Chapter 95 Adjuncts to hepatic resection
Ultrasound and emerging guidance systems
Overview
Hepatic surgery performed without a parenchyma-sparing policy carries relevant risks for patient survival because of postoperative liver failure; for this reason, the application of hepatic surgery is limited (Vauthey et al, 2004). In particular, the coexistence of liver cirrhosis in most patients with hepatocellular carcinoma (HCC) has a considerable adverse effect on the surgical results (see Chapter 80), and recent series are still associated with mortality rates higher than 5% (Liu et al, 2004) and with resectability rates of 30% to 35%, which is relatively low (Sotiropoulos et al, 2006). For this reason, and for the broadening of ultrasound (US)-guided percutaneous therapies (N’Kontchou et al, 2009), the surgical treatment of HCC has surrendered its role as first choice treatment for all patients and is now reserved only for those patients with a normal bilirubin level, no signs of portal hypertension, and a single, small HCC (Bruix & Llovet, 2009).
Conversely, for colorectal cancer liver metastases (CLM), surgical treatment remains the gold standard (see Chapter 81A). The definition of resectability has shifted from a focus on tumor characteristics, such as tumor number and size, to determination of whether both intrahepatic and extrahepatic disease can be completely resected, and whether such an approach is appropriate from an oncologic standpoint. Therefore limiting surgery in the case of CLM has increasingly focused on the technical feasibility, although not entirely (Minagawa et al, 2000; Torzilli et al, 2009b). In this sense, progress with chemotherapeutic regimens has improved the resectability rate (Wicherts et al, 2008), although it remains generally around 15% to 25% of patients (Garcea et al, 2008). A major limit of resectability is the need for major hepatectomies, which still represents a significant factor impacting the risk of perioperative mortality and morbidity (Schroeder et al, 2006; Cucchetti et al, 2006). Portal vein embolization (PVE) was devised with the double aim of increasing the safety of major liver resection and allowing more patients to benefit from the surgical treatment (Jaeck et al, 2004; see Chapter 93A, Chapter 93B ). However, liver failure occurs in 33% of patients who did not undergo PVE, and it still happens in 10% of those who did (Hemming et al, 2003). As with HCC, alternative therapies have been devised for treating CLM, such as percutaneous interstitial treatment (Siperstein et al, 2007). Although these new therapies initially enabled patients otherwise excluded from the surgical program to be treated, the apparently good results obtained have generated confusion in the scientific community as to which treatment modalities should be chosen to manage these patients.
Imaging techniques have been introduced as aids for surgeons in performing liver resection. In fact, since the early 1980s, intraoperative ultrasonography (IOUS) has been used in hepatic surgery, initially in patients with liver cirrhosis (Makuuchi et al, 1980; see Chapter 21). Now liver resections can be carried out with very low mortality, even if cirrhosis is present (Imamura et al, 2003; Torzilli et al, 1999a). There are many reasons for this improvement, but a large part can be attributed to better operative technique, including IOUS. Indeed, US guidance allowing the so-called radical but conservative policy (Torzilli et al, 2005a, 2006b) makes feasible an alternative to major hepatectomies, having the aim of reducing the need for major parenchymal removal while keeping an acceptable oncologic radicality. This policy has allowed resectability rates from outpatient evaluation to hepatectomy of 68% and 69% for HCC and CLM, respectively (Torzilli et al, 2008b, 2009b). Recently, the demonstration of feasibility and efficacy of contrast-enhanced US performed intraoperatively (CE-IOUS) has furthermore stressed the relevance of IOUS guidance during liver surgery (Torzilli et al, 2007b, 2008a).
Technical Aspects
Probe Selection
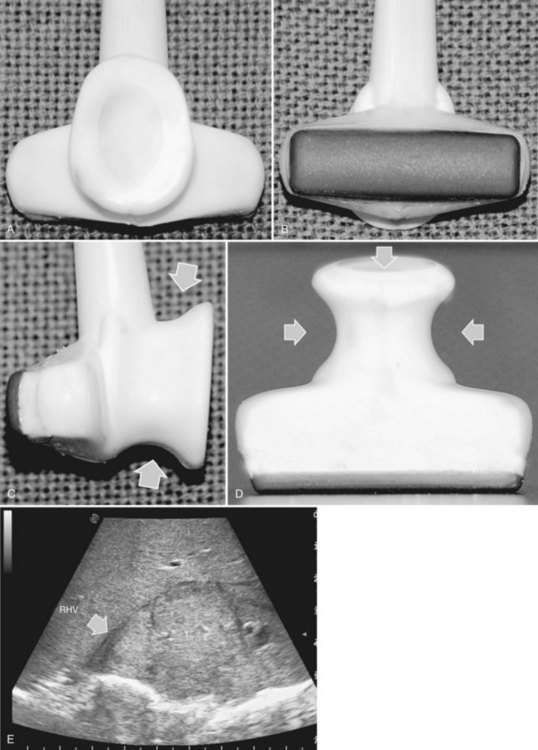
High-frequency (7.5 to 10 MHz) echoprobes are commonly recommended to perform IOUS, because they allow a higher spatial resolution than those that operate at lower frequencies (3.5 to 5 MHz); however, the latter are also useful, at least along the initial exploration, providing a better panoramicity that helps compensate for the lower spatial resolution (Fig. 95.1). On the other hand, it should be pointed out that often higher spatial resolution at the superficial portion of the liver is less important than the overall visibility of the deeper structures, because the most superficial portions are also those appreciable with palpation. In the latter condition, once a nodule is slightly visible at IOUS but is not palpable, especially in a cirrhotic liver, a surgical glove filled with deaerated sterile water can be positioned between the probe and the liver surface for making the lesion more visible (Fig. 95.2). Lower frequency echoprobes are also useful for allowing CE-IOUS; therefore, having both low- and high-frequency probes would be the optimal solution, and new probes with wider ranges of frequencies (3.5 to 10 MHz) are expected.
The most frequently used probes are the T-shaped (Fig. 95.3), interdigital, and microconvex probes (Fig. 95.4). The microconvex probe represents the best compromise among all the aforementioned requirements. Indeed, the T-shaped probe, although it remains more stable and is associated with higher image resolution, has a lower ratio between lateral length and US scanning window than the microconvex probe. Linear transducers with enlarged scanning windows are under development that combine the stability of the linear probes and their higher image resolution with larger scanning windows and a limited volume (Fig. 95.5). When evaluating probes, another aspect that should be considered is the possibility of using them for surgical maneuvers, as described below; newer probes are being designed from this perspective (see Fig. 95.5).
Color Doppler imaging, and particularly new, more sensitive color-flow modes, are progressively assuming greater relevance in hepatic surgery. For example, e-flow intraoperative US (EF-IOUS) is useful for studying the inflow and outflow of the liver intraoperatively (Fig. 95.6), and it shows the flow modifications during surgical maneuvers; this represents crucial data for allowing surgical strategies that would not be feasible otherwise. These promising new technologies will be discussed in this chapter.
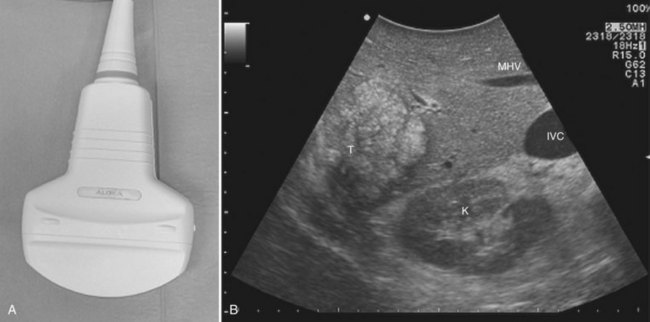
CE-IOUS is one of the latest advancements in intraoperative application of US, but dedicated probes built to carry out this diagnostic modality in direct contact with the targeted organ are few; furthermore, CE-IOUS requires high-quality digital US machines. We use the Alpha 10 (Aloka, Tokyo; Fig. 95.7), which is equipped with a convex probe working at 3- to 6-MHz frequency and at 1.88- to 3.76-MHz harmonic frequency (see Fig. 95.1). The contrast agent used, SonoVue (Bracco Imaging, Milan, Italy), consists of sulfur hexafluoride microbubbles stabilized by a phospholipid shell; the anesthesiologist injects 4.8 mL of SonoVue intravenously for exploration through a peripheral vein. The purpose of the contrast enhancement is to evaluate the lesion’s vascularity, aid in the characterization of those eventually found at IOUS, and to detect new ones, taking advantage of the enhanced brightness of the liver parenchyma, especially along the delayed phases (Fig. 95.8). New liver-specific contrast agents, such as those containing perfluorobutane (Sonazoid, Daiichi Sankyo, Tokyo), will soon become commercially available for clinical use; however, initially, these are being used clinically and only in Japan. This contrast agent, which has properties similar to liver-specific contrast medium used in magnetic resonance imaging (MRI), could improve nodule differentiation (Kudo, 2007) and detection (Nakano et al, 2008).
Preparation for Liver Exploration
The US machine should be positioned opposite the first operator, who should be able to simultaneously view both the screen and the operative field (Fig. 95.9). The screen must be large enough to allow optimal visibility at that distance, and lights should be positioned with caution so as not to interfere with the visibility of the US screen. An assistant familiar with the US keyboard should stay beside it, or a transparent and sterile covering pad should be available that allows the lead member of the surgical team to handle the keyboard directly (see Figs. 95.7 and 95.9).
After entering the abdominal cavity, liver mobilization, division of the round and falciform ligaments, and division of adhesions to free the anterosuperior and inferior surfaces of the liver are the steps that should precede liver exploration with IOUS (Fig. 95.10). Of course, adhesions from the tumor to other organs or structures should not be approached, as they may represent areas of tumor infiltration; in this situation, IOUS can help exclude or confirm tumor invasion, which could change the surgical strategy.
Ultrasound Liver Anatomy
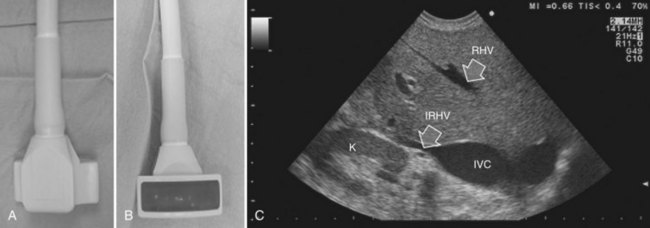
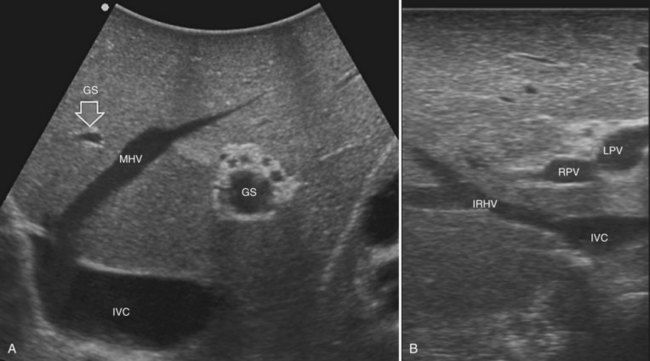
Solid knowledge of the liver anatomy is required to perform IOUS properly (see Chapter 1B). For surgical anatomy, the Brisbane terminology is considered here (Pang, 2002). The three main hepatic veins are readily identified at their junction with the inferior vena cava (IVC), positioning the probe at this level and tilting it upward once the confluence of the hepatic veins into the IVC is recognized. Then, gently withdrawing the probe, the hepatic vein paths can be traced into the liver. Hepatic veins appear as echo-free zones in the liver parenchyma, and the vessel wall is invisible or appears as a thin, hyperechogenic line (Fig. 95.11A); the walls of hepatic veins can be thicker in the cirrhotic liver, and their lumens thinner, as a result of the stiffness of the diseased liver (Fig. 95.11B).
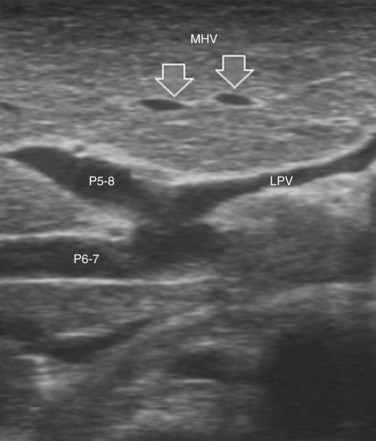
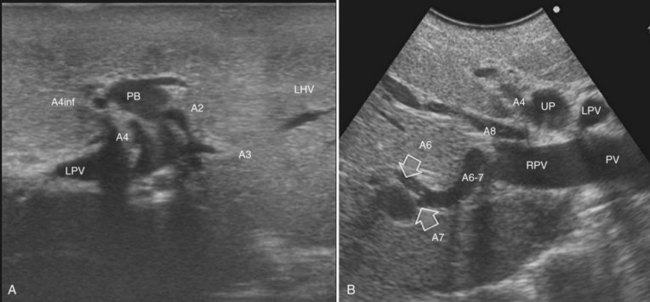
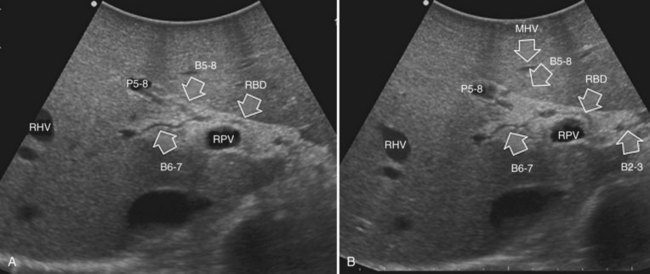
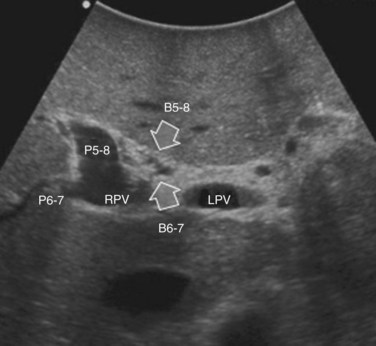
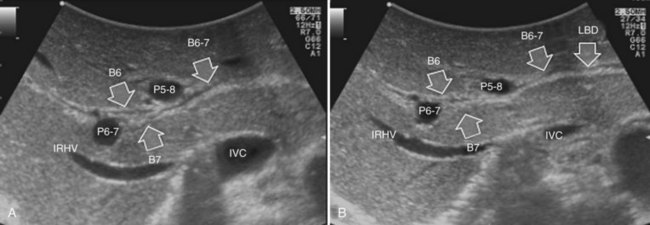
The portal vein branches can be followed by initially positioning the probe horizontally above the inferior portion of segment IV (see Fig. 95.10) to visualize the first-order bifurcation (Fig. 95.12), then the first-, second-, and third-order portal branches can be followed. Because of the existence of the Glisson capsule, the portal pedicles—which include portal vein branches, arteries, and bile ducts—have thicker vessel walls compared with the hepatic veins, and for this reason, they appear at IOUS as echo-free zones surrounded by a thicker hyperechogenic layer (see Fig. 95.11); furthermore, other parallel, thinner vascular structure are visible, which are the arteries (Fig. 95.13), and the bile ducts of the glissonian triad are also visible (Fig. 95.14). The newer, more sensitive color-flow modalities, such as EF-IOUS, help in this differentiation by allowing the recognition of arterial and portal elements that also lie within thin glissonian sheaths (Fig. 95.15); however, in principle, distinction between hepatic veins and portal branches should be based not only on their appearance but mainly on their anatomy. Indeed, in the cirrhotic liver, as already mentioned, the vessel wall of the hepatic vein could be thicker and not easily differentiated from a peripheral portal branch. Following the portal pedicles at the sectional, segmental, and subsegmental level, and positioning them in relation to the hepatic veins, it is possible to precisely define the location of the IOUS target in terms of sections and segments (Fig. 95.16).
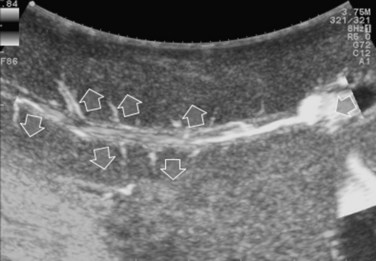
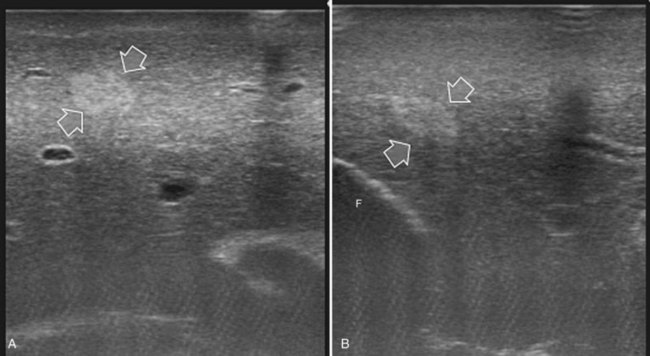
The appearance of the bile ducts on IOUS is worth mentioning because of their peculiarity. Whereas normally they result as thin echo-free zones in the glissonian triad (see Fig. 95.14), once dilated, they appear as more evident echo-free zones with a serpiginous path (Fig. 95.17). The element that is difficult to recognize in the IOUS study of the bile ducts is their segmental anatomy; bifurcation of sectional and segmental ducts is closer to the hilum, compared with the portal branches (Fig. 95.18), and because of that, it is possible to visualize more than one segmental bile duct with one scan; however, with enough US background, IOUS can allow exact definition of the bile duct anatomy, not only in pathologic conditions but also in the normal state. This possibility also allows assessment of variations in the normal anatomy, such as confluence of the right posterior sectional bile duct into the left hepatic duct (Fig. 95.19), which is clearly critical information, particularly if a left hepatectomy is planned.
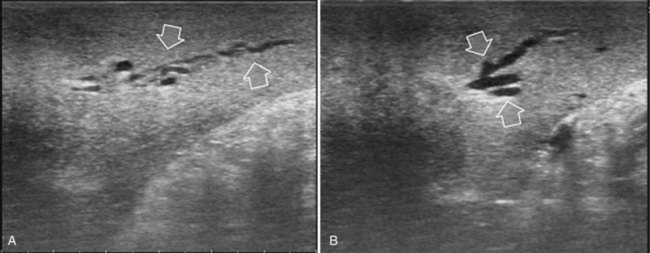
FIGURE 95.17 A and B show the dilation of segmental bile ducts, which assume a serpiginous path (arrows).
Indications
Liver Exploration
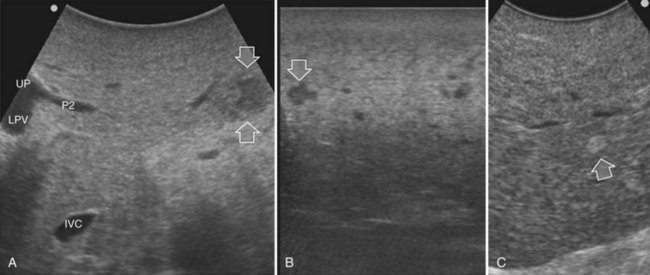
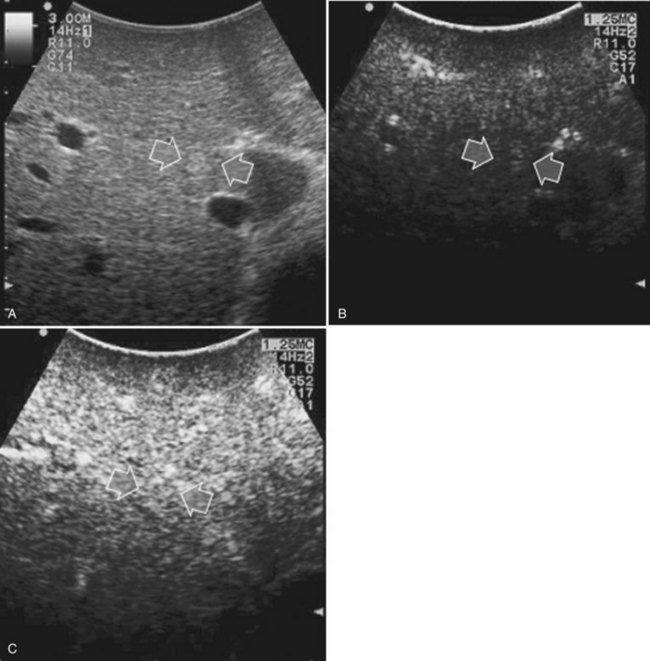
Detection and differentiation are the two main targets of IOUS exploration of the liver. The hard and irregular surface of cirrhotic livers makes difficult the detection of small nodules by palpation; conversely, IOUS allows the detection of new lesions in around 30% of cases (Kokudo et al, 1996), although most of the nodules detected by IOUS in the cirrhotic liver are not tumors but more often are regenerative nodules. In this way, IOUS introduces the risk of overestimating tumor stage (Fig. 95.20). Indeed, except for those nodules with a mosaic pattern evident on US (Fig. 95.21A), which are malignant in 84% of cases, only 24% to 30% of hypoechogenic (dark) nodules (Fig. 95.21B) and 0% to 18% of those hyperechogenic (bright) nodules (Fig. 95.21C) are neoplasms (Kokudo et al, 1996; Takigawa et al, 2001).
To overcome this problem, even biopsy seems to be inadequate. The only nodule that can be easily differentiated intraoperatively from HCC or liver metastases is the small hemangioma, often discovered primarily at IOUS (see Chapter 79A, Chapter 79B ); it displays a typical ultrasonographic pattern, and when compressed, it changes in size and appearance (Fig. 95.22; see Chapter 13). The problem of differentiating benign from malignant lesions on IOUS exploration becomes crucial. Some attempts are now ongoing using elastography, which should allow lesion differentiation based on tissue stiffness expressed on the IOUS screen and indicated by different colors (Fig. 95.23); however, to date, limited data are available to describe its use in this application. In the next section, it will be shown how CE-IOUS will play an increasingly important role in this context and will be even more relevant in the near future.
Rather than differentiation, in the case of CLM, their clue is their accurate detection, particularly in the event of multinodular hepatic involvement. Indeed, in patients with CLM, the detection of any tiny nodule undiscovered preoperatively becomes crucial for attempting a reduction of the still high postoperative early recurrence rate (Yoshidome et al, 2008). From 10% to 40% of patients with colon cancer have nonpalpable CLM (Agrawal et al, 2006; Machi et al, 1991); as a consequence, IOUS exploration of the liver remains crucial, and CE-IOUS seems able to enhance its role in this context.
Contrast-Enhanced Intraoperative Ultrasonography
Hepatocellular Carcinoma
CE-IOUS is now used for characterizing the new lesions of HCC eventually detected at IOUS (Torzilli et al, 2007b). The rationale is to check the vascular pattern during contrast enhancement of each new lesion. Because it is very important to identify the arterial vascularization in HCC, which can take 20 to 30 seconds, each nodule must be carefully evaluated, and this necessity demands multiple injections in the presence of multiple nodules. This requirement may be obviated by the new liver-specific contrast agents. Using these, the contrast enhancement remains visible from several minutes to even hours after injection, and CE-IOUS should become easier to use and will likely be more effective for detection of HCC.
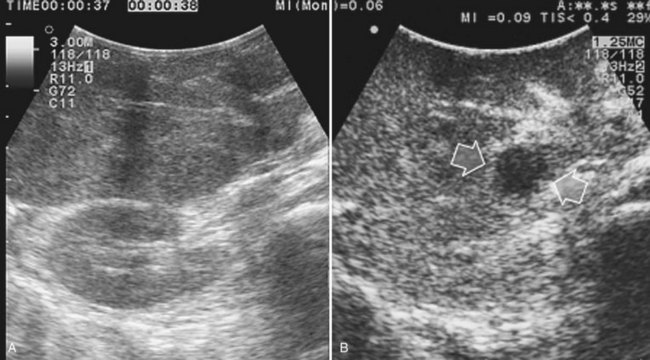
Tumor vascularity as a criterion for differentiating regenerative or dysplastic nodules from HCC correlates with the histologic evidence of a progressive increase in unpaired arteries from dysplastic to neoplastic nodules in a cirrhotic liver (Roncalli et al, 1999). Certainly, the pattern of vascular enhancement by itself is not sufficient for differentiating malignant from nonmalignant nodules with 100% specificity. Indeed, although percutaneous contrast-enhanced US (CE-US) provides differential diagnosis of hepatic nodules based on their vascular pattern with 95% specificity (Quaia et al, 2004), intraoperatively there is the need to differentiate lesions smaller than 1 cm. For these nodules, the vascularity as a criterion for differential diagnosis is less specific, although some improvements compared with conventional IOUS could be expected. Therefore, in the early 1990s, attempts were made to apply CE-IOUS using carbon dioxide as a contrast material, but the need for arterial catheterization made this technique too invasive (Takada et al, 1990). In my preliminary experience, CE-IOUS has provided remarkable findings, either by adding information on nodular vascularity in patients with HCC, or by detecting nodules that were not visible at IOUS, in patients with CLM (Torzilli et al, 2004).
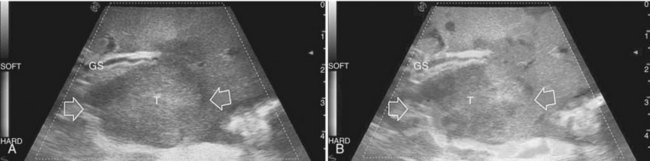
For patients undergoing operation for HCC, my colleagues and I have introduced a classification of the enhancement pattern of lesions seen at CE-IOUS, upon which the surgical decision making is established (Fig. 95.24; Torzilli et al, 2007b). In brief, any pathologic lesion should appear as hypoechogenic at the late phases, with or without inner vessels and with or without an arterial phase, in which it is enhanced prior to the remnant liver parenchyma. Lesions with this type of appearance should be removed (Fig. 95.25). Those lesions that disappear once the contrast enhances the liver are not considered neoplastic and are not removed (Fig. 95.26). With these criteria, we obtained with CE-IOUS a specificity of 69% (Torzilli et al, 2007b). This value is probably not that high, especially when compared with that reported for CE-US (Quaia et al, 2004); however, as mentioned before, the small size of the lesions targeted for CE-IOUS study could explain this discrepancy. For these tiny nodules, the neovascularity as a criterion for differentiation between malignant and benign lesions has limits that are independent from the method we use; therefore CE-IOUS can be helpful in a certain percentage of nodules but not in all, although the rate of 69% specificity is encouraging, as it means that we can provide proper information with this new technique in 7 of 10 lesions we detect at laparotomy. For those lesions remaining, even histology may be lacking, as we know no common agreement exists among Western and Eastern pathologists on the definition of early HCC and dysplastic lesions (International Consensus Group for Hepatocellular Carcinoma, 2009). New perspective may be provided by a more extensive use of the new contrast agent, for now clinically available only in Japan (Kudo, 2007). This agent (Sonozoid) has a Kupffer phase, and it adds further criteria for differentiating those nodules detected at IOUS.
Colorectal Liver Metastases
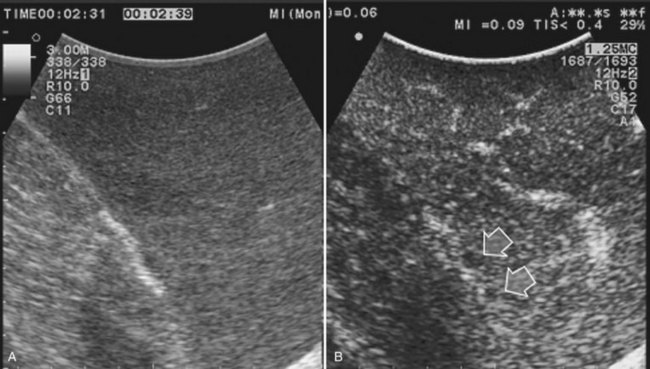
Improving detection is the main target of CE-IOUS in the case of CLM. In the 1990s, half of the patients who underwent surgery for CLM had a surgical strategy modified by IOUS findings (Kane et al, 1994). More recently, progress in preoperative imaging has reduced this rate; in fact, some authors have reported that just 4% of the operative decision making is modified by IOUS (Sahani et al, 2004). Conversely, adding CE-IOUS to IOUS exploration modified operative decision making in 38% of patients with CLM (Torzilli et al, 2008a). If this discrepancy in the rates of modified surgical strategy among series is partially the result of different surgical approaches, particularly more parenchyma-sparing procedures, then probably CE-IOUS is playing a role, too. Indeed, with CE-US, the CLM shows the so-called “black hole” effect (Fig. 95.27). The metastastic nodule in the late phase, which lasts from 2 to 5 minutes after injection, remains unenhanced and black in comparison with the surrounding enhanced liver parenchyma (Fig. 95.28). This is particularly useful in those patients who undergo surgery after chemotherapy, in whom some nodules may not be well visualized by IOUS because of the histologic rearrangements induced by the chemotherapeutic agents. CE-IOUS allows better nodule visibility and has allowed my colleagues and me to detect 9% of adjunctive nodules (Torzilli et al, 2008a); therefore CE-IOUS application resulted in an increased sensitivity, and in patients with CLM, it appears to have an important role. The only condition under which it may not help is in the event of a bright liver at IOUS (Chen et al, 2008), which correlates with the degree of fatty change and accounts for 10% of our patients (Torzilli et al, 2008a). Indeed, in these circumstances, the visibility of CLM, generally hypoechogenic, is enhanced by the brightness of the surrounding liver parenchyma, mimicking the effect of the contrast enhancement (Fig. 95.29). As a confirmation of this, we never found new sites of disease at CE-IOUS in those patients having a bright liver at IOUS.
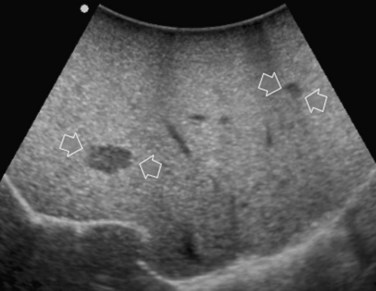
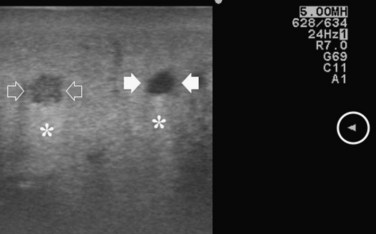
FIGURE 95.29 Clear visibility of two hypoechoic masses (arrows) in a so-called bright (fatty) liver.
For CLM, new perspectives could be obtained by using liver-specific contrast agents, which allow prolonged exploration. A preliminary experience of eight patients showed new lesions in two detected only at CE-IOUS (Nakano et al, 2008).
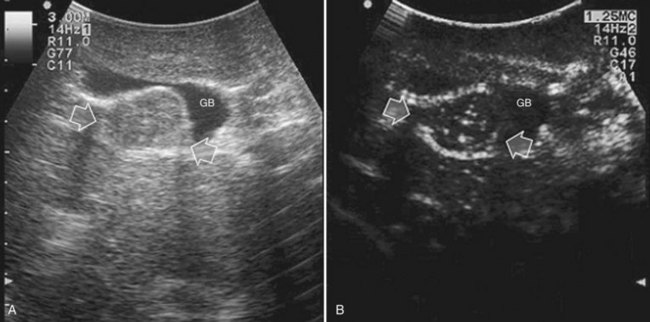
Care should be used in CLM patients who also have coexisting liver cysts, which may appear similar to the CLM along the delayed phases of contrast enhancement; however, the cysts should have been already mapped based on the preoperative imaging and should have been identified at exploration with conventional IOUS. Therefore, any new “black hole” detected in the liver in locations other than where cysts were eventually detected should be considered suspicious for malignancy. Liver cyst features at unenhanced IOUS have an anechoic content and a posterior enhanced echo (Fig. 95.30; see Chapter 13), and it must be taken into account that the anechoic pattern could be mimicked by tiny CLM (Fig. 95.31).
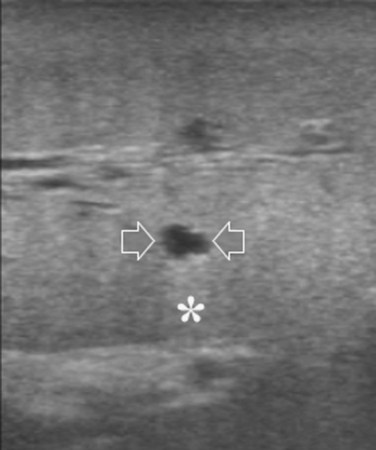
FIGURE 95.30 A small, simple hepatic cyst (arrows) with its typical posterior echo (asterisk) and the echo-free (black) content.
Other Intraoperative Techniques
Recently, imaging using indocyanine green (ICG) as a fluorescent source has been proposed (Ishizawa et al, 2009; Gotoh et al, 2009). Used in many centers to assess liver function, ICG is injected intravenously approximately 3 days before surgery and during laparotomy, and the liver is explored to detect any sign of fluorescence on its surface by means of a fluorescent imaging system that comprises a control unit and a camera. Once removed, the specimen is explored to detect undisclosed fluorescent areas. With this device, Ishizawa and colleagues (2009) disclosed 13 of 91 lesions with only an ICG fluorescent-imaging technique, obtaining a sensitivity of 100% for HCC and 93% for CLM. Although in a smaller series that included only patients with HCC, Gotoh and colleagues (2009) similarly showed that all the new lesions detected in the examined patients were visible only because of the ICG fluorescent-imaging technique.
Planning of the Surgical Strategy
IOUS exploration of the liver could have a significant impact on the surgical strategy. More recently, impact of IOUS on the operative decision making, when compared with that of preoperative imaging techniques, is reported to be around 4% to 7% (Cerwenka et al, 2003; Jarnagin et al, 2001). The problem of the impact of IOUS on the operative decision making depends on two main factors: the surgical policy of each specific team and the type of tumor. Indeed, the relatively low rates reported (Cerwenka et al, 2003; Jarnagin et al, 2001) are also influenced by the surgeon’s resection policy; when a considerable number of patients undergo major hepatectomies, new nodules detected by IOUS in the same hemiliver would still be resected, and the surgical strategy would not be modified.
Recently, it was shown that major hepatectomies are carried out in a minority of patients, even in extremely complex presentations (Torzilli et al, 2009b), simply because of the extensive use of IOUS guidance for achieving parenchyma-sparing resections; from this perspective, detection of new nodules is more likely to change the surgical strategy.
IOUS allows an accurate three-dimensional reconstruction of the relationship between the tumor, the portal branches, and the hepatic veins, which is a fundamental step in defining the proper surgical strategy. Definition of tumor-vessel relationships is relevant for planning the type of resection (Torzilli et al, 2008c, 2010a). IOUS allows the surgeon to easily recognize whether an HCC is separated by some normal parenchyma from the vessel, or if it is in contact with the vessel without invading its wall, or conversely whether the HCC is invading the vessel wall or is determining the proximal bile duct dilation, or if it is associated with a tumor thrombus. Similarly, definition of the relationship between a CLM and intrahepatic vascular structures is precisely defined by IOUS; once the vessel wall is found to be intact, the circumferential extent of the contact is relevant for planning a resection that spares the vessel in contact with the CLM (Torzilli et al, 2009b). Based on these features, the decision whether the vessel should be resected is considered, and a precise surgical strategy can be pursued.
Tumor in Contact with a Glissonian Pedicle
The glissonian pedicle may be spared when in contact with an encapsulated HCC with integrity of the vessel wall appreciable at IOUS without any sign of bile duct dilation (Fig. 95.32
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree