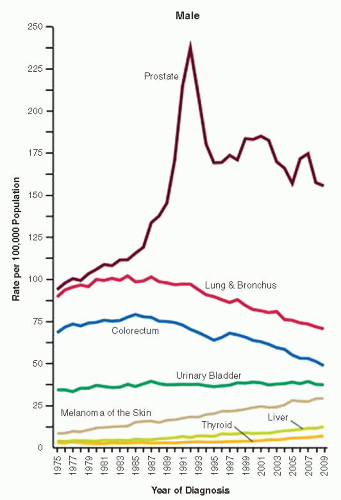
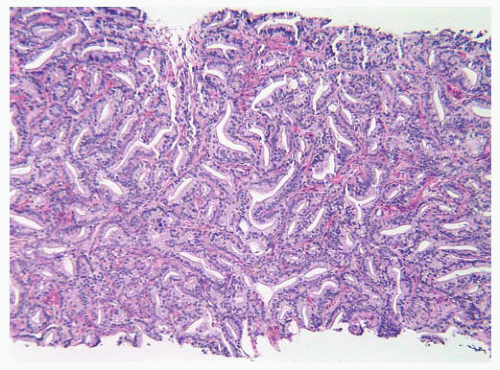
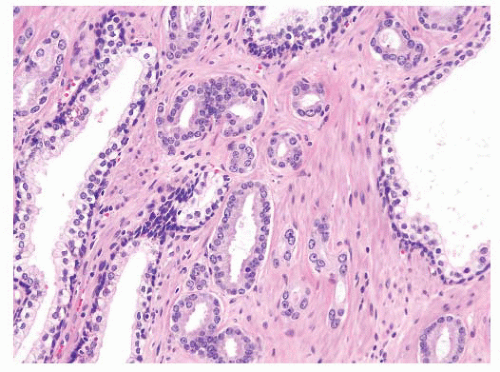
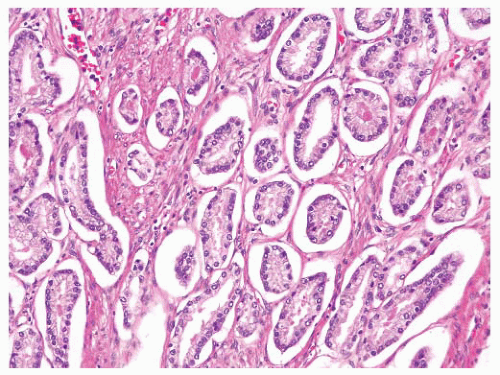
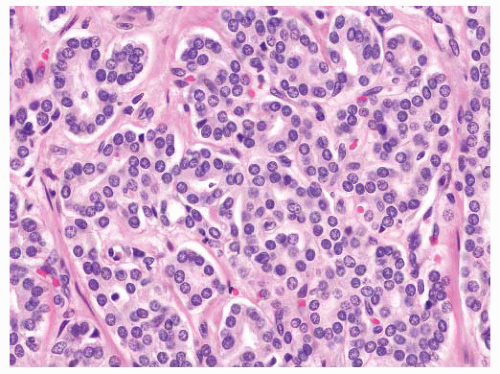
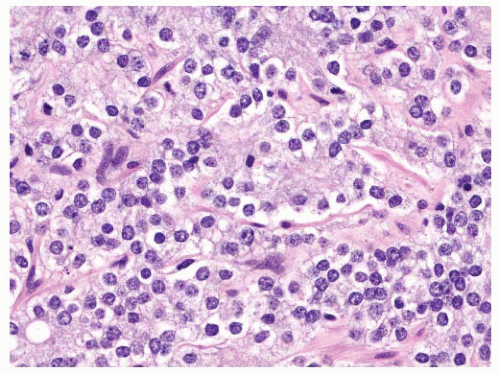
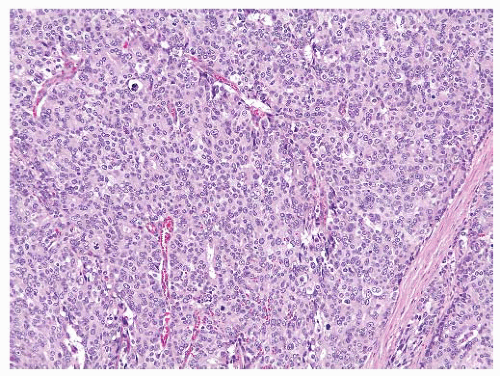
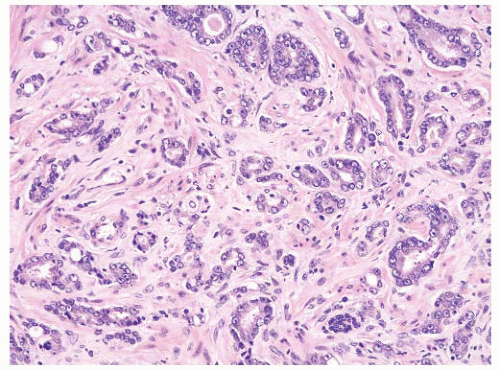
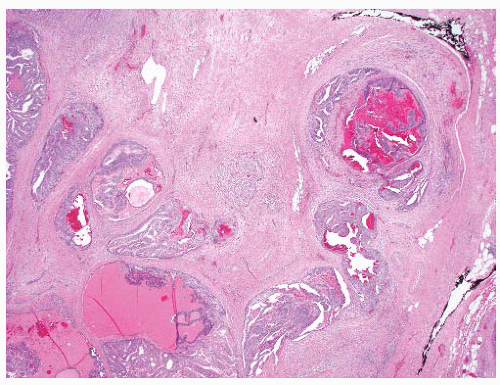
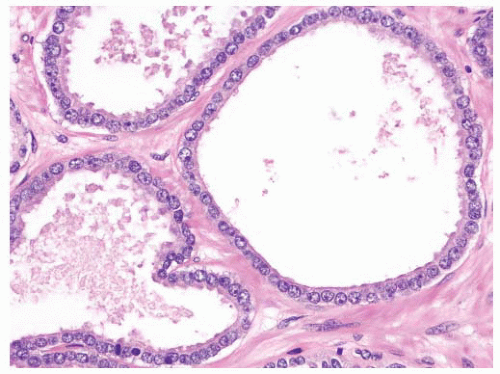
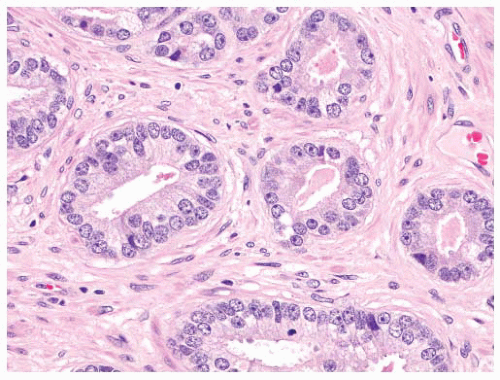
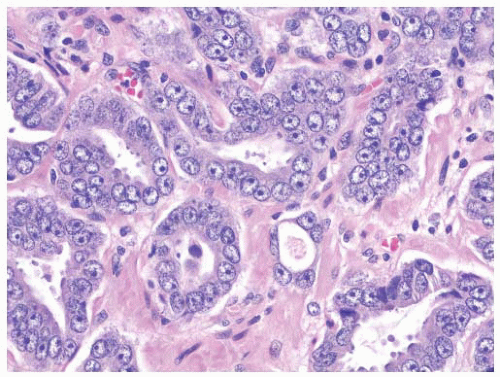
the percentage prevalence of colorectal cancer (9%) and melanoma of the skin (7%).13 United States statistics for 2009 indicate that there were approximately 2,496,784 men alive who had a history of prostate cancer.3
Table 9-1 ▪ AGE OF DIAGNOSIS OF PROSTATE CANCER | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
Table 9-2 ▪ PROSTATE CANCER INCIDENCE BY RACE | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||
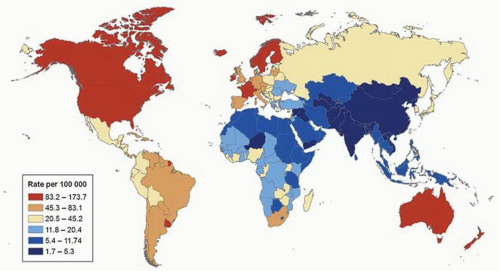
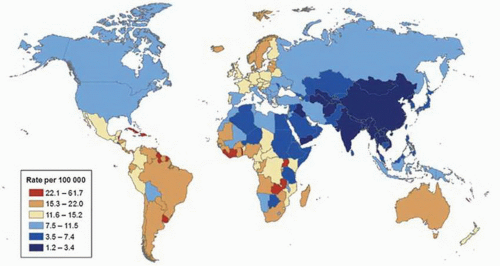
mortality rates are observed in most parts of Asia, northern Africa, and North America (Fig. 9-2).17 Differences overall are less marked for mortality compared to incidence, but are high in Trinidad and Tobago (53.6 per 100,000 men), which are twice the rate of second place Cuba (22.6 per 100,000 men) and 25 times that of lowest place Uzbekistan (1.6 per 100,00 men). During the past several years, prostate cancer mortality rates have been decreasing in many countries, but also increasing in some nations.17 High average increases in mortality rates occurred in Korea (7.8% per year), Moldova (6.5% per year),
and Trinidad and Tobago (4.5% per year), whereas high average decreases occurred in the United States (−4.3% per year), Austria (−4% per year), and Israel (−3.7% per year).
Table 9-3 ▪ PROSTATE CANCER MORTALITY BY RACE | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||
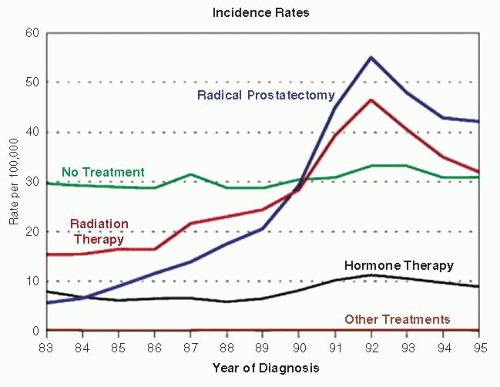
is indirectly linked as a reason for decrease prostate cancer mortality. However, this is currently controversial, as recent large randomized PSA screening studies for prostate cancer in the United States did not show benefit and, in Europe, only 20% reduction in mortality.10,11 Further, decline in prostate cancer mortality was observed even in countries with low PSA screening such as United Kingdom.20
the highly prevalent metabolic syndrome including central obesity, insulin resistance, dyslipidemia, and hypertension is suggested to have an association with prostate cancer.45
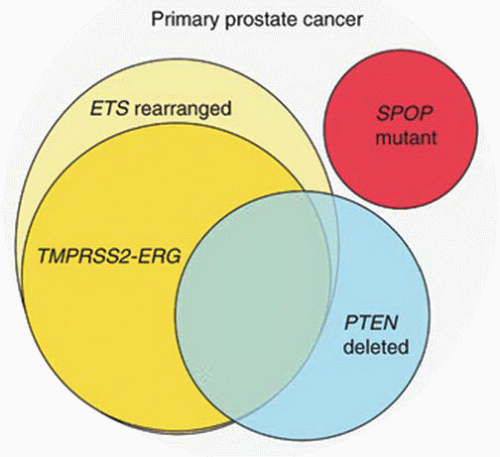
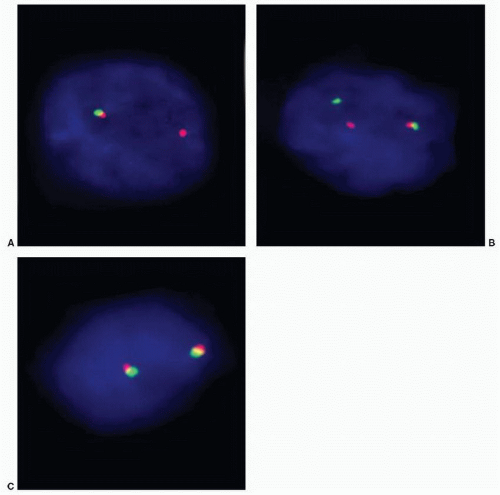
ETS transcription factors.95,96 ETS family of transcription factors includes ERG, ETV1, ETV4, and ETV5 as 3′ end fusion partners, and ERG is the most commonly fused gene at 5′ end with TMPRSS2 comprising about 90% of cases. Several other 5′ end fusion partners of ETS gene have been identified (Table 9-5). With fusion, ERG is brought under the control of an androgen-regulated promoter causing overexpression. Fusion of the two genes occurs by intra- and interchromosomal genetic rearrangements. In about two-thirds of cases, fusion results from deletion of the intervening 3 Mb between TMPRSS2 and ERG (Fig. 9-6).94 Fusion may also occur by more complex rearrangements such as translocation. The most commonly reported fusion transcript is between exon 1 of TMPRSS2 and exon 4 of ERG. About 20 TMPRSS2:ERG transcripts have now been identified. Morphologic features of prostate cancer associated with TMPRSS2:ERG include blue-tinged mucin, cribriform pattern, intraductal spread, macronucleoli, and signet ring cells.97 Only 24% of tumors without any of these features displayed TMPRSS2:ERG, whereas 93% of cases with three or more features harbor the fusion.97 The clinical significance of this gene fusion on prostate cancer behavior is not yet fully understood, and studies correlating it with outcome and pathologic variables have conflicting results.98, 99, 100, 101, 102, 103, 104, 105, 106, 107
Table 9-4 ▪ MUTATED GENES IN PROSTATE CANCER | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
biopsies leading to cancer diagnoses are elevated PSA and/or abnormal digital rectal examination. Prostate cancer is also a common incidental finding in 28.5% cystoprostatectomy specimens, of which only 25.3% are considered clinically significant, defined by most experts as tumors with a volume of 0.5 mL or more and Gleason score of 7 or higher.106,107
Table 9-5 ▪ DETECTED ETS GENE FUSIONS IN PROSTATE CANCER | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
used to enhance sensitivity and specificity in diagnosis of prostate cancer discussed below.
study and control groups. In a larger European Randomized Study of Screening for Prostate Cancer (ERSPC) study that involved 7 countries, 162,243 men were randomized to care with and without PSA testing.11 Results of the ERSPC study with median follow-up of 9 years showed that PSA-based screening reduced prostate cancer death rates by 20% but was associated with high detection and treatment of indolent tumors. In practical terms, 1,410 men must be screened, and additional 48 men must be treated to prevent one death from prostate cancer.11
cutoff to perform a biopsy. Others however have found this test to be inaccurate. The problems are that the prostate may have a larger size also because of increase in stromal volume and TRUS is not accurate in measuring prostate volume. The positive predictive value of PSA density is only slightly higher than the 4 ng/mL cutoff for serum PSA. Further, performing TRUS is still an uncomfortable procedure for the patient even without biopsy. A modification of PSA density adjusts for the transition zone volume.
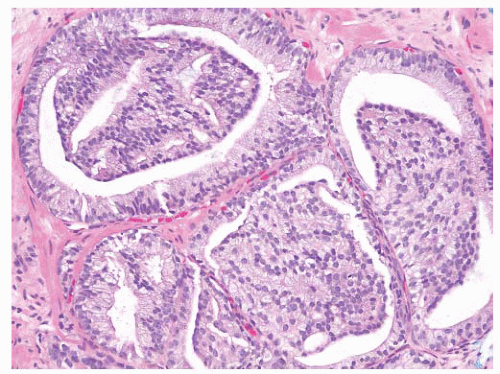
being detected in 40% to 60% of cases.161, 162, 163, 164, 165, 166, 167, 168 However, contemporary data in the era of extended prostate biopsy sampling have shown that the risk of cancer following diagnosis of HGPIN is between 21% and 27.5%.144,169 This risk of finding cancer after unifocal HGPIN is not significantly different from the risk following a benign diagnosis.144,155,156 Extended biopsy sampling has resulted in higher sensitivity for cancer detection, and therefore, less carcinoma is detected on subsequent samples.155
stratification, and irregular spacing. Nuclei are mildly enlarged, and, in particular, nucleoli are inconspicuous to only rarely prominent.
Table 9-6 ▪ COMMON AND UNUSUAL PATTERNS OF HGPIN | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
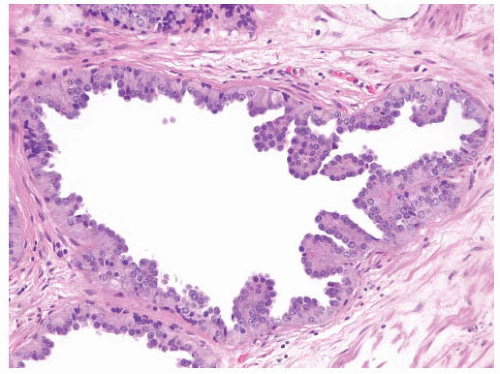
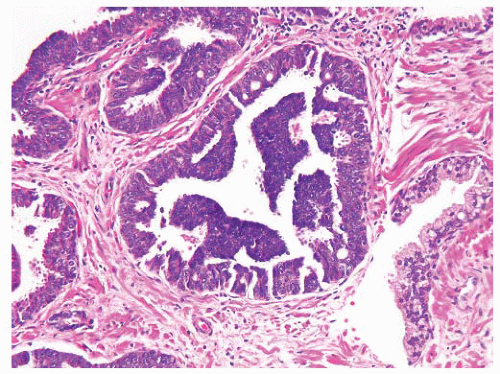
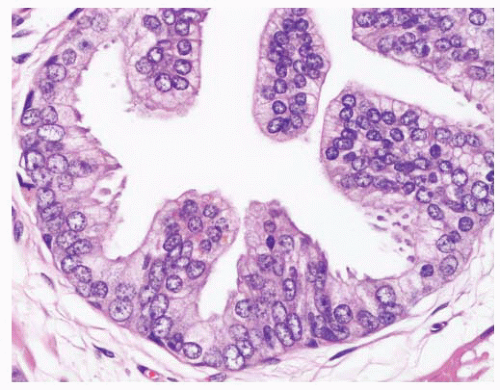
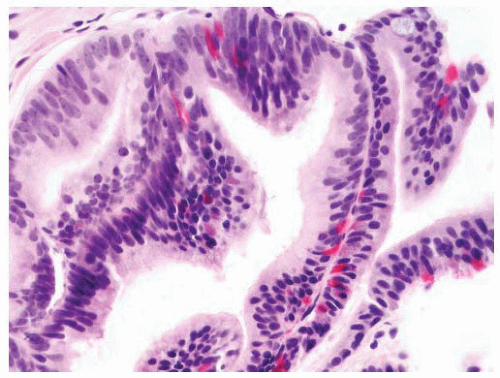
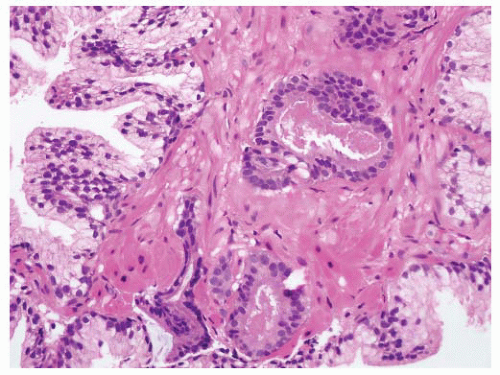
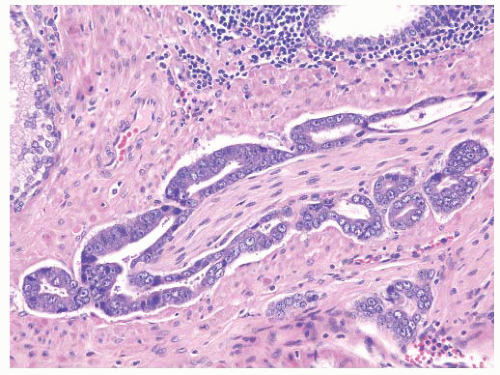
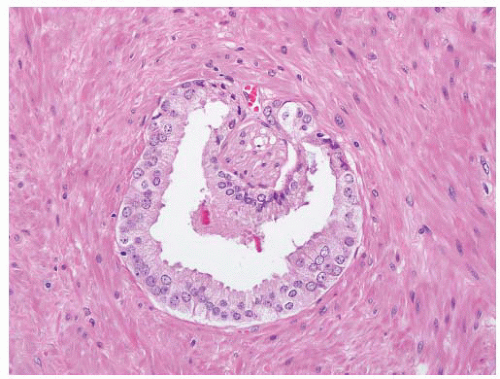
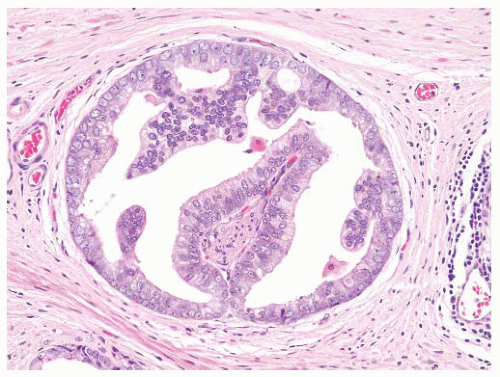
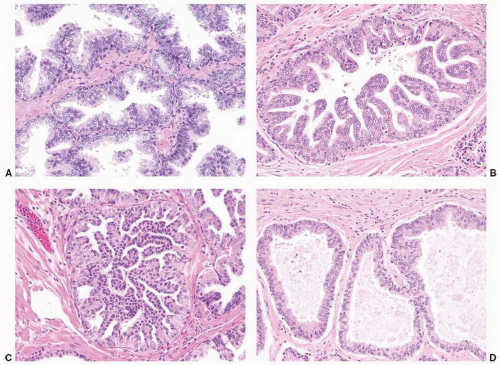 FIGURE 9-8 ▪ Basic patterns of HGPIN include (A) tufted, (B) micropapillary, (C) cribriform, and (D) flat architectures. Note the presence of cell maturation in cribriform HGPIN. |
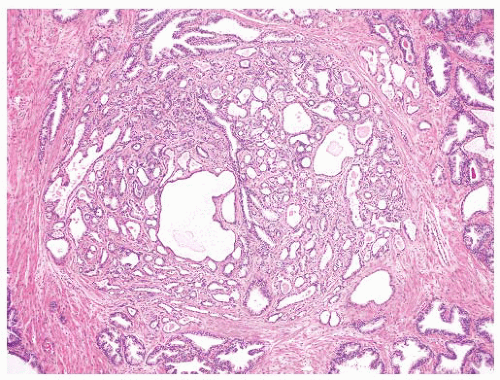
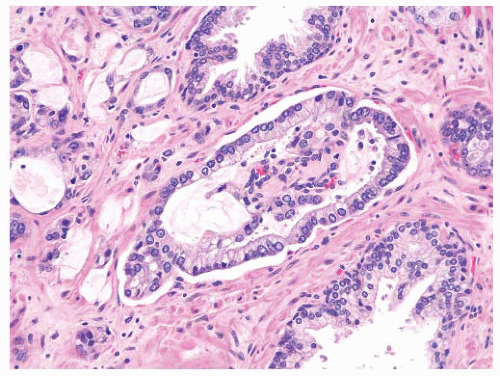
cribriform adenocarcinoma exhibits more complexity including the presence of large expansile glands (bigger than preexisting glands), confluence or back-to-back glands, consistent cribriform architecture, and associated solid nests or may have intraluminal necrosis (Gleason pattern 5). Basal cells are absent in invasive cribriform carcinoma, but this may need to be confirmed with basal cell immunostains. Ductal adenocarcinoma may resemble the different architectures of HGPIN. Ductal adenocarcinoma is characteristically composed of tall columnar cells that are frequently pseudostratified and can be mitotically active. Unlike HGPIN, ductal adenocarcinoma may have large expansile glands and papillae with true fibrovascular stalks, and the cells do not exhibit luminal “maturation.” Invasive features such as crowded back-to-back glands, stromal fibrosis, perineural invasion (PNI), and extraprostatic extension (EPE) are present in ductal adenocarcinoma. Basal cells are absent in the majority of ductal adenocarcinomas that can be confirmed with basal cell immunostains. IDC of the prostate can be very difficult to distinguish from HGPIN due to the lack of a consistent morphologic cutoff separating these two entities. IDC like HGPIN involves large glands containing markedly atypical cells and notably has preserved basal cells (see subsequent section). Unlike HGPIN, the glands of IDC can be expansile and show extensive cribriform change, sometimes with comedonecrosis. A lumen-spanning proliferation of cells with marked atypia and mitoses is usually seen associated with high-volume and high-grade carcinoma (Gleason pattern 4 or 5). However, without an invasive focus, IDC can be very difficult to distinguish from HGPIN in limited biopsy material. Sometimes, a descriptive term such as “markedly atypical cribriform proliferation” or “atypical cribriform lesion” can be used and the differential diagnosis explained in a comment. Generally such cases require additional biopsies.
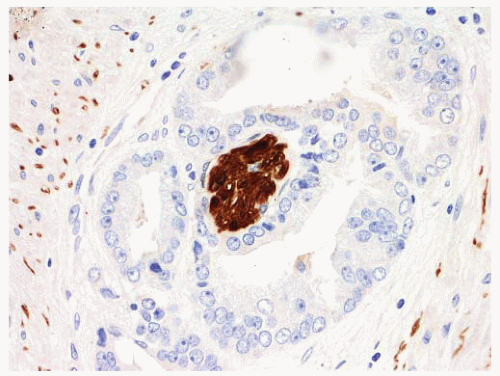
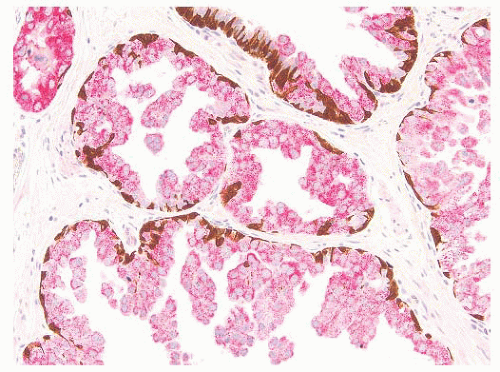 FIGURE 9-15 ▪ Cocktail of AMACR, HMWK, and p63 on HGPIN shows the presence of basal cells (brown) and increased AMACR expression (red). |
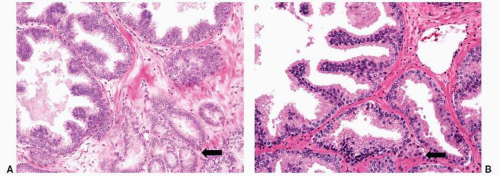 FIGURE 9-16 ▪ Examples of (A) HGPIN with adjacent Gleason pattern 3 carcinoma (arrow) and (B) HGPIN with adjacent atypical glands that may represent outpouching (HGPIN-ATYP). |
adenocarcinoma has no basal cells and may be associated or mingled with typical acinar carcinoma. Basal cell/adenoid cystic carcinoma contains basaloid-appearing cells with smaller nuclei and exhibits solid or adenoid cystic patterns and basement membrane material. Unlike HGPIN, basal cell/adenoid cystic carcinoma expresses p63 and keratin 34βE12 and is usually negative for PSA and PSAP. Urothelial carcinoma originating in the urethra or prostate may spread within the prostatic ducts and acini without invading stroma and thus mimic HGPIN. Urothelial carcinoma, however, tends to fill and expand the ductal or acinar lumen exhibiting solid growth and has significant cell pleomorphism and increased mitotic activity. The cells of urothelial carcinoma may have dense eosinophilic cytoplasm and may show squamoid features. Randomly distributed atypical cells with a pagetoid pattern may be present in glands involved by urothelial carcinoma. Urothelial carcinoma, unlike HGPIN, expresses GATA3 positivity and p63, HMCK (34βE12) staining in the nonbasally situated cells. PSA and PSAP stains are negative.
should have complete abscence of basal cell immunostaining in order to support a diagnosis of adenocarcinoma.
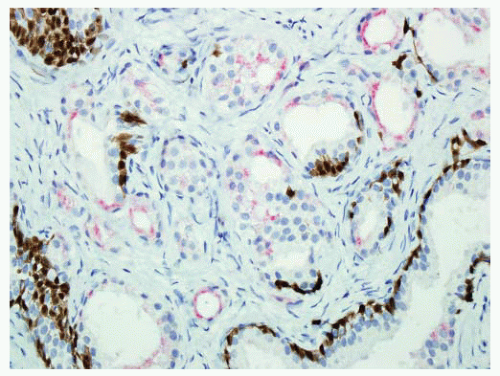 FIGURE 9-19 ▪ Cocktail of AMACR, HMWK, and p63 on AAH shows patchy basal cell immunostaining (brown). AMACR can be positive in a small subset of AAH like in this case. |
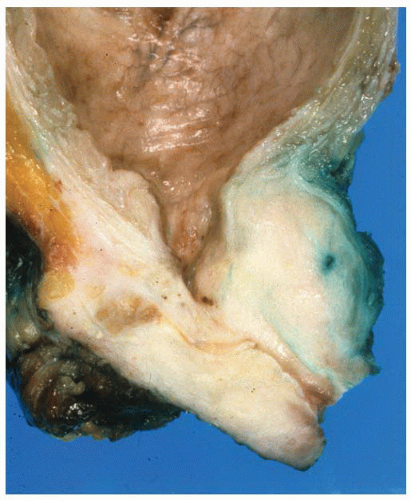
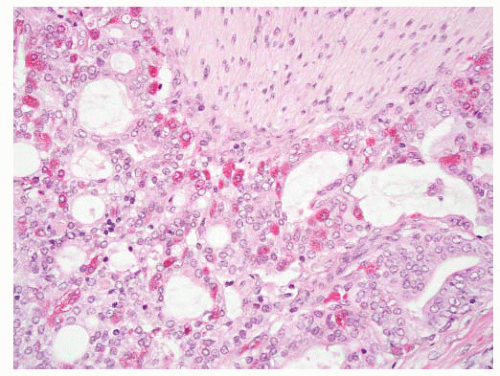
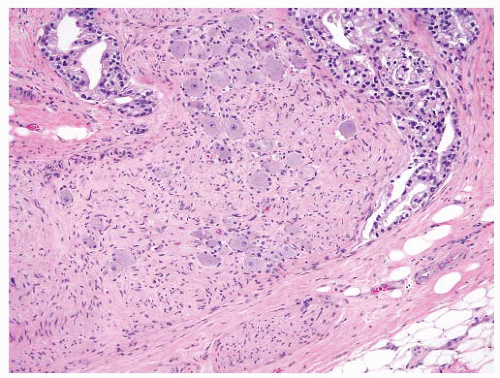
The tumor tissue has a smoother and more solid appearance and firmer texture than the spongy or sometimes cystic benign tissue. Carcinoma often has a yellow, yellow-orange, white, or gray color compared to the tan or beige appearance of benign prostate. The edges of the lesion are often indistinct, and microscopy usually reveals more tumor than was appreciated grossly. Extensive tumor can be overlooked since the whole gland may have a uniform appearance (Fig. 9-22). Many benign lesions such as stromal nodules, infarcts, and areas of prostatitis and atrophy may be grossly mistaken for carcinoma attesting to the nonspecificity of the macroscopic findings.236 Sometimes, extension of tumor beyond the confines of the gland may be seen macroscopically, but the observation may not be accurate, and it always requires histologic confirmation (Fig. 9-23). In one study,238 only one-half of carcinomas thought to show gross extension demonstrated EPE or margin positivity microscopically. In another study, 80% of cases with extraprostatic or margin involvement were correctly identified on gross assessment.239 Necrosis and hemorrhage are rarely seen in prostate carcinoma.
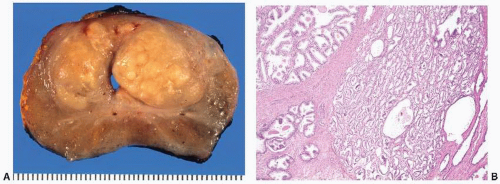
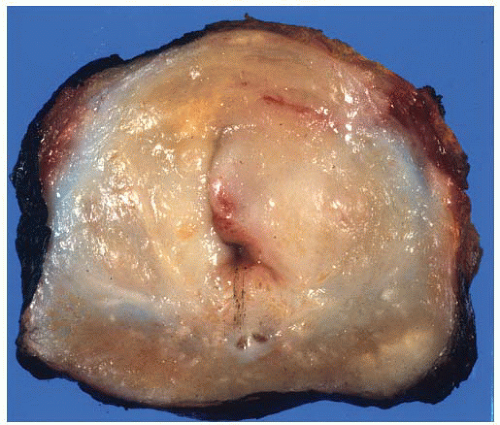 FIGURE 9-21 ▪ Transverse section of RP specimen showing peripheral nodule, right. Periurethral nodularity corresponding to a nodular hyperplasia is also noted. |
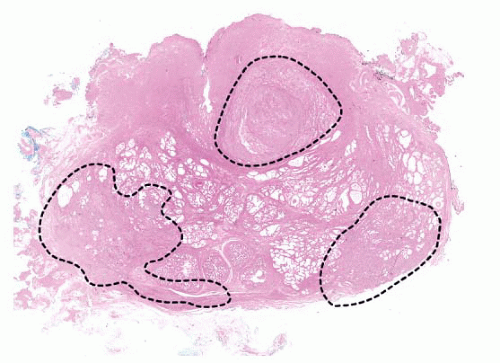
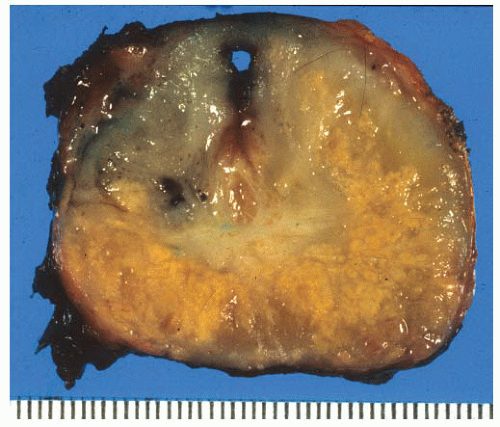 FIGURE 9-22 ▪ Transverse section of RP. Note widespread irregular yellow areas corresponding to extensive adenocarcinoma. |
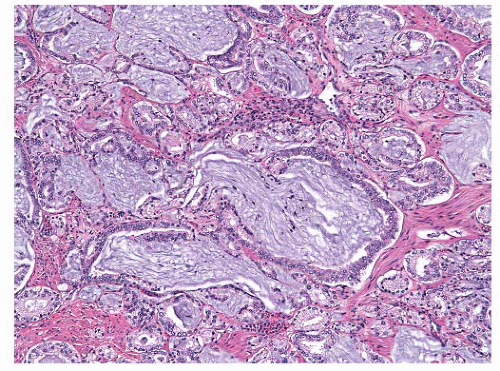
sampling protocols.246 Additionally, low-grade, high-volume transition zone tumors may be found, and these cases may be present with a high serum PSA (Fig. 9-24).247 Some have suggested that transition zone tumors have a distinct appearance with acini lined by columnar cells with basally located nuclei, but this latter can be seen in peripheral zone carcinomas as well.248 Interestingly, there have been some papers discussing differences in biomarker and molecular genetic findings between zones, but all of these changes may be related to the underlying Gleason score.249 The outcome data on differences between peripheral and transition zone tumors are mixed, some studies showing better outcomes and others showing no difference at all.249,250 Central zone carcinomas are uncommon and, in at least one study, have been associated with high Gleason score and high rates of extraprostatic involvement.251 The specific location of a tumor does not appear to have any independent prognostic significance.
that study, minor criteria included basophilic luminal secretions, pink amorphous secretions, intraluminal crystalloids, amphophilic cytoplasm, mitotic figures, and adjacent high-grade PIN. Our approach to diagnosing prostatic adenocarcinoma also utilizes major and minor criteria (Table 9-7). In addition to architectural pattern, absence of basal cells, and nuclear abnormalities including nucleolar enlargement, we include tinctorial alteration as a major criterion for diagnosis. Tinctorial changes are usually appreciated on scanning magnification, and like architectural disarray, they often draw one’s attention to sometimes subtle glandular abnormalities.
Table 9-7 ▪ MICROSCOPIC DIAGNOSIS OF ADENOCARCINOMA | ||||
|---|---|---|---|---|
|
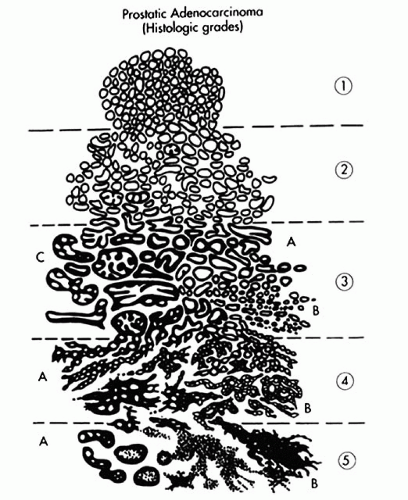
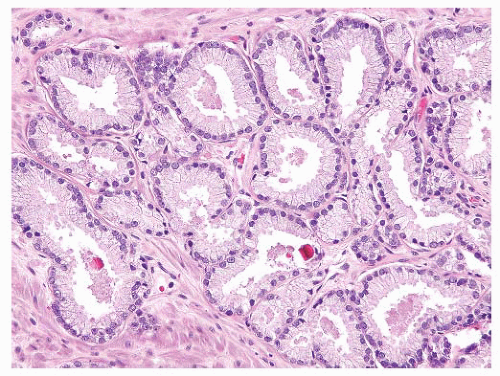
of the lesion is more irregular. Furthermore, there is more intervening stroma between proliferating glands in Gleason pattern 2 compared to pattern 1 (Fig. 9-28). Gleason pattern 2 is an uncommon pattern of carcinoma that may be seen in the transition zone and is therefore identified in prostatic chips, enucleations, and RP specimens. In modern practice, Gleason grade 2 is virtually never diagnosed in prostate needle biopsy specimens.262
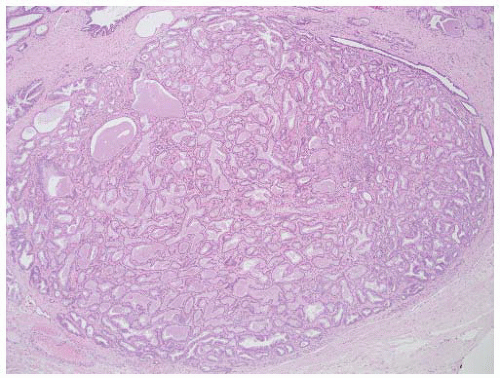 FIGURE 9-27 ▪ Gleason pattern 1. Closely packed small- to medium-sized acini show only slight size and shape variation. The edge of the lesion is visualized as a smooth pushing interface with stroma. |
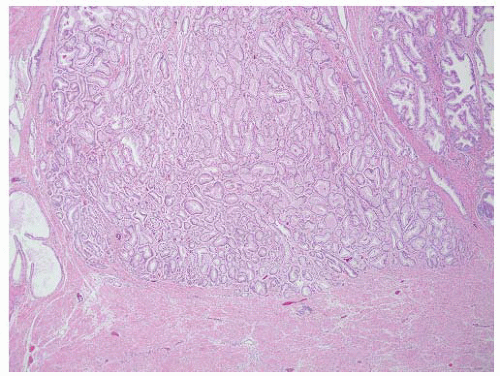 FIGURE 9-28 ▪ Gleason pattern 2 adenocarcinoma in the lesion is well circumscribed but contains acini showing some size and shape variation with more interweaving stroma than in Gleason pattern 1. |
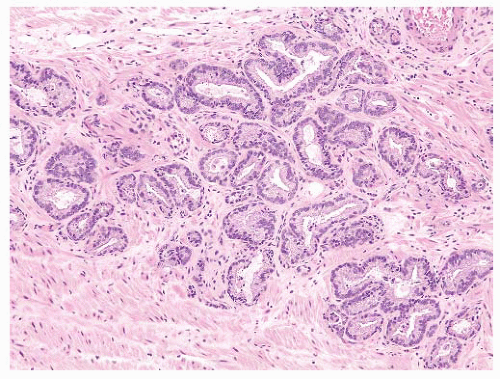 FIGURE 9-29 ▪ Gleason pattern 3A. Note proliferation of single separate acini showing size and shape variation. This lesion is more irregular and infiltrative than Gleason pattern 2. |
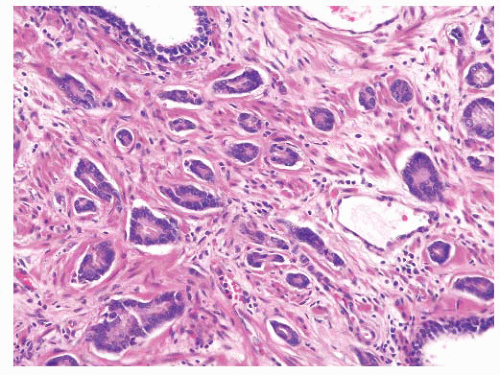 FIGURE 9-31 ▪ Gleason pattern 3B. Note small irregular acini infiltrating between nonneoplastic ones. All acini have at least partially discernable lumina. |
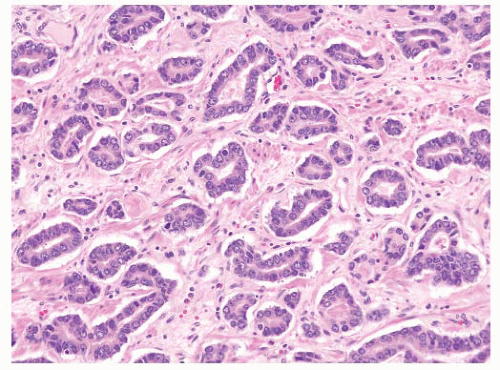 FIGURE 9-33 ▪ Adenocarcinoma characterized by irregular permeation of stroma without any nearby nonneoplastic glands. |
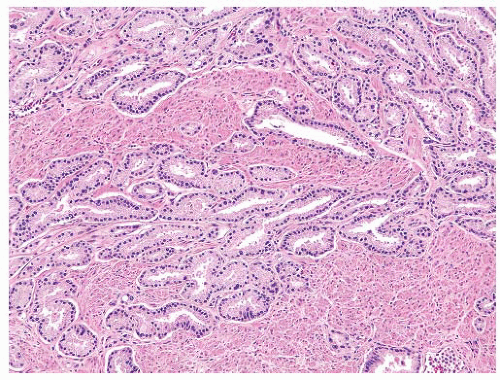 FIGURE 9-34 ▪ Gleason pattern 3 adenocarcinoma showing splitting and permeation between stromal muscle elements. |
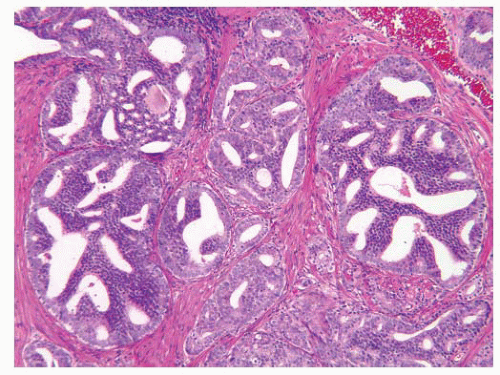 FIGURE 9-36 ▪ Classic Gleason pattern 3C rounded cribriform glands with punched-out lumina. In contemporary useage, this pattern is considered grade 4. |
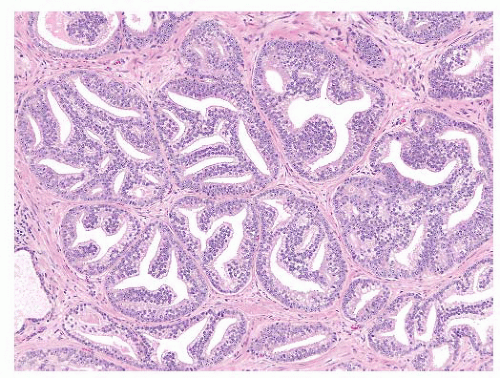 FIGURE 9-37 ▪ Rounded cribriform structures showing stromal infiltration. No basal cells identified. These cribriform structures are considered Gleason pattern 4. |
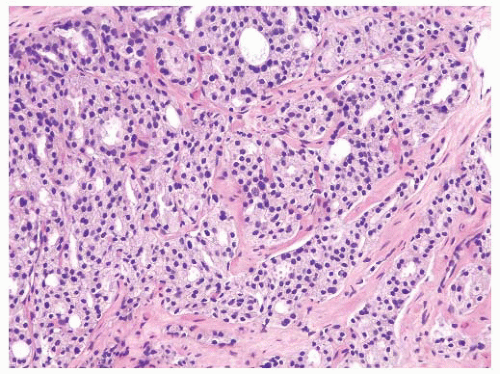 FIGURE 9-39 ▪ Gleason pattern 4A. Note striking fusion of acini and formation of occasional elongated acinar chains (lower right). |
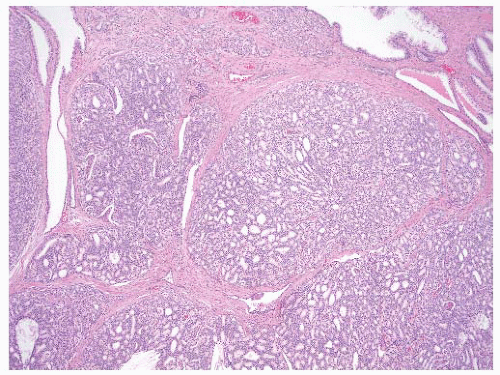 FIGURE 9-41 ▪ Gleason pattern 4A. Note large irregular and consulate cribriform structures with extensive infiltration of stroma. |
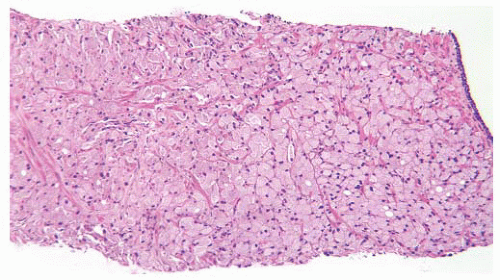 FIGURE 9-42 ▪ Gleason pattern 4B in needle biopsy. Note a fused acinar morphology with little or no gland formation. Individual cells have abundant clear to foamy cytoplasm and small dark nucleoli. |
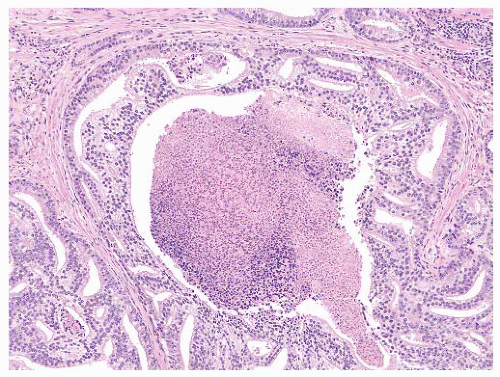 FIGURE 9-43 ▪ Gleason pattern 5A. Note rounded cribriform structures with prominent central comedonecrosis. |
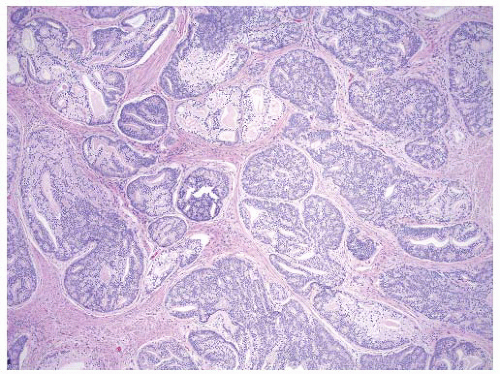 FIGURE 9-44 ▪ Cribriform carcinoma. Note extensive infiltration of stroma by cylindrical and sheet-like cribriform structures. |
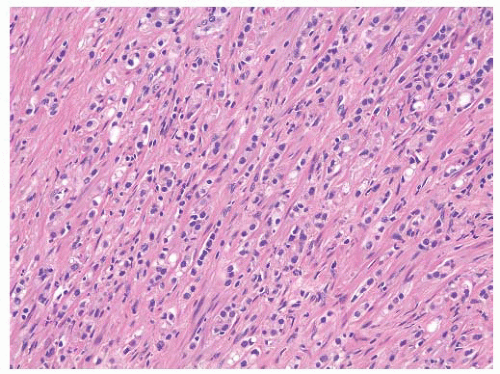 FIGURE 9-45 ▪ Gleason pattern 5B. Note extensive tissue involvement by Indian file-like cords and spindle cells. |
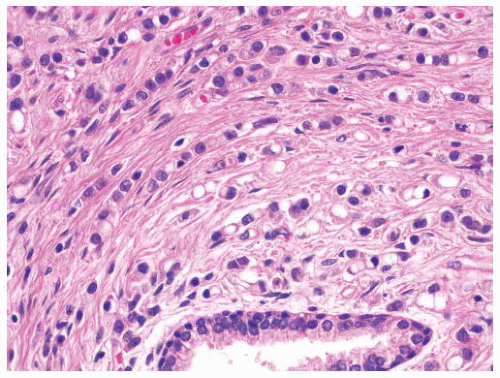 FIGURE 9-47 ▪ Gleason pattern 5. Note poorly differentiated cells arranged in cords, and individually. Many cells show prominent signet ring cell change. |
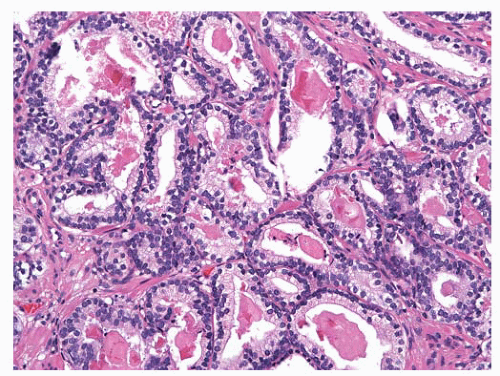
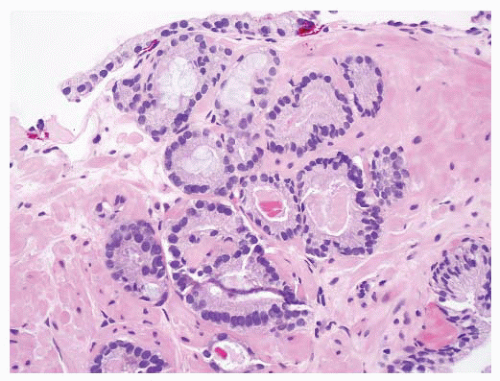
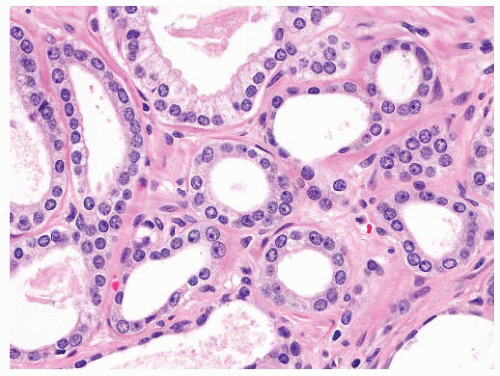
difficult to discern with an H&E-stained slide. Most benign mimickers of adenocarcinoma have complete or incomplete layers of basal cell.203,266,271 The usual patterns of complete atrophy show complete basal cell layers, while partial atrophy, postatrophic hyperplasia, and AAH typically have a discontinuous layer.214,216,272, 273, 274, 275, 276, 277 Some benign glands especially in areas of partial atrophy totally lack a basal cell layer, and therefore, an absence of basal cells is not a specific criterion of malignancy.274, 275, 276,278 Nevertheless, in adenocarcinoma, basal cells are absent, and this feature is an important aid in establishing a malignant diagnosis (Fig. 9-54).
employ basal cell markers (see later section) in some cases especially when the issue of periglandular stromal cells is a problem or when there is more than one layer with cellular overlapping or in situations where there are only very few glands on which to base a judgment (Fig. 9-57). A basal cell marker should not be considered a “malignant stain” since one is looking for a negative observation, a finding that may also be rarely seen in benign glands.203,266
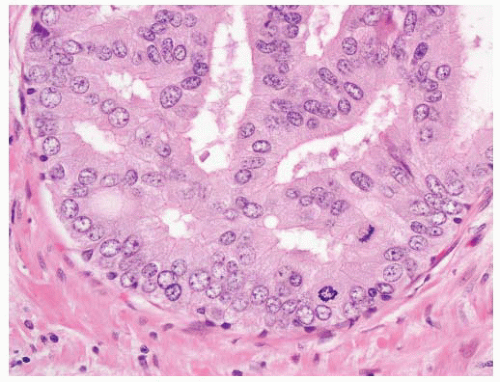
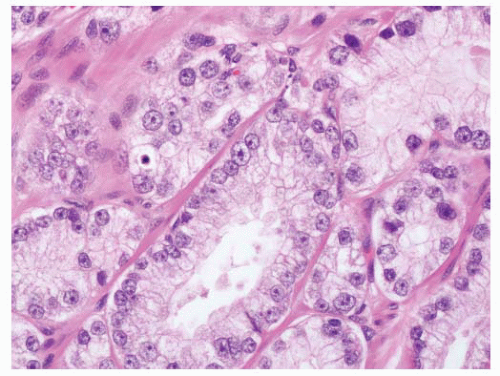
patterns of adenocarcinoma, especially hypernephroid carcinoma (Gleason pattern 4B), and the nuclei are often dark and have a pyknotic appearance with inconspicuous nucleoli (Fig. 9-61). The key point here is that the nuclear atypia does not always equate to nucleolar prominence.282 In the absence of the latter, there are other important attributes of nuclear atypia to look for including nuclear enlargement, nuclear shape and membrane irregularities, hyperchromasia, and parachromatin clearing (Fig. 9-62). These features need to be assessed using high-power microscopy.
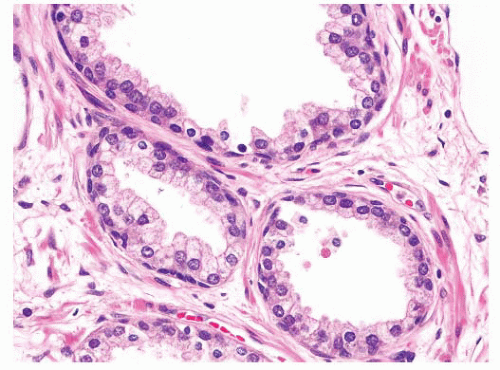 FIGURE 9-55 ▪ Nonneoplastic prostatic glands showing prominent basal cells with elongate and triangular forms. |
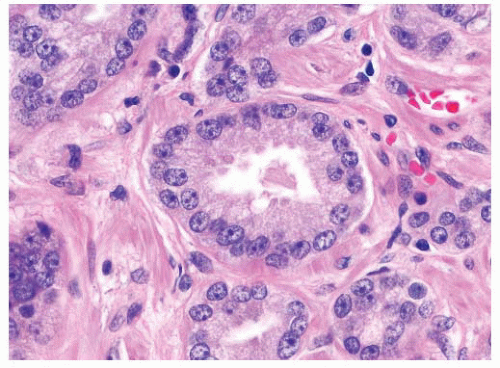 FIGURE 9-59 ▪ Adenocarcinoma showing prominent nucleoli including spindle cells with multiple nucleoli. |
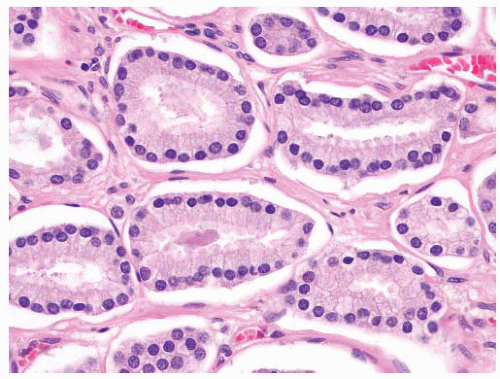 FIGURE 9-60 ▪ Gleason pattern 3 adenocarcinoma showing mildly enlarged nuclei without prominent nucleoli. |
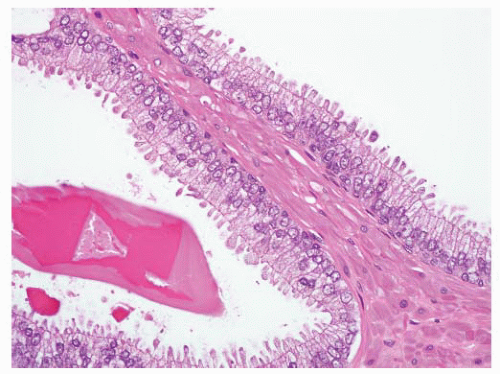
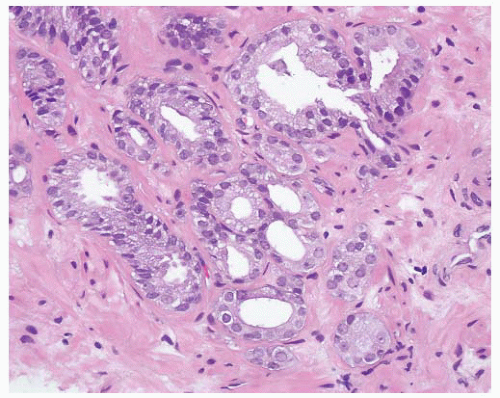
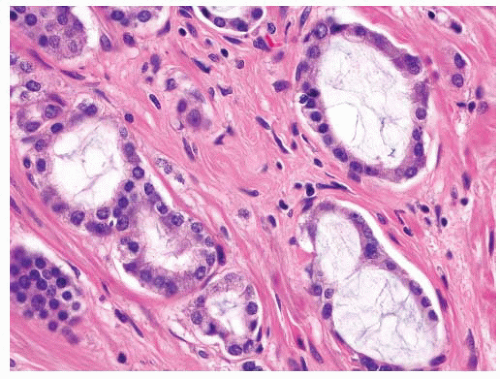
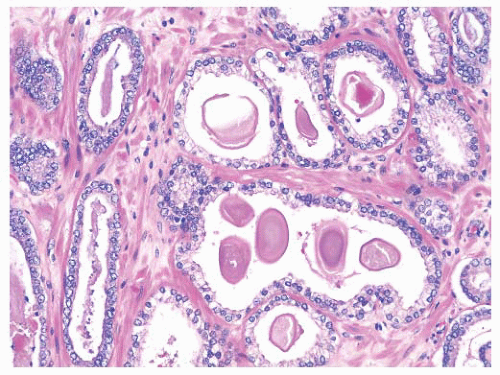
and distend glands (Fig. 9-65). On occasion, there is extensive involvement of acini (Fig. 9-66). The mucin stains with Alcian blue at pH 2.5 indicating its acidic nature. The blue mucin can be present on its own, or it may be accompanied by granular eosinophilic secretions or crystalloids. Blue mucin is present in up to 72% of completely embedded RP specimens and in 18% to 32% of limited adenocarcinomas in needle biopsies.282,287 Blue mucin is not specific for carcinoma and may be seen in a variety of other processes including mucinous metaplasia, basal cell hyperplasia, sclerosing adenosis, and high-grade PIN.288 Importantly, basophilic mucin may also be seen occasionally in atrophy and AAH (adenosis), both of which are common mimickers of small acinar carcinoma289 (Fig. 9-67).
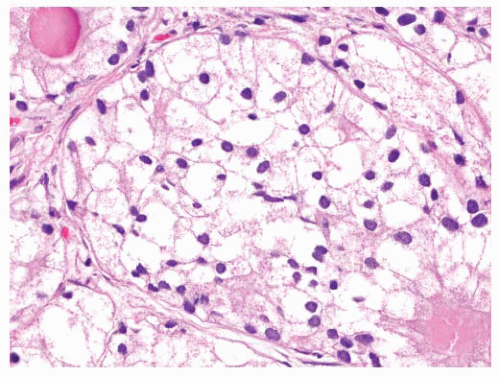 FIGURE 9-61 ▪ Gleason pattern 4B adenocarcinoma composed of cells with small dark nuclei with no nucleoli. |
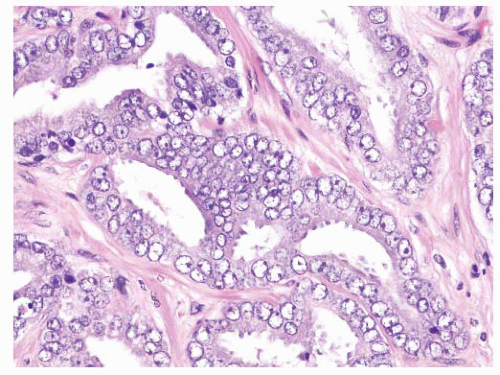 FIGURE 9-62 ▪ High-power photomicrograph showing marked nuclear atypia. Note nuclear membrane irregularities, prominent parachromatic clearing, and focally prominent nucleoli. |
Table 9-8 ▪ SPECIFIC DIAGNOSTIC CRITERIA OF CARCINOMA | |
|---|---|
|
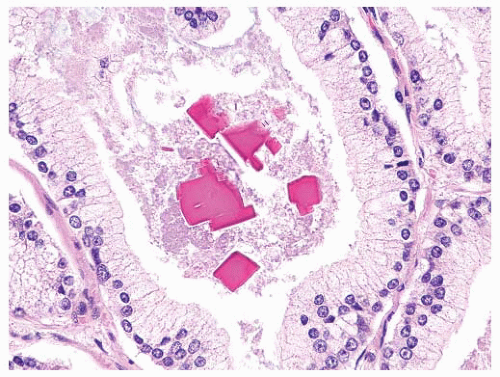
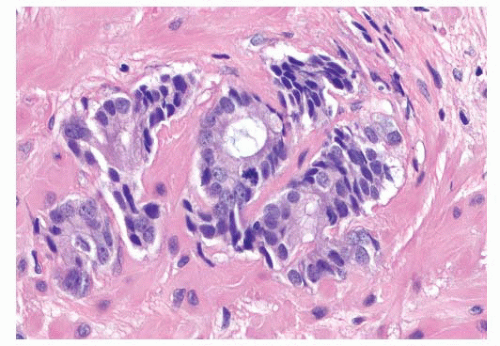
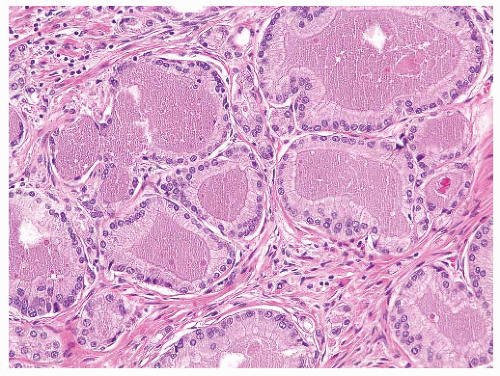 FIGURE 9-63 ▪ Gleason pattern 3 adenocarcinoma with prominent granular eosinophilic secretions. A crystalloid is seen on the right. |
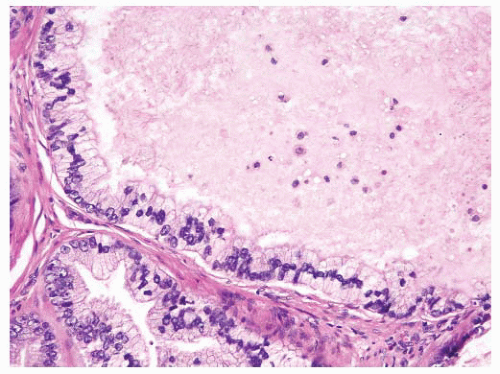 FIGURE 9-64 ▪ High-powered photomicrograph of carcinomatous glands showing eosinophilic luminal secretions and scattered nuclear debris. |
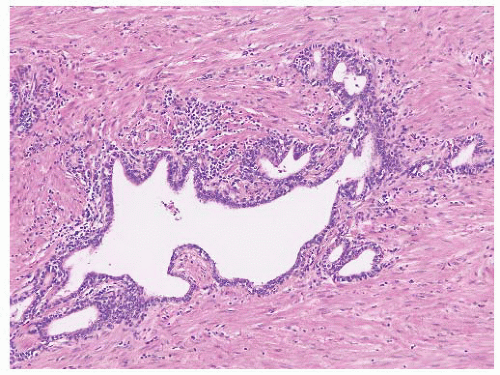
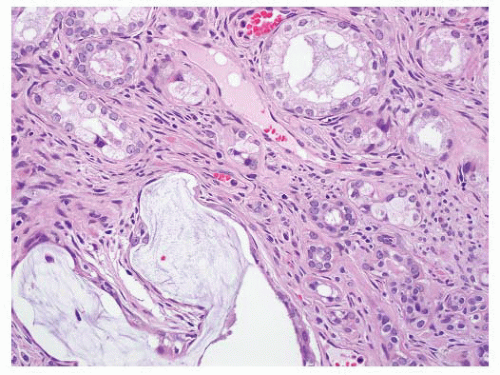
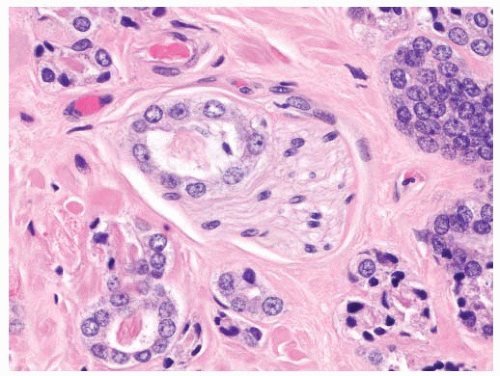
proper, this finding is generally diagnostic of prostatic carcinoma and suggests that the intraprostatic tumor has not been sampled. One must be cautious in such situations since metastatic carcinoma (e.g., gastrointestinal signet-ring carcinoma) may also present in this fashion, so prostatic marker studies may be useful. It is important to be aware that intraprostatic fat can rarely be seen. Involvement of seminal vesicle or bladder tissue by atypical prostatic glands is a good indicator of malignancy.
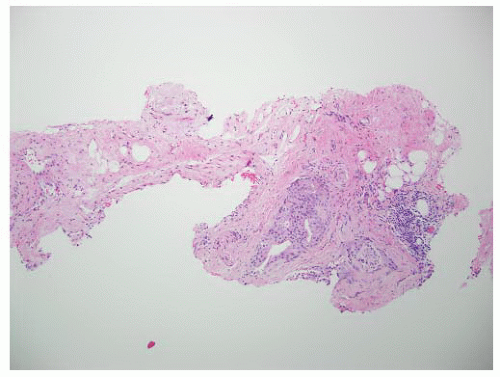 FIGURE 9-75 ▪ Low-power photomicrograph showing adenocarcinoma in periprostatic fibroadipose connective tissue. No actual involvement of prostate parenchyma was present. |
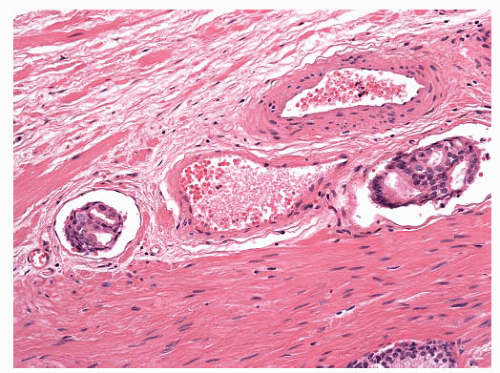
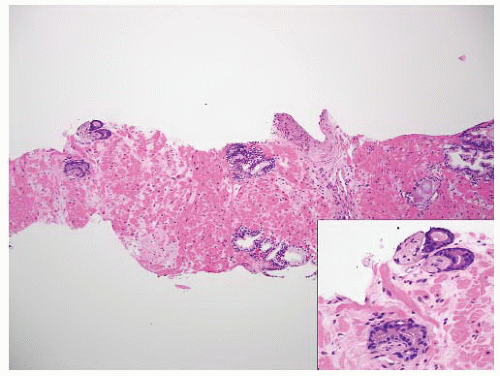 FIGURE 9-77 ▪ Low-power photomicrograph of prostatic core. Note perineural carcinoma near tip on left (inset). |
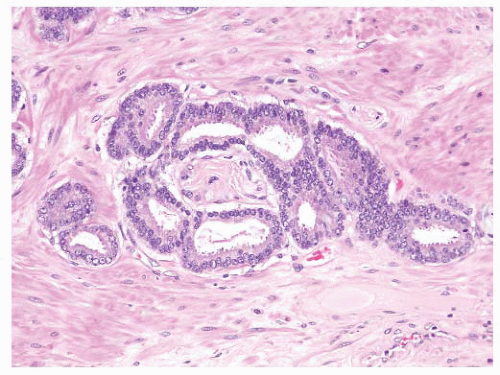 FIGURE 9-79 ▪ Circumferential perineural involvement by atypical glands is diagnostic of adenocarcinoma. |
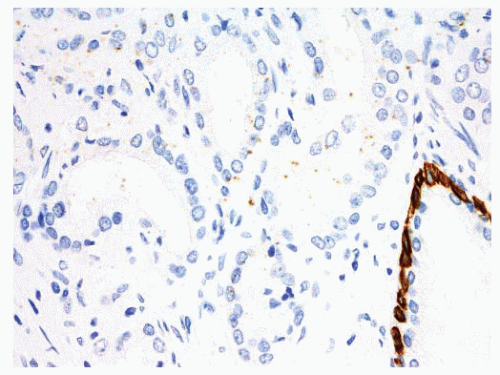
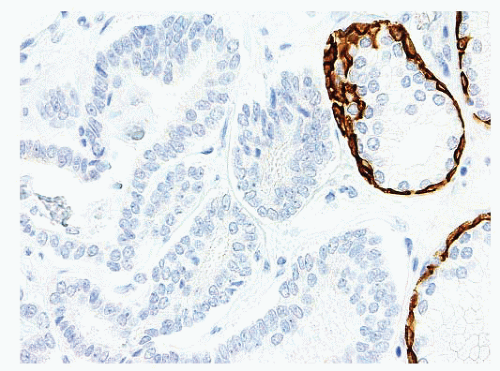
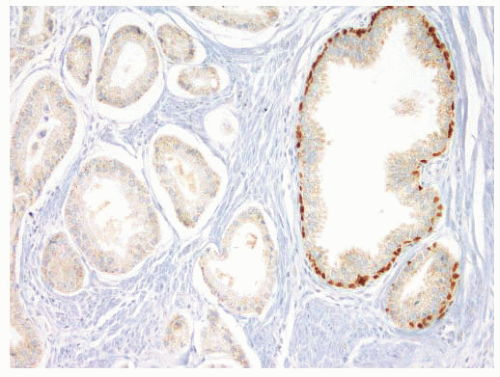 FIGURE 9-88 ▪ p63 staining in adenocarcinoma case. Note absence of p63-positive basal cells in adenocarcinoma (upside) and internal control positivity in nonneoplastic glands on right. |
atrophic foci.312,313 In an early study, looking at the utility of 34βE12 staining in small foci of atypical glands, the stain established a diagnosis of carcinoma in 14% of cases and was confirmatory for carcinoma in 58% of cases, equivocal in 18%, and of no value in 8%.305
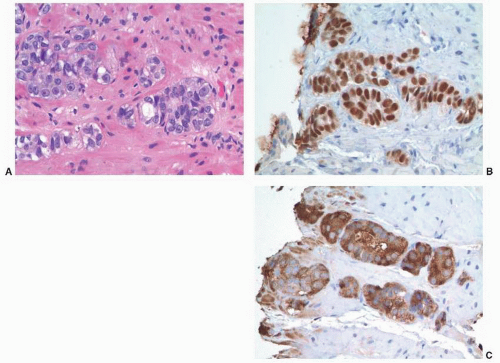 FIGURE 9-90 ▪ p63-positive prostatic adenocarcinoma. A. Note nests and poorly formed glands. B. Diffusely positive p63 nuclear staining. C. Strong racemase staining in atypical cells. |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree