some cases, and indeed some centers in Japan do not have an upper limit of baseline titer in consideration for transplantation. There are however some caveats: we have had to carry out repeated attempts (on separate occasions) in a single patient, which is costly and psychologically difficult for both recipient and donor. Furthermore, when repeated sessions of antibody removal are performed, the patient will become physically exhausted and edematous, even when immunoabsorption rather than plasmapheresis is used. There is conflicting evidence regarding the likelihood of poorer outcomes with higher baseline titers; some have suggested that outcome does depend on baseline titer [43], while others have found no association [37,44]. Our approach has been to avoid attempting ABOi in patients with titers above 1 in 1024; for those with titers of 1 in 512 or 1 in 1024, we may suggest one or two runs in the UK paired scheme prior to ABOi, depending on patient preference. All other patients are offered ABOi, but can opt for one or more runs in the paired scheme first if they wish.
Pediatric ABOi
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree