Alvaro Morales, MD, FACS, FRCS
Definition
Normal aging frequently results in a progressive functional dwindling of the hypothalamic-pituitary-gonadal axis (Liu et al, 2007). In men this process exhibits significant interindividual variability in the age at onset and speed and depth of the decline. The syndrome is variously known as andropause, androgen decline in the aging male (ADAM), late-onset hypogonadism (LOH), or, more accurately, testosterone deficiency syndrome (TDS). It is characterized by multiple clinical manifestations (Table 29–1) (Liu et al, 2004). Urologists often see the same presentation more acutely and dramatically in men subjected to medical or surgical castration for advanced prostate cancer (Shahani et al, 2008).
Table 29–1 Clinical Manifestations of Late-Onset Hypogonadism and Their Anticipated Response to Treatment
| SYSTEM/FUNCTION | AGING | RESPONSE TO TESTOSTERONE |
|---|---|---|
| Erectile function | ↓ | ↑ |
| Sexual desire | ↓ | ↑ |
| Mood/cognition | →/↓ | ↑* |
| Tiredness/lack of motivation | ↓ | ↑ |
| Sleep disturbances | →/↓ | → |
| Spatial cognition | ↓ | ↑* |
| Vasomotor (hot flashes) | ↑ | ↓ |
| Quality of life | ↓ | ↑ |
| Hematocrit | ↓ | ↑ |
| Leptin production | ↑ | ↓ |
| LDL and HDL cholesterol | → | ↓ |
| Fat mass | ↑ | ↓ |
| Muscle mass | ↓ | ↑ |
| Bone mass | ↓ | ↑ |
| Hair and skin changes | ↓ | → |
↑ increases/improves; ↓ decreases/deteriorates; → no change; ↑* suspected/not proved.
HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Historical Perspective
Aging and Hormones: From Antiquity to the 21st Century
The importance of the gonads in the maintenance of homeostasis has been recognized since antiquity. The association of advancing age with hypogonadism has been widely acknowledged since the end of the 19th century with the report in the scientific literature by Brown-Séquard on his observations about the improvement in his own physical strength and intellectual capacity after administration of a liquid testiculaire prepared by him from animal gonads. These reports gave origin to a series of medical claims that exploited testicular extracts and heterologous transplants as effective treatments for the infirmities of aging. They were eventually exposed as hoaxes (Current Comment, 1927).
Key Point
Testosterone Deficiency Syndrome
Concurrently with the pseudoscience at the turn of the last century, serious investigators were exploring the effects of substances produced and secreted by the testes, eventually resulting in the isolation of androsterone and testosterone by Laqueur (David et al, 1935). By 1935, almost simultaneously and independently, three research teams led by Adolf Butenandt, Kàroly Gyula, and Leopold Ruzicka, who were individually sponsored by three different corporations (Schering, Organon, and Ciba, respectively), synthesized the more powerful testicular hormone, ultimately named testosterone (T). For this work, Butenandt and Ruzicka received the Nobel Prize for Chemistry in 1939. After synthesis of the hormone, injectable preparations for human use were developed, and shortly before the onset of World War II clinical trials on T were under way. Interest grew in the use of T for a variety of ailments, ranging from sexual difficulties to the prevention of benign prostatic hyperplasia (Cuneo and Jomain, 1938). By the end of World War II the clinical picture of a syndrome named “male climacteric” associated with low T levels was fully recognized (Werner, 1946). T substitution became so widely used that warnings about the indiscriminate use and abuse of the hormone were already sounded by the 1940s (Thompson, 1946).
Epidemiology of Hypogonadism in Aging
The mean life span in Imperial Rome was about 20 years. United Nations estimates and projections of world population trends over a 75-year period showed that in the last decade of the 20th century the number of humans increased by 1 billion, and it will become close to 2 billion over the next 25 years. More importantly, life expectancy over this period will increase by more than 30 years (United Nations Secretariat, 2008). It follows that the prevalence of hormonal alterations in general and hypogonadism in particular is bound to rise significantly in the first half of this century.
The global prevalence of TDS is not accurately known, but it can be inferred from population projections that it is on the rise. In an attempt to alleviate the scarcity of descriptive epidemiology of androgen deficiency, the Massachusetts Male Aging Study (MMAS) reported a crude incidence rate of 12.3 per 1000 person-years, leading to a prevalence of 481,000 new cases of TDS per year in American men 40 to 69 years old (Araujo et al, 2004). Further assessment with data from the Boston Area Community Health (BACH) survey documented a prevalence of symptomatic T deficiency in men 30 to 70 years of age of 5.6% (Araujo et al, 2007). These observations are further confirmed by an Australian study (Liu et al, 2007) and the European Male Aging Study with larger populations of very different geographic, cultural, and ethnic backgrounds (Wu et al, 2008).
Physiologic Principles
Regulation of Testosterone Production—Central Mechanisms
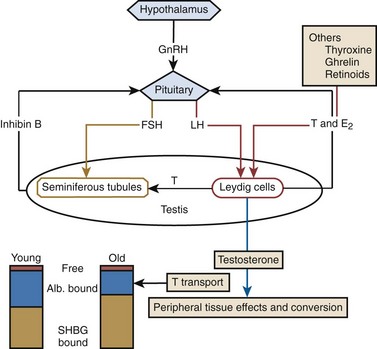
Luteinizing hormone (LH) modulates T biosynthesis by Leydig cells and also controls their population (Habert et al, 2001). An example exists in aged animals, in which “hibernation” of Leydig cells develops after prolonged LH suppression (Chen and Zirkin, 1999). In rodents, aging of the hypothalamus results in an apoptotic process leading to decreased production of gonadotropins (Morales and Heaton, 2003). Although not proved in humans, this process may explain a fundamental cause of TDS. In the testis the local cellular environment has the potential for impact on regulation and function in a paracrine fashion, mostly in the form of T acting in seminiferous tubules (Fig. 29–1). Sertoli cells do not appear to have a major impact as long as LH control is active (Young et al, 2000). Neural influences on Leydig cells have been demonstrated, independent of the pituitary, in the paraventricular nucleus of the hypothalamus (Selvage and Rivier, 2003), and the peptide ghrelin actively regulates Leydig cell activity (Barreiro et al, 2004). These findings have implications for T production independent of central endocrine mechanisms.
Androgen Production in Aging—Peripheral Mechanisms
T and dihydrotestosterone (DHT) are the main androgens playing the key regulatory roles in determining and later supporting the male phenotype. Androgen effects require the delivery of androgens to the site of action, penetration into the cells, metabolic conversion where necessary, and action with the androgen receptor (AR) on the genome. T is the predominant circulating androgen; the adrenal cortex secretes 19-carbon steroids with weak androgenic activity that serve as precursors for estrogens and active androgens such as T. Very small amounts of steroids are produced in brain cells, but local production may be functionally important (King et al, 2002) and not be represented in serum-based measurements. Leydig cells decrease in numbers in the testes of older men and these men have less T secreted per burst and a decline in secretory bursts in response to LH, suggesting partial desensitization of Leydig cells to LH with aging. Taken together, these studies demonstrate that a component of the decline in T with aging in men is due to testicular failure.
Transport and Metabolism of Testosterone
T is exchanged readily through cellular membranes with the extracellular environment. The passive transfer of T through membranes means that serum concentrations reflect general tissue androgen levels except where local sources of production or conversion exist. Circulating T is 98% bound to proteins (sex hormone–binding globulin [SHBG] and albumin (see Fig. 29–1), in which form it is relatively spared hepatic metabolism. The binding to SHBG and that to albumin have different affinities, providing the concept of bioavailable T; T bound to albumin is more readily unbound for use.
T levels are determined by the relative rates of production and metabolism. T can be aromatized to 17β-estradiol or reduced to 5α-DHT. Aromatization by cytochrome P450 arom and the supply of T substrate determine estrogen availability in estrogen-dependent cells such as those in the testis, fat, liver, hair follicles, and brain (de Ronde et al, 2003). There are two isoforms of 5α-reductase, and the cellular availability of 5α-DHT depends on T reduction and the rate of DHT metabolism. The balance between T and DHT metabolic enzyme activity is more critical to the cells in which it is occurring than to the overall rate of T metabolism. With aging, changes occur in enzyme functions that can potentially skew cellular activities in a way that is hidden from any systemic measures of androgen status. T catabolism takes place mostly in the liver, prostate, and skin, and catabolic products are largely excreted in the urine.
Androgen Receptor
T and DHT act through the androgen receptor (AR), which is a ligand-inducible transcription factor. The AR structure includes a COOH-terminal ligand binding domain, a DNA binding domain, a hinge region, a variable-length CAG repeat region, and an NH2-terminal transactivation domain (Glass et al, 1997). Most of the transactivational activity is associated with the NH2 terminus. The CAG repeat stretch is maximal in man (about 22 triplets) and decreases with evolutionary distance from man (Choong and Wilson, 1998). The hinge region is responsible for nuclear function after hormone binding. The ligand binding domain acts with the help of molecular chaperones, which increase the affinity for the hormone of the unbound AR.
T is transported through the cellular membrane, binds to the inactive AR complex, and is translocated to the nucleus. The binding of T to DNA activates the basal transcription machinery, thus regulating the transcription of target genes. Although the time frame for this genomic activity is 30 to 60 minutes there are nongenomic actions that take place too rapidly to follow this pathway. These rapid actions may be due to activation or possibly binding to undetermined membrane-bound receptors. The AR may be activated without the presence of ligand, for example, by protein kinase C, a factor that has great importance in the androgen interaction with prostate cancer cells (Culig, 2004).
CAG Repeat Polymorphism and Aging
The CAG repeat lengths of the AR vary from 6 to 39, depending on race (Valdehuis et al, 2005). In general, increased androgenic effect is seen at any level of T in men with shorter CAG repeat length. In older men, lower T is associated with fewer CAG repeats. The association of polymorphic AR with TDS manifestations showed that older men with more than 23 CAG repeats had less elevated LH, normal T, and less decreased potency but more depression, more anxiety, more deterioration of general well-being, and decreased beard growth (Harkonen et al, 2003; Lapauw et al, 2007). Changes in the AR can play a major role in the symptomatic response to T, potentially explaining some of the variability in clinical presentation in men with similar serum T. To complicate the picture, the AR CAG repeat length has been reported to correlate not only with serum T but also with estradiol in aging males (Huhtaniemi et al, 2009).
Androgen Actions
Testosterone and Glucose Metabolism
The metabolic syndrome is a complex association of several interrelated abnormalities that increase the risk for cardiovascular disease and progression to type 2 diabetes mellitus. Insulin resistance is the key factor for the clustering of risk factors (Grossman et al, 2008). Hypogonadism is seen frequently in association with the metabolic syndrome (Dandona et al, 2008), and several studies show that the fundamental cardiovascular risk parameters can be improved by the administration of T to these men. Hypogonadal men have increased leptin, obesity, and insulin levels that become normal with exogenous T treatment, which also reduces abdominal fat mass and improves insulin action (Zitzmann and Nieschlag, 2003). T administration to older men is associated with decreased visceral fat and glucose concentrations and increased insulin sensitivity (Bhasin, 2003). Hypogonadism correlates with high serum glucose, high triglycerides, high body mass, high waist-to-hip ratio, high total body fat mass, and high fasting insulin resistance index. Treatment with T improves the metabolic syndrome, especially in overweight older men (Schroeder et al, 2004).
Androgens and the Brain
A relationship between hypogonadism and depression has not been conclusively established. Although castration for the treatment of advanced cancer of the prostate has been shown to correlate with an increase in psychiatric illness, mostly depression (DiBlasio et al, 2008), there are studies showing improvements in measures of mood and well-being in aging men with hypogonadism (Almeida et al, 2008). There are well-researched gender differences in selected areas of cognition such as verbal skills and spatial tests that point to the developmental influence of T. Studies of hypogonadal men suggest that some cognitive skills can be improved with replacement therapy (Zitzmann, 2006), but this is not invariably seen because there are clearly many potential causes of impaired cognition.
Diagnosis
Clinical Diagnosis
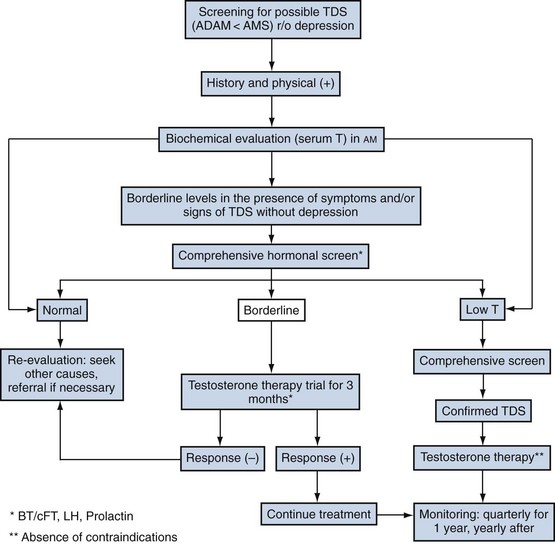
Clinical diagnosis of TDS is challenging because neither a low serum T level nor symptoms are truly diagnostic of the condition. The presence of symptoms, however, is a sine qua non for diagnosis and should be coupled with a low or borderline-low objective biochemical measure of T. In practice this ideal is not always achievable. Thus it was suggested that (Black et al, 2004) in the presence of symptoms but without strong biochemical support that a therapeutic trial would be an acceptable diagnostic approach; it should be noted that a sustained placebo response can be observed for up to a year (Haren et al, 2005). Morley (2007) further advanced this notion by suggesting that the diagnosis of TDS requires the presence of symptoms, a low level of serum T, and a positive response to treatment. The concept was further supported by a recent set of multidisciplinary recommendations (see Recommendation 4 later; Wang et al, 2009).
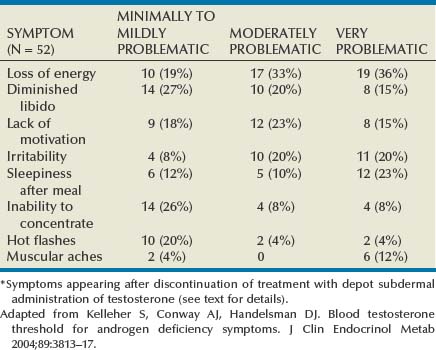
The clinical picture is well recognized (see Table 29–1). Among the most prominent symptoms are tiredness, hypoactive sexual desire, and dysphoria. Erectile dysfunction (ED) is, however, the most frequent reason for consultation. These manifestations generally correlate with the degree of T deficiency (Zitzmann et al, 2006) but need not all be present to identify the syndrome. In addition, the severity of one or more of them does not necessarily match the severity of the others, nor do we yet understand the uneven appearance of these manifestations. TDS symptoms recur after discontinuation of therapy at highly reproducible intraindividual T levels, but the trigger level differs widely among individuals (Table 29–2) (Kelleher et al, 2004). The increasingly recognized association of TDS and ED and cardiovascular disease (Billups et al, 2008) has brought to the forefront of clinical practice the need for a more in-depth assessment of men presenting with TDS-ED (Corona et al, 2009).
Screening Questionnaires for TDS
Three questionnaires have been developed to detect hypogonadism in adult men: (1) St. Louis University’s ADAM, (2) the Aging Male Survey (AMS), and (3) the MMAS. The first two are symptom questionnaires, and the last one represents a mixture of symptoms and epidemiologic findings. The ADAM questionnaire (Table 29–3) in the original validation had a sensitivity and specificity of 88% and 60%, respectively, against serum bioavailable T (BT) levels (Morley et al, 2000). In a follow-up study, BT and calculated free T (cFT) were lower in ADAM-positive men than in ADAM-negative men. The effectiveness of the ADAM questionnaire as a screening tool has been confirmed by most independent investigators (Tancredi et al, 2004). The AMS (Fig. 29–2) (Moore et al, 2004), in the author’s hands, has sensitivity (83%) and specificity (39%) similar to those of the ADAM, but in a European study none of the three AMS domains correlated significantly with serum levels of total, bioavailable, or free T in men older than 70 (T’Sjoen et al, 2004). In contrast, the MMAS showed a sensitivity of 60% and a specificity of 59%. These questionnaires are useful as screening tools but fall short of expectation for diagnostic purposes. Their value as outcome measures after treatment with T remains undetermined (Morales et al, 2007).
Table 29–3 The Androgen Deficiency in Aging Male (ADAM) Questionnaire
| Yes | No | 1. Do you have a decrease in libido (sex drive)? |
| Yes | No | 2. Do you have a lack of energy? |
| Yes | No | 3. Do you have a decrease in strength and/or endurance? |
| Yes | No | 4. Have you lost height? |
| Yes | No | 5. Have you noticed a decreased enjoyment of life? |
| Yes | No | 6. Are you sad and/or grumpy? |
| Yes | No | 7. Are your erections less strong? |
| Yes | No | 8. Have you noticed a recent deterioration in your ability to play sports? |
| Yes | No | 9. Are you falling asleep after dinner? |
| Yes | No | 10. Has there been a recent deterioration in your work performance? |
If you answered Yes to questions 1 or 7 or any 3 other questions, you may be experiencing androgen deficiency (low testosterone level).
Biochemical Diagnosis
T values decline while SHBG levels increase with aging. In young men, about 60% of circulating T is bound to SHBG, 38% is bound to albumin, and 1% to 2% is free (see Fig. 29–1). The T that is free and bound to albumin is considered to be capable of entering tissues and to be responsible for the actions of T. SHBG-bound T is inaccessible to tissues and not active. In some tissues, such as the prostate, T bound to SHBG can activate SHBG receptors and produce effects within the cell (Rosner et al, 1999). The active component (free plus albumin bound) is called bioavailable T (BT). Epidemiologic studies suggest that BT correlates better with symptoms associated with T deficiency than total T.
Assays for Testosterone
Key Point
Biochemistry
Although testosterone measurements should be restricted to morning hours at all ages (Brambilla et al, 2009), the importance of repeated measurements when the values are not conclusive cannot be emphasized enough. The biologic variation of repeated measurements of T is about 20%. Values between 250 and 360 ng/dL (9 to 12 nmol/L) are considered “borderline.” In the presence of a clear picture of TDS, a therapeutic trial of 3 months is justified (see Fig. 29–3).
The urologist should become familiar with the range and quality of the T assays offered by the local laboratory as well as the intraindividual and interindividual variations in serum T levels. Assay techniques have improved greatly, but many laboratories have paid scant attention to the accuracy or the normal range of their assays. Three assays for measurement of total T are commonly used: (1) radioimmunoassay, (2) nonradioactive immunoassay kits, and (3) automated platform assays that use chemiluminescent detection. There is significant interassay variability: a value of 297 ng/dL (10.3 nmol) showed a variation from 160 to 508 ng/dL (5.5 to 17.6 nmol). In addition, the coefficients of variation ranged from 5.1% to 22.7%. Fortunately, most of the assay techniques had regression slopes close to 1 and correlation coefficients of 0.92 to 0.97 compared with liquid chromatography/tandem mass spectroscopy (Wang et al, 2004a).
In young men, but much less in older men, there is a substantial circadian rhythm, with higher values being obtained in the morning. In addition, in all men there is substantial variability (±20%) from week to week (Morley et al, 2002). Thus, two samples obtained a week or two apart, ideally in the morning, would seem to be a minimal criterion. The assays that more closely reflect tissue available T are (1) free T only if measured by ultracentrifugal ultrafiltration or dialysis techniques, (2) BT, generally measured by an ammonium sulfate precipitation technique, and (3) calculated free T or BT that can be obtained by measuring T and SHBG with or without an albumin level (see later).
Assays for BT and free T are cumbersome, costly, and not readily available. Vermeulen and colleagues (1999) provided a solution for situations in which the more reliable BT or free T determinations are not accessible: a calculation can be made from the values obtained for total T and the determination of SHBG. It has become known as calculated free T, or simply cFT. This method provides a good correlation with the values of nonbound T. (The formula is available at http://www.issam.ch.) Although this approach, in general, works well, there are pitfalls: (1) different SHBG levels varying up to twofold with different assays and (2) altered binding characteristics (either SHBG or serum) related to aging or illness. The relative advantages, drawbacks, and usefulness of the various assays are given in Table 29–4.
Table 29–4 Assays for Testosterone and Their Attributes
| ASSAY | UTILITY | COMMENTS |
|---|---|---|
| Total testosterone | Low/intermediate | Variable normal ranges; below 200 ng/dL very likely to be hypogonadal; above 600 ng/dL unlikely to be hypogonadal |
| Free testosterone | ||
| Dialysis | High | Difficult to do; requires 3H-T |
| Ultrafiltration | High | |
| Analog | Poor | Commonly available in NA |
| Calculated free | Intermediate | Requires SHBG and T measurements |
| Bioavailable testosterone |