The connective tissue in the portal zones is supplied by the hepatic artery.
Hepatic Arterial Flow
In man, during surgery, the hepatic artery supplies 35% of the hepatic blood flow and 50% of the liver’s oxygen supply [5]. The hepatic arterial flow serves to hold total hepatic blood flow constant. It regulates blood levels of nutrients and hormones by maintaining blood flow, and thereby hepatic clearance, as steady as possible [6].
The proportion of hepatic arterial flow increases greatly in cirrhosis, related to the extent of portal–systemic venous shunting. It is the main blood supply to tumours. A drop in systemic blood pressure from haemorrhage, or any other cause, lowers the oxygen content of the portal vein and the liver becomes more and more dependent on the hepatic artery for oxygen. The hepatic artery and the portal vein adjust the volume of blood and oxygen they supply to the liver according to demand [6].
Hepatic Arteriography
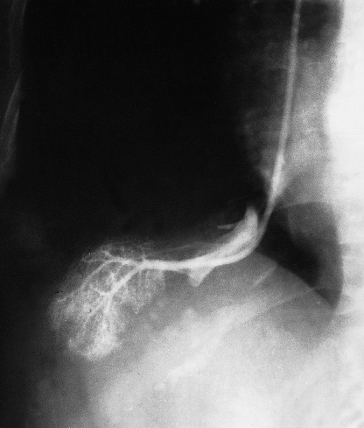
Hepatic arteriography can be used for the diagnosis of space-occupying lesions of the liver, but cross-sectional imaging has greatly reduced this indication. Lesions include cysts, abscesses and benign and malignant tumours (Chapter 35), as well as vascular lesions such as aneurysms (Fig. 9.2) or arteriovenous fistulae. Embolization via a catheter is used for treating tumours and hepatic trauma, and in the management of hepatic arterial aneurysms or arteriovenous fistulae (Figs 9.3, 9.4).
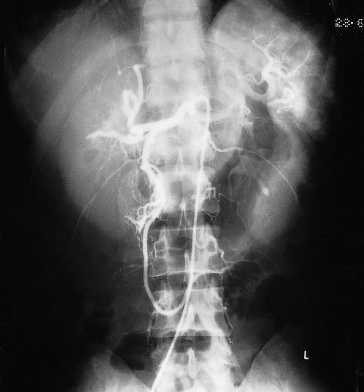
Fig. 9.2. Hepatic artery aneurysm in a patient with subacute bacterial endocarditis. CT scans of the upper abdomen: (a) before and (b) after contrast enhancement. The aneurysm shows as a filling defect (arrow) which highlights following contrast injection.
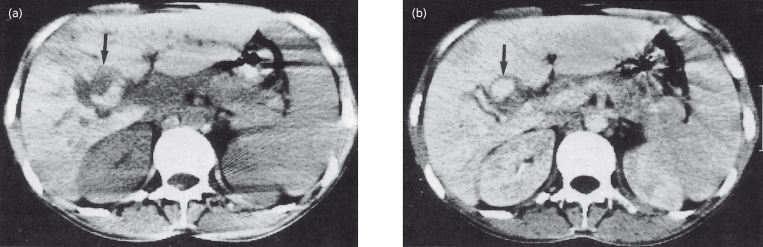
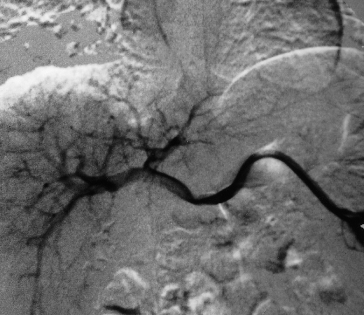
Fig. 9.3. Subacute bacterial endocarditis. Coeliac arteriogram showing a 3-cm false aneurysm (arrow) of one of the intrahepatic branches of the right hepatic artery, 2.5 cm lateral to its major bifurcation.
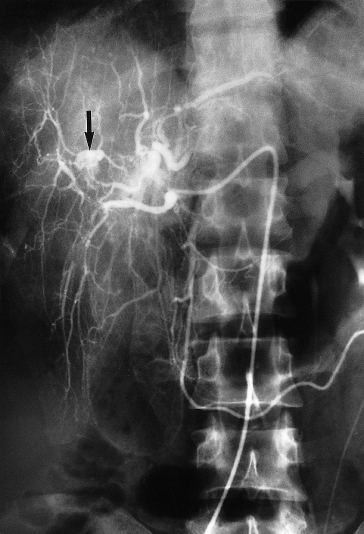
Fig. 9.4. Same patient as in Fig. 9.3. Coeliac angiogram immediately postembolization showing obliteration of the aneurysm and its feeding vessels [8].
Hepatic arterial catheterization is used to introduce cytotoxic drugs or radioactive beads into hepatocellular neoplasms and for pump perfusion in patients with metastases, particularly from colorectal cancer (Chapter 35).
Spiral CT is of great value in diagnosing hepatic arterial thrombosis after liver transplant [7] and variations in intrahepatic anatomy before liver resection [8].
Hepatic Artery Occlusion
The effects depend on the site and extent of available collateral circulation. If the division is distal to the origins of the gastric and gastroduodenal arteries the patient may die. Survivors develop a collateral circulation. Slow thrombosis is better than sudden block. Simultaneous occlusion of the portal vein is nearly always fatal.
The size of the infarct depends on the extent of the collateral arterial circulation. It rarely exceeds 8 cm in diameter and has a pale centre with a surrounding congested haemorrhagic band. Liver cells in the infarcted area are jumbled together in irregular collections of eosinophilic, granular cytoplasm without glycogen or nuclei. Subcapsular areas escape because they have an alternative arterial blood supply.
Hepatic infarction can develop without arterial occlusion in shock, cardiac failure, diabetic ketosis, toxaemia of pregnancy [9], after liver transplant or systemic lupus erythematosus [10]. If sought by scanning, small hepatic infarcts are frequent after percutaneous liver biopsy.
Aetiology
Occlusion of the hepatic artery is very rare. Hitherto it was regarded as a fatal condition. However, hepatic angiography has allowed earlier diagnosis and the prognosis has improved. Some of the causes are polyarteritis nodosa, giant cell arteritis and embolism in patients with acute bacterial endocarditis. A branch of the artery may be tied during cholecystectomy but recovery is usual. Trauma to the right hepatic or cystic artery may complicate laparoscopic cholecystectomy [11]. Hepatic arterial dissection may follow abdominal trauma or hepatic arterial catheterization. Gangrenous cholecystitis can complicate hepatic artery embolization [12].
Clinical Features
The condition is rarely diagnosed ante-mortem. The patient exhibits the features of the cause, such as bacterial endocarditis or polyarteritis nodosa, or has undergone a difficult upper abdominal operation. Sudden pain in the right upper abdomen is followed by collapse and hypotension. Right upper quadrant tenderness develops and the liver edge is tender. Jaundice deepens rapidly. There is usually fever and leucocytosis and liver function tests show hepatocellular damage. The prothrombin time rises precipitously and haemorrhages develop. With major occlusions the patient passes into coma and is dead within 10 days.
Hepatic Arteriography.
This is essential. The obstruction to the hepatic artery may be shown. Intrahepatic arterial collaterals develop in the portal zones and subcapsular areas. Extrahepatic collaterals form in the suspensory ligaments and with adjacent structures.
Scanning.
The infarcts are round, oval or wedge-shaped and are centrally located. Early lesions are hypoechoic on ultrasound. CT shows infarcts as low attenuation, peripheral wedged-shaped lesions. Occluded arterial vessels may be identified. Later lesions are confluent with distinct margins. MRI shows a lesion of low signal intensity on T1-weighted images and with high signal intensity on T2-weighted images [10]. Bile lakes follow large infarcts and these may contain gas.
Treatment.
The causative lesion must be treated. Antibiotics and antifungals may prevent secondary infection in the anoxic liver. The general management is that of acute hepatocellular failure. Trauma to the artery is treated by percutaneous arterial embolization.
Hepatic Arterial Lesions Following Liver Transplantation
The term ischaemic cholangitis is used to describe bile duct damage due to ischaemia [13]. It follows post-transplant-associated thrombosis or stenosis of the hepatic artery or occlusion of peribiliary arteries [14] and is associated with a poor quality donor liver such as one from a non-heart-beating donor. Later, thrombosis or stenosis of the hepatic artery or occlusion of peribiliary arterials leads to segmental hepatic infarction with abscesses and biloma [14]. The picture may be asymptomatic or present as relapsing bacteraemia.
Early diagnosis is made by duplex ultrasound. Spiral CT is highly accurate [7].
Retransplantation is the only management for lesions of the hepatic artery following transplant.
Ischaemic cholangitis manifesting as segmental strictures and cholangiectases with resultant impaired bile flow can also follow hepatic arterial chemotherapy and systemic vasculitis.
Aneurysms of the Hepatic Artery
These are rare but make up about one-fifth of all visceral aneurysms. The aneurysm may complicate bacterial endocarditis, polyarteritis nodosa or arteriosclerosis. Trauma is becoming increasingly important, including motor vehicle accidents and iatrogenic causes such as biliary tract surgery, liver biopsy and interventional radiological procedures. Pseudoaneurysms may complicate chronic pancreatitis with pseudocyst formation. Bile leaks are significantly associated with pseudoaneurysm [15]. It may be congenital. The aneurysm may be extra- or intrahepatic and may vary in size from a pin point to a grapefruit: it may be congenital.
Clinical Presentation.
The classical triad of jaundice [16], abdominal pain and haemobilia is present in only about one-third. Abdominal pain is frequent and may last as long as 5 months before the aneurysm ruptures. Between 60 and 80% of patients present for the first time with rupture into the peritoneum, biliary tree or gastrointestinal tract with resultant haemoperitoneum, haemobilia or haematemesis.
Diagnosis.
The diagnosis is suggested by sonography and confirmed by hepatic arteriography and a CT scan after enhancement (Fig. 9.2) [17]. Pulsed Doppler ultrasound may show turbulent flow in the aneurysm [18].
Treatment.
Intrahepatic aneurysms are treated by angiographic embolization (Figs 9.3, 9.4). Aneurysms of the common hepatic artery may also be treated surgically by proximal and distal ligation.
Hepatic Arteriovenous Shunts
These are usually secondary to blunt trauma, liver biopsy or neoplasms, usually primary liver cancer. Multiple shunts may be part of hereditary haemorrhagic telangiectasia, when they can be so extensive that congestive heart failure follows.
Large shunts cause a bruit in the right upper quadrant. The diagnosis is confirmed by hepatic angiography. Embolization with particles and/or placement of occluding devices is the usual treatment.
The Portal Venous System
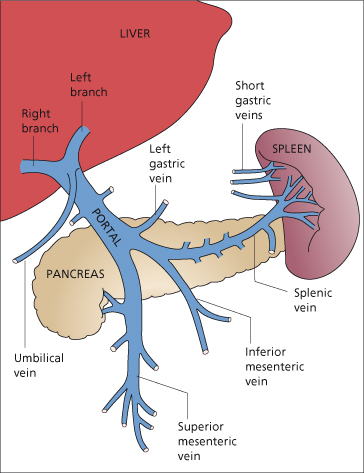
The portal system includes all veins that carry blood from the abdominal part of the alimentary tract, the spleen, pancreas and gallbladder. The portal vein enters the liver at the porta hepatis in two main branches, one to each lobe; it is without valves in its larger channels (Fig. 9.5) [19].
The portal vein is formed by the union of the superior mesenteric vein and the splenic vein just posterior to the head of the pancreas at about the level of the second lumbar vertebra. It extends slightly to the right of the midline for a distance of 5.5–8 cm to the porta hepatis. The portal vein has a segmental intrahepatic distribution, accompanying the hepatic artery.
The superior mesenteric vein is formed by tributaries from the small intestine, colon and head of the pancreas, and irregularly from the stomach via the right gastroepiploic vein.
The splenic veins (5–15 channels) originate at the splenic hilum and join near the tail of the pancreas with the short gastric vessels to form the main splenic vein. This proceeds in a transverse direction in the body and head of the pancreas, lying below and in front of the artery. It receives numerous tributaries from the head of the pancreas, and the left gastroepiploic vein enters it near the spleen. The inferior mesenteric vein, bringing blood from the left part of the colon and rectum, usually enters its medial third. Occasionally, however, it enters the junction of the superior mesenteric and splenic veins.
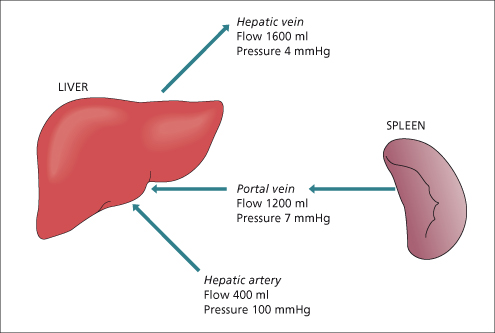
Portal blood flow in man is about 1000–1200 mL/min.
The fasting arterioportal oxygen difference is only 1.9 volumes per cent (range 0.4–3.3 volumes per cent) and the portal vein contributes 40 mL/min or 72% of the total oxygen supply to the liver. During digestion, the arterioportal venous oxygen difference increases due to increased intestinal utilization.
Stream-lines in the portal vein: there is no consistent pattern of hepatic distribution of portal inflow. Sometimes splenic blood goes to the left and sometimes to the right. Crossing-over of the bloodstream can occur in the portal vein. Flow is probably stream-lined rather than turbulent.
Portal pressure is about 7 mmHg (Fig. 9.6).
Collateral Circulation
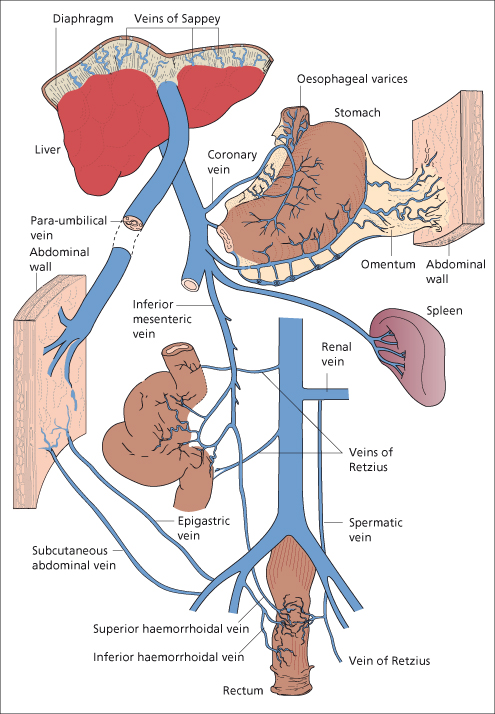
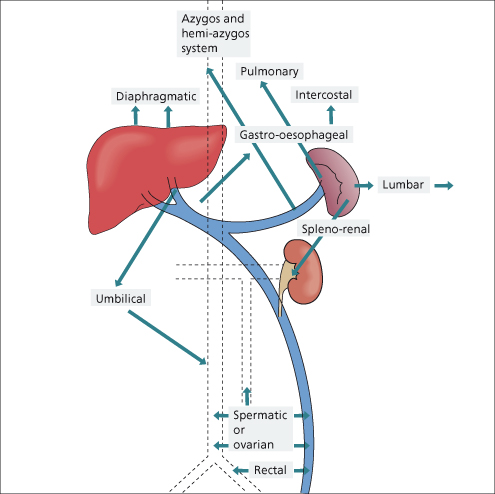
When the portal circulation is obstructed, whether it be within or outside the liver, a remarkable collateral circulation develops to carry portal blood into the systemic veins (Figs 9.7, 9.8).
Fig. 9.8. The sites of the collateral circulation in the presence of intrahepatic portal vein obstruction.
Intrahepatic Obstruction (Cirrhosis)
Normally 100% of the portal venous blood flow can be recovered from the hepatic veins, whereas in cirrhosis only 13% is obtained [20]. The remainder enters collateral channels which form four main groups.
Group I: where protective epithelium adjoins absorptive epithelium:
(a) At the cardia of the stomach, where the left gastric vein, posterior gastric [21] and short gastric veins of the portal system anastomose with the intercostal, diaphragmo-oesophageal and azygos minor veins of the caval system. Deviation of blood into these channels leads to varicosities in the submucous layer of the lower end of the oesophagus and fundus of the stomach.
(b) At the anus, the superior haemorrhoidal vein of the portal system anastomoses with the middle and inferior haemorrhoidal veins of the caval system. Deviation of blood into these channels may lead to rectal varices.
Group II: in the falciform ligament through the paraumbilical veins, relics of the umbilical circulation of the fetus (Fig. 9.9).
Group III: where the abdominal organs are in contact with retroperitoneal tissues or adherent to the abdominal wall. These collaterals run from the liver to diaphragm and in the splenorenal ligament and omentum. They include lumbar veins and veins developing in scars of previous operations or in small or large bowel stomas.
Group IV: portal venous blood is carried to the left renal vein. This may be through blood entering directly from the splenic vein or via diaphragmatic, pancreatic, left adrenal or gastric veins.
Blood from gastro-oesophageal and other collaterals ultimately reaches the superior vena cava via the azygos or hemiazygos systems. A small volume enters the inferior vena cava. An intrahepatic shunt may run from the right branch of the portal vein to the inferior vena cava [22]. Collaterals to the pulmonary veins have also been described.
Extrahepatic Obstruction
With extrahepatic portal venous obstruction, additional collaterals form, attempting to bypass the block and return blood towards the liver. These enter the portal vein in the porta hepatis beyond the block. They include the veins at the hilum, venae comitantes of the portal vein and hepatic arteries, veins in the suspensory ligaments of the liver and diaphragmatic and omental veins. Lumbar collaterals may be very large.
Effects
When the liver is cut off from portal blood by the development of the collateral circulation, it depends more on blood from the hepatic artery. It shrinks and shows impaired capacity to regenerate. This might be due to lack of hepatotrophic factors, including insulin and glucagon, which are of pancreatic origin.
Collaterals usually imply portal hypertension, although occasionally if the collateral circulation is very extensive portal pressure may fall. Conversely, portal hypertension of short duration can exist without a demonstrable collateral circulation.
A large portal–systemic shunt may lead to hepatic encephalopathy, septicaemias due to intestinal organisms, and other circulatory and metabolic effects.
Pathology of Portal Hypertension
Collateral venous circulation is disappointingly insignificant at autopsy. The oesophageal varices collapse.
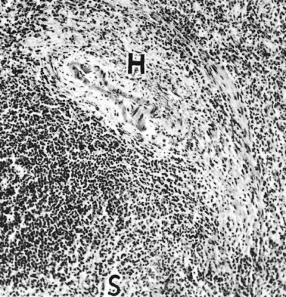
The spleen is enlarged with a thickened capsule. The surface oozes dark blood (fibrocongestive splenomegaly). Malpighian bodies are inconspicuous. Histologically, sinusoids are dilated and lined by thickened epithelium (Fig. 9.10). Histiocytes proliferate with occasional erythrophagocytosis. Periarterial haemorrhages may progress to siderotic, fibrotic nodules.
Fig. 9.10. The spleen in portal hypertension. The sinusoids (S) are congested and the sinusoidal wall is thickened. A haemorrhage (H) lies adjacent to an arteriole of a Malpighian corpuscle. (H & E,×70.)
The splenic artery and portal vein are enlarged and tortuous and may be aneurysmal. The portal and splenic vein may show endothelial haemorrhages, mural thrombi and intimal plaques and may calcify (see Fig. 9.7). Such veins are usually unsuitable for portal surgery. In 50% of patients with cirrhosis small, deeply placed splenic arterial aneurysms are seen [23].
Hepatic changes depend on the cause of the portal hypertension.
The height of the portal venous pressure correlates poorly with the apparent degree of cirrhosis and in particular of fibrosis. There is a much better correlation with the degree of nodularity.
Varices
Oesophageal
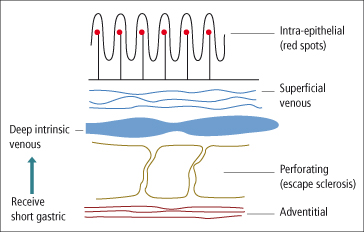
The major blood supply to oesophageal varices is the left gastric vein. The posterior branch usually drains into the azygos system, whereas the anterior branch communicates with varices just below the oesophageal junction and forms a bundle of thin parallel veins that run in the junction area and continue in large tortuous veins in the lower oesophagus. There are four layers of veins in the oesophagus (Fig. 9.11) [24]. Intraepithelial veins may correlate with the red spots seen on endoscopy and which predict variceal rupture. The superficial venous plexus drains into larger, deep intrinsic veins. Perforating veins connect the deeper veins with the fourth layer which is the adventitial plexus. Typical large varices arise from the main trunks of the deep intrinsic veins and these communicate with gastric varices.
The connection between portal and systemic circulation at the gastro-oesophageal junction is extremely complex [25]. Its adaptation to the cephalad and increased flow of portal hypertension is ill-understood. A palisade zone is seen between the gastric zone and the perforating zone (Fig. 9.12). In the palisade zone, flow is bidirectional and this area acts as a water shed between the portal and azygos systems. Turbulent flow in perforating veins between the varices and the perioesophageal veins at the lower end of the stomach may explain why rupture is frequent in this region [26]. Recurrence of varices after endoscopic sclerotherapy may be related to the communications between various venous channels or perhaps to enlargement of veins in the superficial venous plexus. Failure of sclerotherapy may also be due to failure to thrombose the perforating veins.
Fig. 9.12. Radiograph of a specimen injected with barium–gelatine, opened along the greater curvature. Four distinct zones of normal venous drainage are identified: the gastric zone (GZ), palisade zone (PZ), perforating zone (PfZ) and truncal zone (TZ). A radio-opaque wire demarcates the transition between the columnar and stratified squamous epithelium. GOJ, gastro-oesophageal junction [25].
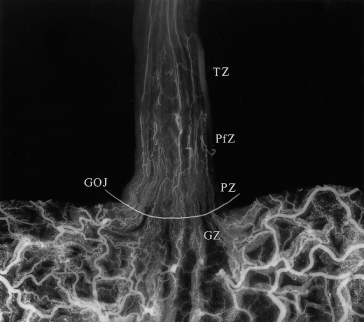
Gastric
These are largely supplied by the short gastric veins and drain into the deep intrinsic veins of the oesophagus. They are particularly prominent in patients with extrahepatic portal obstruction.
Duodenal varices show as filling defects. Bile duct collaterals may be life-threatening at surgery [27].
Colorectal
These develop secondary to inferior mesenteric–internal iliac venous collaterals [28]. They may present with haemorrhage. They are visualized by colonoscopy. Colonic varices are more frequent in association with splanchnic thrombosis.
Collaterals between the superior haemorrhoidal (portal) veins and the middle and inferior haemorrhoidal (systemic) veins lead to anorectal varices [29].
Portal Hypertensive Intestinal Vasculopathy
Chronic portal hypertension may not only be associated with discrete varices but with a spectrum of intestinal mucosal changes due to abnormalities in the microcirculation [30].
Portal Hypertensive Gastropathy.
This is almost always associated with cirrhosis and is seen in the fundus and body of the stomach. Histology shows vascular ectasia in the mucosa. The risk of bleeding is increased, for instance from non-steroidal anti-inflammatory drugs (NSAIDs). These gastric changes may be increased after sclerotherapy. They are relieved only by reducing the portal pressure [31].
Gastric Antral Vascular Ectasia.
This is marked by increased arteriovenous communications between the muscularis mucosa and dilated precapillaries and veins [32]. Gastric mucosal perfusion is increased. This must be distinguished from portal hypertensive gastropathy. It is not directly related to portal hypertension, but is influenced by liver dysfunction [33].
Congestive Jejunopathy and Colonopathy.
Similar changes are seen in the duodenum and jejunum. Histology shows an increase in size and number of vessels in jejunal villi [34]. The mucosa is oedematous, erythematous and friable [35]. Congestive colonopathy is shown by dilated mucosal capillaries with thickened basement membranes but with no evidence of mucosal inflammation [30].
Others
Portal–systemic collaterals form in relation to bowel–abdominal wall adhesions secondary to previous surgery or pelvic inflammatory disease. Varices also form at mucocutaneous junctions, for instance, at the site of an ileostomy or colostomy.
Haemodynamics of Portal Hypertension
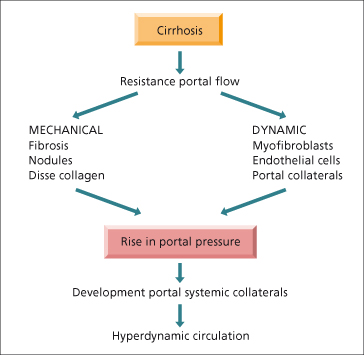
This has been considerably clarified by the development of animal models such as the rat with a ligated portal vein or bile duct or with carbon tetrachloride-induced cirrhosis. Portal hypertension is related both to vascular resistance and to portal blood flow (Fig. 9.13). The fundamental haemodynamic abnormality is an increased resistance to portal flow. This is mechanical due to the disturbed architecture and nodularity of cirrhosis or due to an obstructed portal vein and also due to dynamic changes related to dysfunction of the endothelium and reduced bioavailability of nitric oxide (NO) [36]. Other intrahepatic factors such as collagen deposition in the space of Disse [37] leading to loss of fenestrae (capillarization of the sinusoids), hepatocyte swelling [38,39] and the resistance offered by portal–systemic collaterals contribute.
There is also a dynamic increase in intrahepatic vascular resistance [36].
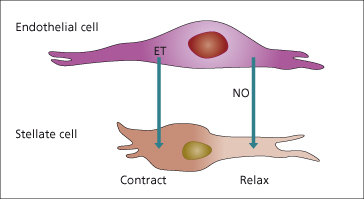
Stellate (Ito) cells have contractile properties that can be modulated by vasoactive substances [40]. These include NO which is vasodilatory [41] (Chapter 7) and endothelin which is a vasoconstrictor [42]. These may modulate intrahepatic resistance and blood flow, especially at a sinusoidal level (Fig. 9.14) [43].
Fig. 9.14. Regulation of sinusoidal blood flow. Endothelial and stellate cells are potential sources of endothelin (ET) which is contractile on stellate cells. Nitric oxide (NO) relaxes stellate cells. NO synthase is the precursor of NO and is produced by endothelial and stellate cells.
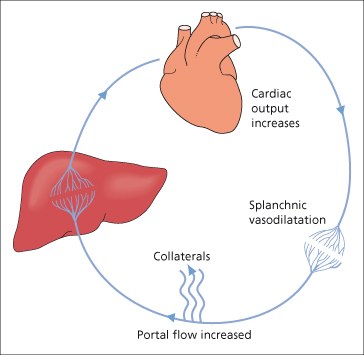
Collaterals develop when the pressure gradient between the portal vein and hepatic vein rises above a certain threshold, a process which involves angiogenic factors [44]. At the same time portal flow increases in the splanchnic bed due to splanchnic vasodilatation and increased cardiac output. It is uncertain whether the hyperdynamic circulation is the cause or the consequence of the portal hypertension or both. It is related to the severity of liver failure. Cardiac output increases further and there is generalized systemic vasodilatation (Fig. 9.15). Arterial blood pressure is normal or low (Chapter 7).
Splanchnic vasodilatation is probably the most important factor in maintaining the hyperdynamic circulation. Azygous blood flow is increased. Gastric mucosal blood flow rises. The increased portal flow raises the oesophageal variceal transmural pressure. The increased flow refers to total portal flow (hepatic and collaterals). The actual portal flow reaching the liver is reduced. The factors maintaining the hyperdynamic splanchnic circulation are multiple. There seems to be an interplay of vasodilators and vasoconstrictors. These might be formed by the hepatocyte, fail to be inactivated by it or be of gut origin and pass through intrahepatic or extrahepatic venous shunts.
Endotoxins and cytokines, largely formed in the gut, are important triggers [45]. NO and endothelin-1 are synthesized by vascular endothelium in response to endotoxin. Prostacyclin is produced by portal vein endothelium and is a potent vasodilator [46]. It may play a major role in the circulatory changes of portal hypertension due to chronic liver disease.
Glucagon is vasodilatory after pharmacological doses but is not vasoactive at physiological doses. It is not a primary factor in the maintenance of the hyperkinetic circulation in established liver disease [47].
Clinical Features of Portal Hypertension
History and General Examination (Table 9.1)
Cirrhosis is the commonest cause. Aetiological factors should be looked for. Past abdominal inflammation, especially neonatal, is important in extrahepatic portal vein thrombosis. Prothrombotic factors, inherited or acquired, and drugs, such as sex hormones, predispose to portal and hepatic venous thrombosis.
Table 9.1. Investigation of a patient with suspected portal hypertension
| History |
| Relevant to cirrhosis or chronic hepatitis (Chapter 7) |
| Gastrointestinal bleeding: number, dates, amounts, symptoms, treatment |
| Results of previous endoscopies |
| Patient history: alcoholism, blood transfusion, hepatitis B, hepatitis C, intra-abdominal, neonatal or other sepsis, oral contraceptives, myeloproliferative disorder |
| Examination |
| Signs of hepatocellular failure |
| Abdominal wall veins: |
| site |
| direction of blood flow |
| Splenomegaly |
| Liver size and consistency |
| Ascites |
| Oedema of legs |
| Rectal examination |
| Endoscopy of oesophagus, stomach and duodenum |
| Additional investigations |
| Liver biopsy |
| Hepatic vein catheterization |
| Splanchnic arteriography |
| Hepatic ultrasound, CT scan or MRI |
Haematemesis is the commonest presentation. The number and severity of previous haemorrhages should be noted, together with their immediate effects, whether there was associated confusion or coma and whether blood transfusion was required. Melaena, without haematemesis, may result from bleeding varices. The absence of dyspepsia and epigastric tenderness and a previously normal endoscopy help to exclude haemorrhage from peptic ulcer.
The stigmata of cirrhosis include jaundice, vascular spiders and palmar erythema. Anaemia, ascites and precoma should be noted.
Abdominal Wall Veins
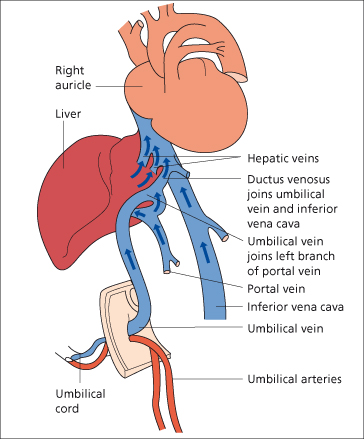
In intrahepatic portal hypertension, some blood from the left branch of the portal vein may be deviated via paraumbilical veins to the umbilicus, whence it reaches veins of the caval system (Fig. 9.16). In extrahepatic portal obstruction, dilated veins may appear in the left flank.
Fig. 9.16. Distribution and direction of blood flow in anterior abdominal wall veins in portal venous obstruction (left) and in inferior vena caval obstruction (right).
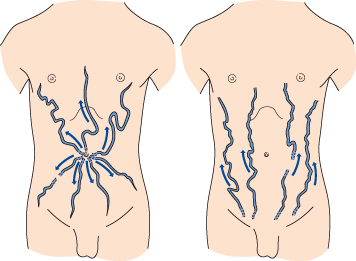
Distribution and Direction.
Prominent collateral veins radiating from the umbilicus are termed caput Medusae. This is rare and usually only one or two veins, frequently epigastric, are seen (Figs 9.16, 9.17). The blood flow is away from the umbilicus, whereas in inferior vena caval obstruction the collateral venous channels carry blood upwards to reach the superior vena caval system (Fig. 9.16). Tense ascites may lead to functional obstruction of the inferior vena cava and cause difficulty in interpretation.
Murmurs.
A venous hum may be heard, usually in the region of the xiphoid process or umbilicus. A thrill, detectable by light pressure, may be felt at the site of maximum intensity and is due to blood rushing through a large umbilical or paraumbilical channel to veins in the abdominal wall. A venous hum may also be heard over other large collaterals such as the inferior mesenteric vein. An arterial systolic murmur usually indicates primary liver cancer or alcoholic hepatitis.
The association of dilated abdominal wall veins and a loud venous murmur at the umbilicus is termed the Cruveilhier–Baumgarten syndrome [48,49]. This may be due to congenital patency of the umbilical vein, but more usually to a well-compensated cirrhosis [48–50].
The paraxiphoid umbilical hum and caput Medusae indicate portal obstruction beyond the origin of the umbilical veins from the left branch of the portal vein. They therefore indicate intrahepatic portal hypertension (cirrhosis).
Spleen
The spleen enlarges progressively. The edge is firm. Size bears little relation to the portal pressure. It is larger in young people and in macronodular rather than micronodular cirrhosis.
An enlarged spleen is the single most important diagnostic sign of portal hypertension. If the spleen cannot be felt or is not enlarged on imaging, the diagnosis of portal hypertension is questionable.
The peripheral blood shows a pancytopenia associated with an enlarged spleen (secondary ‘hypersplenism’). This is related more to reticuloendothelial hyperplasia than to the portal hypertension and is unaffected by lowering the pressure by a portacaval shunt.
Liver
A small liver may be as significant as hepatomegaly, and size should be evaluated by careful percussion. It correlates poorly with the height of portal pressure.
Liver consistency, tenderness or nodularity should be recorded. A soft liver suggests extrahepatic portal venous obstruction. A firm liver supports cirrhosis.
Ascites
This is rarely due to portal hypertension alone, although a particularly high pressure may be a major factor. The portal hypertension raises the capillary filtration pressure, and determines fluid localization to the peritoneal cavity. Ascites in cirrhosis always indicates liver cell failure in addition to portal hypertension.
Rectum
Anorectal varices are visualized by sigmoidoscopy and may bleed. They are found in 44% of patients with cirrhosis, increasing in those who have bled from oesophageal varices [51]. They must be distinguished from simple haemorrhoids which are prolapsed vascular cushions and which do not communicate with the portal system.
X-Ray of the Abdomen and Chest
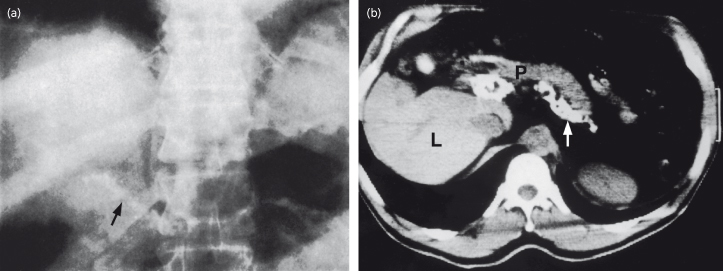
This is useful to delineate liver and spleen. Rarely, a calcified portal vein may be shown (Fig. 9.18) [52].
Fig. 9.18. (a) Plain X-ray of the abdomen. Calcification can be seen in the line of the splenic and portal vein (arrow). (b) CT scan confirms the calcified splenic vein (arrow). L, liver; P, pancreas.
Branching, linear gas shadows in the portal vein radicles, especially near the periphery of the liver and due to gas-forming organisms, may rarely be seen in adults with intestinal infarction or infants with enterocolitis. Portal gas may be associated with disseminated intravascular coagulation. CT and ultrasound may detect portal gas more often, for instance in suppurative cholangitis when the prognosis is not so grave [53].
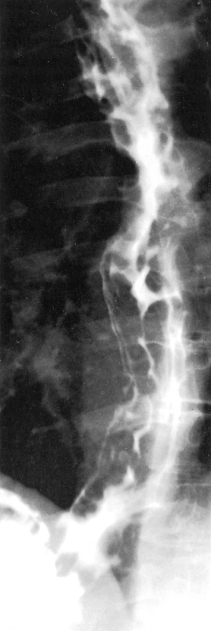
Tomography of the azygos vein may show enlargement (Fig. 9.19) as the collateral flow enters the azygos system.
Fig. 9.19. Tomography of the mediastinum of a patient with large portosystemic collaterals, showing enlargement of the azygos vein (arrow).
A widened left paravertebral shadow may be due to lateral displacement of the pleural reflection between the aorta and vertebral column by a dilated hemiazygos vein.
Massively dilated paraoesophageal collaterals may be seen on the chest radiograph as a retrocardiac posterior mediastinal mass.
Diagnosis of Varices
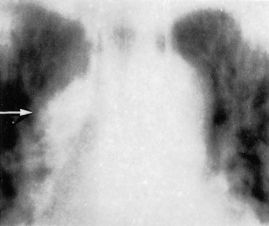
Barium studies have largely been replaced by endoscopy. Oesophageal varices show as filling defects in the regular contour of the oesophagus (Fig. 9.20). They are most often in the lower third, but may spread upwards so that the entire oesophagus is involved. Widening and finally gross dilatation are helpful signs.
Fig. 9.20. Barium swallow X-ray shows a dilated oesophagus. The margin is irregular. There are multiple filling defects representing oesophageal varices.
Gastric varices pass through the cardia, line the fundus in a worm-like fashion and may be difficult to distinguish from mucosal folds.
Occasionally gastric varices show as a lobulated mass in the gastric fundus simulating a carcinoma. Portal venography is useful in differentiation.
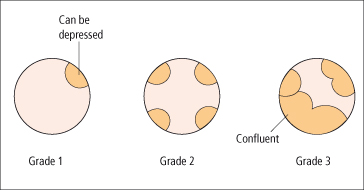
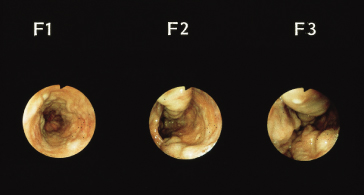
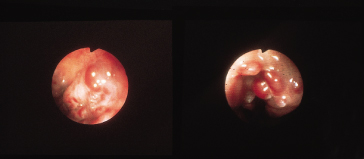
Endoscopy is the best screening test to detect varices. The size of the varix should be graded (Figs 9.21, 9.22) [54]. Varices are small (≤5 mm diameter) or large (>5 mm diameter) when assessed with full insufflation.
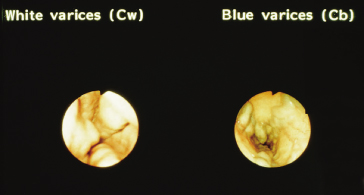
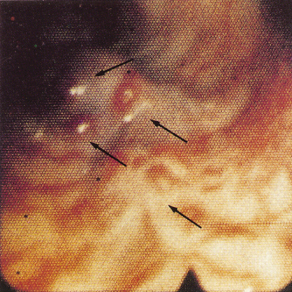
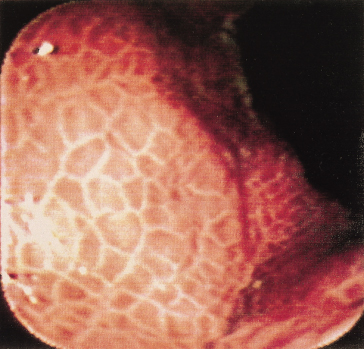
The larger the varix the more likely it is to bleed. Varices usually appear white and opaque (Fig. 9.23). Red colour correlates with blood flow through dilated subepithelial and communicating veins. Dilated subepithelial veins may appear as raised cherry-red spots (Fig. 9.24) and red wheal markings (longitudinal dilated veins resembling whip marks). They lie on top of large subepithelial vessels. The haemocystic spot is approximately 4 mm in diameter (Fig. 9.25). It represents blood coming from the deeper extrinsic veins of the oesophagus straight out towards the lumen through a communicating vein into the more superficial submucosal veins. Red colour is usually associated with larger varices. All these signs are associated with a higher risk of variceal bleeding. Intraobserver error may depend on the skill and experience of the endoscopist. Intraobserver agreement is only good for size and presence of red signs [55].
Portal hypertensive gastropathy is seen largely in the fundus and antrum, but can extend throughout the stomach (Fig. 9.26). It is shown as a mosaic-like pattern with small polygonal areas, surrounded by a whitish-yellow depressed border [56]. Red point lesions and cherry-red spots predict a high risk of bleeding. Black–brown spots are due to intramucosal haemorrhage. Sclerotherapy may increase the gastropathy [57]. Capsule endoscopy is an accurate diagnostic tool to detect oesophageal varices and portal hypertensive gastropathy, but not as good as endoscopy [58]. Its use should be confined to patients in whom endoscopy is contraindicated. If neither type of endoscopy is possible the presence of oesophageal varices can be predicted using platelet count/ spleen diameter ratio [59] with a positive likelihood ratio of 2.77 and negative likelihood ratio of 0.13.
Fig. 9.26. Portal gastropathy. A mosaic of red and yellow is seen together with petechial haemorrhages.
Variceal (azygos) blood flow can be assessed during diagnostic endoscopy by a Doppler ultrasound probe passed down the biopsy channel of the standard gastroscope.
Portal hypertensive colopathy is seen in about half the patients with portal hypertension, usually in those with gastropathy. Colonoscopy may be needed to diagnose lower gastrointestinal bleeding in patients with cirrhosis [60].
Imaging the Portal Venous System
Ultrasound
Longitudinal scans at the subcostal margins and transverse scans at the epigastrium are essential (Fig. 9.27). The portal and superior mesenteric veins can always be seen. The normal splenic vein may be more difficult.
Fig. 9.27. Transverse ultrasound shows a patent portal vein (P); the arrow indicates the inferior vena cava.
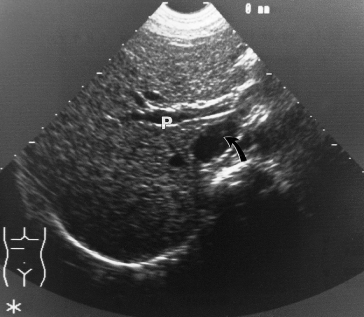
A large portal vein suggests portal hypertension, but this is not diagnostic. If collaterals are seen, this confirms portal hypertension. Portal vein thrombosis is accurately diagnosed and echogenic areas can sometimes be seen within the lumen.
Doppler Ultrasound
Doppler ultrasound demonstrates the anatomy of the portal veins and hepatic artery (Table 9.2). Satisfactory results depend on technical expertise. Small cirrhotic livers are difficult to see as are those of the obese. Colour-coded Doppler improves visualization (Fig. 9.28). Portal venous obstruction is demonstrated by Doppler ultrasound as accurately as by angiography provided the Doppler is technically optimal.
Table 9.2. Clinical uses of Doppler ultrasound
| Portal vein |
| Patency |
| Hepatofugal flow |
| Anatomical abnormalities |
| Portal–systemic shunt patency |
| Acute flow changes |
| Hepatic artery |
| Patency (post-transplant) |
| Anatomical abnormalities |
| Hepatic veins |
| Screening Budd–Chiari syndrome |
Fig. 9.28. Colour Doppler ultrasound of the porta hepatis shows the hepatic artery in red and portal vein in blue.
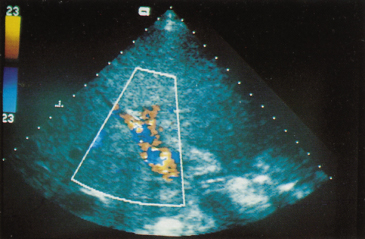
Doppler ultrasound shows spontaneous hepatofugal flow in portal, splenic and superior mesenteric veins in 8.3% of patients with cirrhosis [61]. Its presence correlates with severity of cirrhosis and with encephalopathy. Variceal bleeding is more likely if the flow is hepatopetal.
Abnormalities of the intrahepatic portal veins can be shown. These are important if surgery is contemplated.
Colour Doppler is a good way of demonstrating portal–systemic shunts and the direction of flow in them. These include surgical shunts but also transjugular intrahepatic portosystemic shunts (TIPS). Intrahepatic portal–systemic shunts may be visualized [62].
Colour Doppler screening is useful for patients suspected of the Budd–Chiari syndrome.
The hepatic artery is more difficult than the hepatic veins to locate because of its small size and direction. Nevertheless, duplex Doppler is the primary screening procedure to show a patent hepatic artery after liver transplantation.
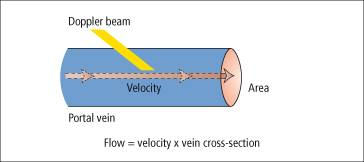
Duplex Doppler has been used to measure portal blood flow. The average velocity of blood flowing in the portal vein is multiplied by the cross-sectional area of the vessel (Fig. 9.29). There are observer errors in measurement. The method is most useful in measuring rapid, large, acute changes in flow rather than monitoring chronic changes in portal haemodynamics.
Portal blood flow velocity correlates with the presence and size of oesophageal varices. In cirrhosis, the portal vein velocity tends to fall and when less than 16 cm/s portal hypertension is likely.
Computed Tomography
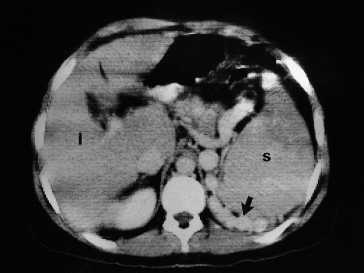
After contrast, portal vein patency can be established and retroperitoneal, perivisceral and paraoesophageal varices may be visualized (Fig. 9.30). Oesophageal varices may be shown as intraluminal protrusions enhancing after contrast. The umbilical vein can be seen. Gastric varices show as rounded structures, indistinguishable from the gastric wall.
Fig. 9.30. Contrast-enhanced CT scan in a patient with cirrhosis and a large retroperitoneal retrosplenic collateral circulation (arrow). l, liver; s, spleen.
CT arterioportography is done by rapid CT scanning during selective injection of contrast into the superior mesenteric vein via a catheter [63]. It is particularly useful in showing focal lesions, the collateral circulation and arteriovenous shunts [64], but is rarely used due to the improvement of dynamic scanning with CT or MR following intravenous contrast.
Magnetic Resonance Angiography
Magnetic resonance angiography gives excellent depiction of blood vessels as regions of absent signal (Figs 9.31–9.33). Portal patency, morphology and flow of velocity may be demonstrated. Magnetic resonance angiography is more reliable than Doppler [65].
Fig. 9.31. Magnetic resonance angiography of a patient with cirrhosis showing the right kidney (K), superior mesenteric vein (SMV), portal vein (PV), left gastric vein (LGV), left branch of portal vein (LBR), gastro-oesophageal collateral veins (V) and the inferior vena cava (IVC).
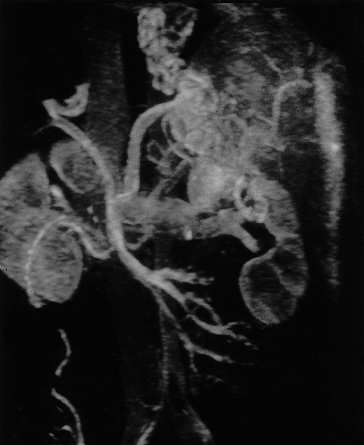
Fig. 9.32. Magnetic resonance angiography in a patient with portal vein thrombosis showing the portal vein replaced by collaterals (PV), the inferior vena cava (IVC) and the aorta (A).
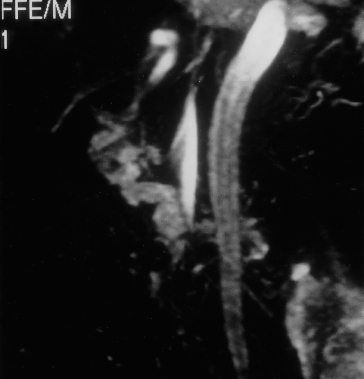
Fig. 9.33. Magnetic resonance angiography showing a spontaneous splenorenal shunt to the inferior vena cava. Black arrow, renal vein; open arrow, vena cava.
Venography
If the portal vein is patent by scanning, confirmation by venography is not necessary even when portal surgery or hepatic transplantation is being considered.
Patency of the portal vein is important, particularly in the diagnosis of splenomegaly in childhood and in excluding invasion by a hepatocellular carcinoma in a patient with cirrhosis.
Anatomy of the portal venous system must be known before such operations as portal–systemic shunt, or transjugular intrahepatic stent shunt, hepatic resection or hepatic transplantation. The patency of a surgical shunt may be confirmed.
The demonstration of a large portal collateral circulation is essential for the diagnosis of chronic hepatic encephalopathy (Figs 9.8, 9.30).
A filling defect in the portal vein or in the liver due to a space-occupying lesion may be demonstrated. Intrasplenic pulp pressure is an index of portal hypertension [66], but has been replaced by direct intrahepatic puncture of the portal vein.
Venographic Appearances
When the portal circulation is normal, the splenic and portal veins are filled but no other vessels are outlined. A filling defect may be seen at the junction of the splenic and superior mesenteric veins due to mixing with non-opacified blood. The size and direction of the splenic and portal veins are very variable. The intrahepatic branches of the portal vein show a gradual branching and reduction in calibre. Later the liver becomes opaque due to sinusoidal filling. The hepatic veins may rarely be seen in later films.
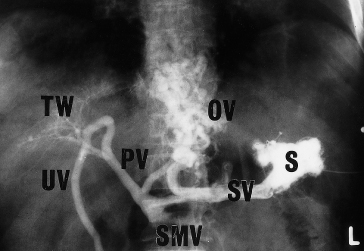
In cirrhosis, the venogram varies widely. It may be completely normal or may show filling of large numbers of collateral vessels with gross distortion of the intrahepatic pattern (‘tree in winter’ appearance) (Fig. 9.34).
Fig. 9.34. Splenic venogram from a patient with cirrhosis of the liver. The gastro-oesophageal collateral circulation can be seen and the intrahepatic portal vascular tree is distorted (‘tree in winter’ appearance). OV, oesophageal veins; PV, portal vein; S, splenic pulp; SMV, superior mesenteric vein; SV, splenic vein; TW, ‘tree in winter’ appearance; UV, umbilical vein.
In extrahepatic portal or splenic vein obstruction, large numbers of vessels run from the spleen and splenic vein to the diaphragm, thoracic cage and abdominal wall. Intrahepatic branches are not usually seen, although, if the portal vein block is localized, paraportal vessels may short circuit the lesion (Fig. 9.32) and produce a delayed but definite filling of the vein beyond.
Visceral Angiography
Safety has increased with the use of smaller (French 5) arterial catheters. New contrast materials are less toxic to kidneys and other tissues and hypersensitivity reactions are rare. However, diagnostic angiography is rarely needed except to demonstrate shunting and when evaluating patients with hepatocellular carcinoma for targeted radioactive bead therapy, and for hepatic arterial problems after liver transplantation.
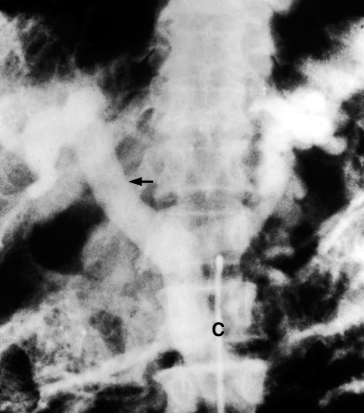
The coeliac axis is catheterized via the femoral artery and contrast is injected. The material that flows into the splenic artery returns through the splenic and portal veins and produces a splenic and portal venogram. Similarly, a bolus of contrast introduced into the superior mesenteric artery returns through the superior mesenteric and portal veins, which can be seen in radiographs exposed at the appropriate intervals (Figs 9.35, 9.36).
Fig. 9.35. Selective coeliac angiogram showing an intrahepatic arterial pattern. A Riedel’s lobe is shown.
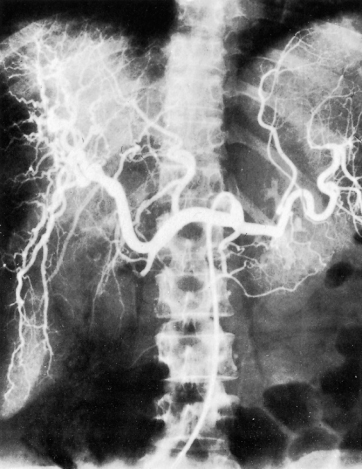
Fig. 9.36. Venous phase of selective coeliac angiogram showing patent portal (arrow) and splenic veins. C, catheter in coeliac axis.
Visceral angiography demonstrates the hepatic arterial system, so allowing space-filling lesions in the liver to be identified. A tumour circulation may diagnose hepatocellular cancer or another tumour.
Knowledge of splenic and hepatic arterial anatomy is useful if surgery is contemplated. Haemangiomas, other space-occupying lesions and aneurysms may be identified.
The portal vein may not opacify if flow in it is hepatofugal or if there is ‘steal’ by the spleen or by large collateral channels. A superior mesenteric angiogram will confirm that the portal vein is in fact patent.
Digital Subtraction Angiography
The contrast is given by selective arterial injection with immediate subtraction of images. The portal system is very well visualized free of other confusing images (Fig. 9.37). Spatial resolution is poorer than with conventional film-based angiography. The technique is particularly valuable for the parenchymal phase of hepatic angiography and for the diagnosis of vascular lesions such as haemangiomas or arteriovenous malformations.
Splenic Venography
Contrast material, injected into the pulp of the spleen, flows into the portal venous system with sufficient rapidity to outline splenic and portal veins (Fig. 9.34). The collateral circulation is particularly well visualized [67]. Splenic venography has now been replaced by less invasive procedures.
Carbon Dioxide Occluded Venography
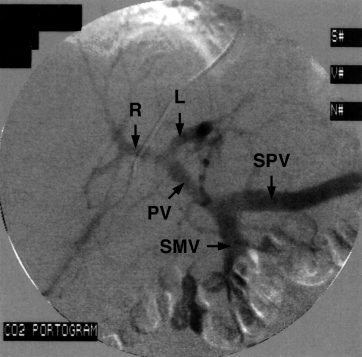
Injection of carbon dioxide into a catheter in the wedged hepatic venous position allows an excellent venogram of the hepatic venous and portal venous tree (Fig. 9.38) [68].
Fig. 9.38. Carbon dioxide portal venography real-time imaging following the injection of carbon dioxide into the wedged hepatic vein. PV, portal vein (L, left branch; R, right branch); SMV, superior mesenteric vein; SPV, splenic vein.
Portal Pressure Measurement
A balloon catheter is introduced into the femoral vein or internal jugular vein and, under fluoroscopic control, into the hepatic vein (Fig. 9.39). Measurements are taken in the wedged hepatic venous pressure (WHVP) and free hepatic venous pressure (FHVP) positions by inflating and deflating the balloon in the tip of the catheter [67,69]. The hepatic venous pressure gradient (HVPG) is the difference between WHVP and FHVP. This is the portal (sinusoidal) venous pressure. When the cause of portal hypertension is mainly sinusoidal (alcohol, viral hepatitis) the WHVP is the same as the portal pressure, but this relationship does not hold when there is a large presinusoidal component [70]. The normal HVPG is 5–6 mmHg and values of 10 mmHg or more represent clinically significant portal hypertension when complications of cirrhosis (decompensation) can occur [71]. Measurements can be performed at the same time as transjugular liver biopsy [72].
Fig. 9.39. A catheter has been inserted into a hepatic vein via the jugular vein. The wedged position is confirmed by introducing a small amount of contrast, which has entered the sinusoidal bed.
HVPG is related to survival [73] and also to prognosis in patients with bleeding oesophageal varices [74]. The procedure may be used to monitor therapy, for instance the effect of beta-blockers such as propranolol, with optimal target reduction of HVPG by 20% from baseline or to less than 12 mmHg, which results in a reduced risk of bleeding [75].
Variceal Pressure
An endoscopic pressure gauge may be fixed to the end of the endoscope. The level of venous pressure is a major factor predicting variceal haemorrhage [76].
Pressure may be recorded by direct puncture of varices at the time of sclerotherapy [77]. It is about 15.5 mmHg in cirrhotic patients, significantly lower than the main portal pressure of about 18.8 mmHg. An endoscopic balloon has been developed to measure variceal pressure and this gives comparable results to direct puncture [78].
Estimation of Hepatic Blood Flow
Constant Infusion Method
Hepatic blood flow may be measured by a constant infusion of indocyanine green (ICG) and catheterization of the hepatic vein [79,80]. Flow is calculated by the Fick principle.
Plasma Disappearance Method
Hepatic blood flow can be measured after an intravenous injection of ICG followed by analysis of the disappearance curve in a peripheral artery and hepatic vein. If the extraction of a substance is about 100%, for instance, using 131I heat-denatured albumin colloidal complex, hepatic blood flow can be determined by peripheral clearance without hepatic vein catheterization. However, in patients with cirrhosis, as up to 20% of the blood perfusing the liver may not go through normal channels and hepatic extraction is reduced, hepatic vein catheterization is necessary to estimate extraction and thus hepatic blood flow.
Azygos Blood Flow
Most of the blood flowing through gastro-oesophageal varices terminates in the azygos system. Azygos blood flow can be measured using a double thermodilution catheter directed under fluoroscopy into the azygos vein [81]. Alcoholic cirrhotic patients who have bled from varices show a flow of about 600 mL/min. Azygos flow is markedly reduced by propranolol.
Experimental Portal Venous Occlusion and Hypertension
Survival following acute occlusion depends on the development of an adequate collateral circulation. In the rabbit, cat or dog this does not develop and death supervenes rapidly. In the monkey or man, the collateral circulation is adequate and survival is usual.
Acute occlusion of one branch of the portal vein is not fatal. The liver cells of the ischaemic lobe atrophy, but bile ducts, Kupffer cells and connective tissues survive. The unaffected lobe hypertrophies.
Experimentally, portal hypertension can be produced by occluding the portal vein, injecting silica into the portal vein, infecting mice with schistosomiasis, by any experimental type of cirrhosis or by biliary obstruction. An extensive collateral circulation develops, the spleen enlarges but ascites does not form.
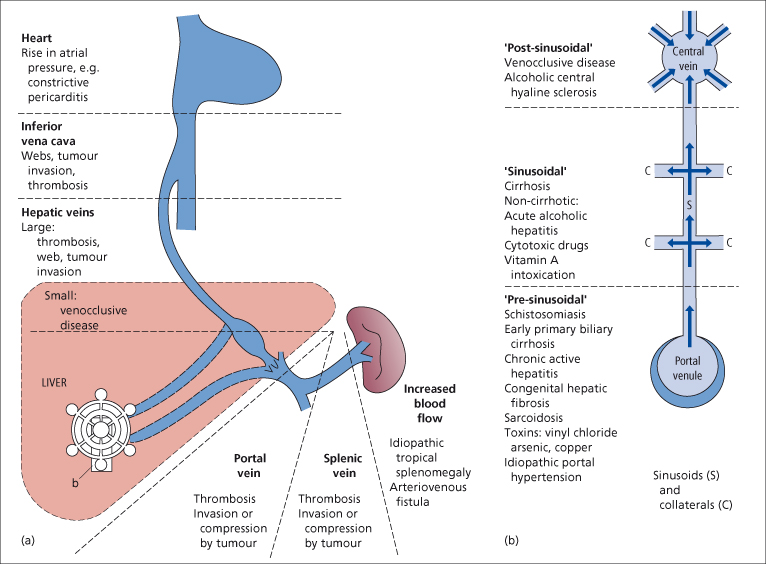
Classification of Portal Hypertension
Portal hypertension usually follows obstruction to the portal blood flow anywhere along its course. Portal hypertension has been classified into two types: (1) presinusoidal (extrahepatic or intrahepatic); and (2) a larger group of hepatic causes (intrahepatic ‘sinusoidal’ and postsinusoidal) (Fig. 9.40, Table 9.3). This distinction is a practical one. The presinusoidal forms, which include obstruction to the sinusoids by Kupffer and other cellular proliferations, are associated with relatively normal hepatocellular function. Consequently, if patients with this type suffer a haemorrhage from varices, liver failure is rarely a consequence. In contrast, patients with the hepatic type may develop liver failure after bleeding.
Table 9.3. Classification of portal hypertension
| Presinusoidal | Extrahepatic | Blocked portal vein |
| Increased splenic flow | ||
| Intrahepatic | Portal zone infiltrates | |
| Toxic | ||
| Hepatoportal sclerosis | ||
| Hepatic | Intrahepatic (sinusoidal) | Cirrhosis |
| Postsinusoidal | Other nodules | |
| Blocked hepatic vein |
Fig. 9.40. Causes of portal hypertension. (a) Pre- and posthepatic. (b) Intrahepatic (NB an overlap exists; wedge hepatic vein pressure may be high in patients with ‘presinusoidal’ causes, especially as the disease progresses, indicating sinusoidal and/or collateral involvement. Some ‘postsinusoidal’ conditions may also have a sinusoidal component).
Extrahepatic Portal Venous Obstruction
This causes extrahepatic presinusoidal portal hypertension. The obstruction may be at any point in the course of the portal vein, usually due to thrombosis. The venae comitantes enlarge in an attempt to deliver portal blood to the liver, so assuming a leash-like cavernous appearance. The portal vein, represented by a fibrous strand, is recognized with difficulty in the multitude of small vessels. This cavernous change follows any block in the main vein (see Fig. 9.32). Confluent thrombosis may extend to the splenic and/or superior mesenteric vein [82].
Aetiology
Infections
Umbilical infection with or without catheterization of the umbilical vein may be responsible in neonates [83]. The infection spreads along the umbilical vein to the left portal vein and hence to the main portal vein. Acute appendicitis and peritonitis are causative in older children.
Portal vein occlusion is particularly common in India, accounting for 20–30% of all variceal bleeding. Neonatal dehydration and infections may be responsible.
Ulcerative colitis and Crohn’s disease can be complicated by portal or hepatic vein thrombosis.
Portal vein obstruction may be secondary to biliary infections due, for instance, to gallstones or primary sclerosing cholangitis.
Postoperative
The portal and splenic veins commonly thrombose after splenectomy, especially when, preoperatively, the patient had a normal platelet count. The thrombosis spreads from the splenic vein into the main portal vein. It is especially likely in patients with myeloid metaplasia. A similar sequence follows occluded surgical portosystemic shunts.
The portal vein may thrombose as a complication of major, difficult hepatobiliary surgery, for instance repair of a stricture or removal of a choledochal cyst.
Trauma
Portal vein injury may rarely follow vehicle accidents or stabbing. Laceration of the portal vein is 50% fatal and ligation may be the only method to control the bleeding.
Hypercoagulable State
This is a frequent cause of portal vein thrombosis in adults and less often in children [84]. It is commonly due to a myeloproliferative disorder which may be latent, or the presence of G20210A prothrombin gene mutation, and/or one or more heterozygous or homozygous deficiency states for protein C, S, antithrombin III or other prothrombotic tendencies [85]. At autopsy, thrombotic lesions are found in macroscopic and microscopic portal veins of patients dying with portal hypertension and myelometaplasia [86]. Ascites and oesophageal varices are associated.
Invasion and Compression
The classic example is hepatocellular carcinoma. Carcinoma of the pancreas, usually of the body, and of other adjacent organs may lead to portal vein thrombosis. Chronic pancreatitis is frequently associated with splenic vein obstruction, but involvement of the portal vein is rare (5.6%) [82,87].
Congenital
Congenital obstruction can be produced anywhere along the line of the right and left vitelline veins from which the portal vein develops. The portal vein may be absent with visceral venous return passing to systemic veins, particularly the inferior vena cava [88]. Hilar venous collaterals are absent.
Congenital abnormalities of the portal vein are usually associated with congenital defects elsewhere [88,89].
Cirrhosis
Portal vein thrombosis is not infrequent as a complication of cirrhosis [90]. Invasion by a hepatocellular carcinoma is a frequent cause. Postsplenectomy thrombocytosis is another aetiological factor. Mural thrombi found at autopsy are probably terminal. It is easy to over-diagnose thrombosis by finding a non-filled portal vein on imaging. This usually represents ‘steal’ into massive collaterals or into a large spleen [90].
Miscellaneous
Portal vein thrombosis has very rarely been associated with pregnancy and with oral contraceptives, especially in older women and with long usage [91] and with thrombophlebitis migrans and other general disease of veins.
In retroperitoneal fibrosis, the portal venous system may be encased by dense fibrous tissue.
Portal vein occlusion with recanalization is a common manifestation of Behçet’s disease [92].
Unknown
In about half of patients the aetiology remains obscure. Some of these patients have associated autoimmune disorders such as hypothyroidism, diabetes, pernicious anaemia, dermatomyositis or rheumatoid arthritis [82]. In some instances, the obstruction may have followed undiagnosed intra-abdominal infections such as appendicitis or diverticulitis.
Clinical Features
The patient may present with features of the underlying disease, for instance polycythaemia rubra vera [86] or primary liver cancer. Children may have growth retardation [93].
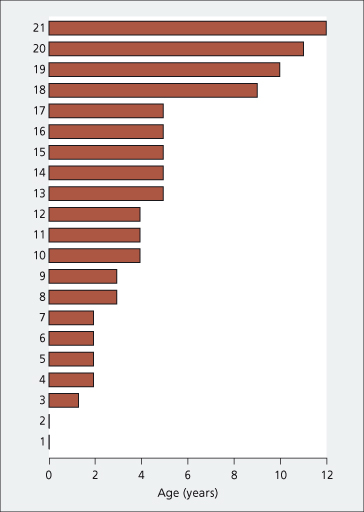
Bleeding from oesophagogastric varices is the most common presentation. In those of neonatal origin, the first haemorrhage is at about the age of 4 years (Fig. 9.41). The frequency increases between 10 and 15 years and decreases after puberty. However, some patients with portal venous thrombosis never bleed and in others haemorrhage may be delayed for as long as 12 years. If blood replacement is adequate, recovery usually ensues in a matter of days. Apart from frank bleeds, intermittent minor blood loss is probably common. This is diagnosed only if the patient is having repeated checks for faecal blood or iron deficiency anaemia.
Fig. 9.41. Portal vein occlusion in neonates. Age at time of first haemorrhage in 21 patients in whom the portal vein block occurred in the neonatal period.
Especially in children, haemorrhage may be initiated by a minor, intercurrent infection. The mechanism is unclear. Aspirin or a similar drug may be the precipitating factor. Excessive exertion or swallowing a large bolus does not seem to initiate bleeding.
The spleen is always enlarged and symptomless splenomegaly may be a presentation, particularly in children. Periumbilical veins are not seen but there may be dilated abdominal wall veins in the left flank.
The liver is normal in size and consistency. Stigmata of hepatocellular disease, such as jaundice or vascular spiders, are absent. With acute portal venous thrombosis, ascites is early and transient, subsiding as the collateral circulation develops. Ascites is usually related to an additional factor that has depressed hepatocellular function, such as a haemorrhage or a surgical exploration. It may be seen in the elderly where it is related to the deterioration of liver function with ageing [94].
Hepatic encephalopathy is not uncommon in adults, usually following an additional insult such as haemorrhage, infection or anaesthetic. Chronic encephalopathy may be seen in elderly patients with a particularly large portal–systemic circulation. Rarely, compression of the common bile duct can occur, termed ‘portal biliopathy’ [95], which may cause jaundice.
Imaging
Ultrasound shows echogenic thrombus within the portal vein and colour Doppler shows slow flow velocity in the cavernous collaterals and no portal venous signal [96,97].
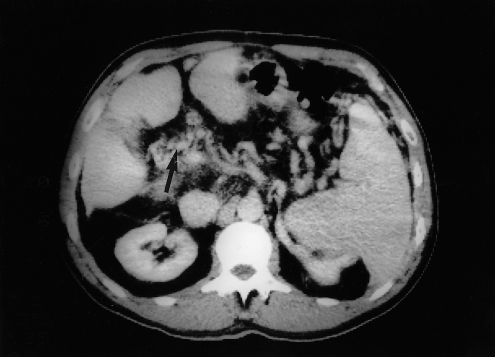
CT shows the thrombus as a non-enhancing filling defect within the lumen of the portal vein and dilatation of many small veins at the hilum (Fig. 9.42).
Fig. 9.42. Abdominal CT scan with contrast showing the main portal vein replaced by a leash of small veins (arrow).
MRI shows an area of abnormal signal within the lumen of the portal vein which appears isointense on a T1-weighted image with a more intense signal on a T2-weighted image.
Angiography in the portal venous phase shows a filling defect or non-opacification of the portal vein. However, the portal vein may not be visualized if blood is diverted away from it into extensive collaterals.
Haematology
Haemoglobin is normal unless there has been blood loss. Leucopenia and thrombocytopenia are related to the enlarged spleen. Circulating platelets and leucocytes, although in short supply, are adequate and function well.
Hypersplenism is not an indication for splenectomy. Blood coagulation is normal.
Serum Biochemistry
All the usual tests of ‘liver function’ are normal. Elevation of serum globulin may be related to intestinal antigens, bypassing the liver through collaterals. Mild pancreatic hypofunction is related to interruption of the venous drainage of the pancreas [98].
Prognosis
This depends on the underlying disease [82]. The outlook is much better than for cirrhosis as liver function is normal. The prognosis is surprisingly good in the child and, with careful management of recurrent bleeding, survival to adult life is expected. The number of bleeds seems to reduce as time passes. Women may bleed in pregnancy but this is unusual; their babies are normal.
Treatment
Any cause must be identified and treated. This may be more important than the portal hypertension. For instance, hepatocellular carcinoma, invading the portal vein, precludes aggressive therapy for bleeding oesophageal varices. If the variceal bleeding is related to polycythaemia rubra vera, reduction of the platelet count must precede any surgical therapy; anticoagulants may be needed [82].
Prophylactic treatment of varices is not indicated. They may never rupture and as time passes collaterals open up.
With acute portal vein thrombosis, anticoagulant therapy will result in recanalization in one-third of patients [99]. If diagnosed early, anticoagulants may prevent spreading thrombosis and intestinal infarction or severe bleeding. Presence of ascites and splenic vein thrombosis should lead to alternative therapies [99].
Children should survive haemorrhage with proper management, including transfusion. Care must be taken to give compatible blood and to preserve peripheral veins. Aspirin ingestion should be avoided. Upper respiratory any other infections should be treated seriously as they seem to precipitate haemorrhage.
Endoscopic therapy is valuable as an emergency procedure; balloon tamponade may be needed.
Major or recurrent bleeds may be treated by repeated sclerotherapy, particularly in children, or ligation. Unfortunately this does not treat gastric fundal varices and the congestive gastropathy remains.
Definitive surgery to reduce portal pressure maybe impossible as there are no suitable veins for a shunt. Even apparently normal-looking veins seen on venography turn out to be in poor condition, presumably related to extension of the original thrombotic process. In children, veins are very small and difficult to anastomose.
Results for all forms of surgery are unsatisfactory. Splenectomy is the least successful.
A shunt (portacaval, mesocaval or splenocaval) is the most satisfactory treatment. In children a mesentericoportal shunt, anastomosing to a patent left portal vein branch, not only prevents bleeding, but improves growth [100].
When the patient is exsanguinating, despite massive blood transfusion, an oesophageal transection may have to be performed. Here again gastric varices are not treated. Postoperative complications are common.
TIPS may be possible providing the superior mesenteric vein is patent [101].
Splenic Vein Obstruction
Isolated splenic vein obstruction causes sinistral (left-sided) portal hypertension. It may be due to any of the factors causing portal vein obstruction (Fig. 9.43). Pancreatic disease such as carcinoma (18%), pancreatitis (65%), pseudocyst and pancreatectomy are particularly important [87].
Fig. 9.43. A 64-year-old man with polycythaemia rubra vera. Transhepatic portal venogram (transhepatic needle marked by upper arrow) showing a thrombosed splenic vein (marked by the lower arrow) with patent superior mesenteric and portal veins. This patient, after preliminary reduction of red cell and platelet count by radioactive phosphorus, was successfully treated by splenectomy.
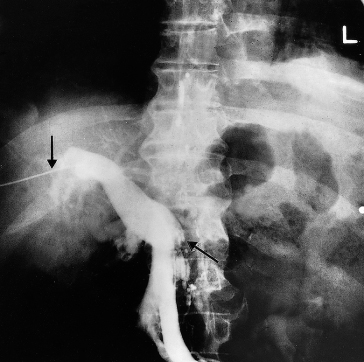
If the obstruction is distal to the entry of the left gastric vein, a collateral circulation bypasses the obstructed splenic vein through short gastric veins into the gastric fundus and lower oesophagus, so reaching the left gastric vein and portal vein. This leads to very prominent varices in the fundus of the stomach but few in the lower oesophagus.
The selective venous phase of an angiogram, an enhanced CT scan or MRI are diagnostic. Splenectomy, by blocking arterial inflow, is usually curative but unnecessary if the patient has not bled from varices [102].
Hepatic Arterioportal Venous Fistulae
Portal hypertension results from increased portal venous flow. Increase in intrahepatic resistance due to a rise in portal flow may also be important. Portal zones show thickening of small portal radicles with accompanying mild fibrosis and lymphocyte infiltration. The increased intrahepatic resistance may persist after obliteration of the fistula.
These fistulae are usually congenital, traumatic (including after liver biopsy) or related to adjacent malignant neoplasm [103]. Inferior mesenteric arteriovenous fistulae may be associated with acute ischaemic colitis.
With large fistulae, a loud arterial bruit is heard in the right upper abdomen. Pain may be pronounced. Others present with portal hypertension.
Ultrasound and enhanced CT show an enlarged hepatic artery and a dilated intrahepatic portal vein. The diagnosis is confirmed by arteriography.
Selective non-invasive embolization of fistulae has replaced surgery.
Portohepatic Venous Shunts
These are probably congenital and represent persistence of the omphalomesenteric venous system. They may be between the main portal and hepatic veins or between the right or left portal vein and hepatic veins [104]. They are diagnosed by ultrasound, enhanced CT scan, MRI and colour Doppler imaging and confirmed by arteriography.
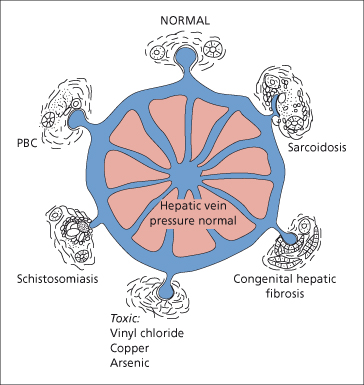
Presinusoidal Intrahepatic and Sinusoidal Portal Hypertension (Fig. 9.44)
Portal Tract Lesions
In schistosomiasis, the portal hypertension results from the ova causing a reaction in the minute portal venous radicles.
Fig. 9.44. The aetiology of presinusoidal intrahepatic portal hypertension. PBC, primary biliary cirrhosis.
In congenital hepatic fibrosis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree