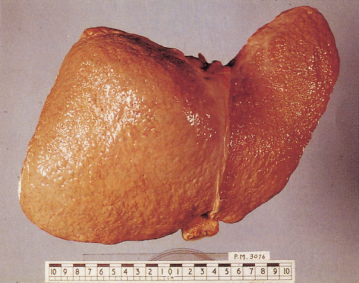
Nodule formation without fibrosis, as in partial nodular transformation (Fig. 7.1), is not cirrhosis.
Causes of Cirrhosis (Table 7.1)
In Western countries the prevalence of alcoholic cirrhosis, NASH cirrhosis (non-alcoholic steatohepatitis) and viral cirrhosis, in particular hepatitis C, are all increasing. In developing countries, the predominant causes are hepatitis virus B and C, but alcohol and autoimmune conditions may be increasing.
Table 7.1. Aetiology and definitive treatment of cirrhosis
| Aetiology | Treatment |
| Viral hepatitis (B, C and D) | Antivirals |
| Alcohol | Abstention |
| NASH | Weight loss |
| Metabolic | |
| iron overload (HFE haemochromatosis) | Venesection |
| copper overload (Wilson’s disease) | Copper chelator |
| α1-antitrypsin deficiency | ? Transplant |
| type IV glycogenesis | ? Transplant |
| galactosaemia | Withdraw milk and milk products |
| tyrosinaemia | Withdraw dietary tyrosine. ? Transplant |
| Primary biliary cirrhosis | ? Transplant |
| Primary sclerosing cholangitis | ? Transplant |
| Hepatic venous outflow block | |
| Budd–Chiari syndrome | Relieve main vein block. ? Transplant |
| heart failure | Treat cardiac cause |
| Autoimmune hepatitis | Immunosuppression |
| Toxins and drugs, e.g. methotrexate, amiodarone | Identify and stop |
NASH, non-alcoholic steatohepatitis.
Cirrhosis where the aetiology cannot be determined is termed cryptogenic. This is a diagnosis of exclusion. With improving diagnostic techniques the proportion of patients labelled cryptogenic is falling. In some cases it may be difficult to determine the aetiology as specific histological features may disappear with burnt out cirrhosis, for example autoimmune hepatitis, non-alcoholic steatohepatitis or sarcoidosis.
Cirrhosis and Co-Factors (Fig. 7.2)
In some forms of liver disease there is a single cause, for example in hepatitis B and C, primary biliary cirrhosis and primary sclerosing cholangitis. However, in many cases co-factors may be important. Thus the prevalence of subjects homozygous for the C282Y mutation for haemochromatosis is between 1/100 and 1/200 in the UK and Ireland. However, only a small fraction of these subjects ever manifest signs of cirrhosis due to haemochromatosis. Suggested co-factors include age, sex, obesity, alcohol, iron intake and other genetic factors as yet unknown. Similarly, many subjects drink excessive quantities of alcohol but only a small proportion ever develop cirrhosis. NASH cirrhosis only develops in a small proportion of obese diabetics.
Fig. 7.2. Many liver diseases have a major initiating factor and a number of co-factors contributing to the development of cirrhosis.
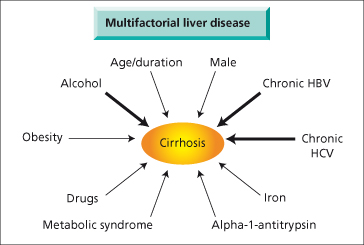
Causes of liver disease also interact. Progressive disease is more likely in patients with hepatitis B or C who drink excess alcohol. Patients heterozygous for α-1-antitrypsin deficiency who are obese are more likely to manifest cirrhosis.
The risk of developing cirrhosis may also depend on the age and sex of the patient, duration of the disease and immunological status. For patients infected with hepatitis C, fibrosis progression is more rapid in patients infected at an older age and increases with duration of infection [1]. Patients with insulin resistance or diabetes mellitus, or who are immunosuppressed, are at higher risk for developing cirrhosis from several aetiologies.
Thus in many cases there can be a principal factor and interacting co-factors which cause a patient to develop cirrhosis (Fig. 7.2). The relative importance of these co-factors may vary from patient to patient.
Anatomical Diagnosis
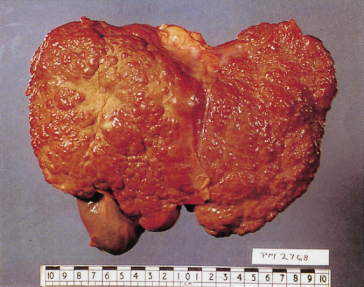
The diagnosis of cirrhosis depends on demonstrating widespread nodules in the liver combined with fibrosis. Cirrhosis may be classified as micronodular (Fig. 7.3), macronodular (Fig. 7.4) or mixed.
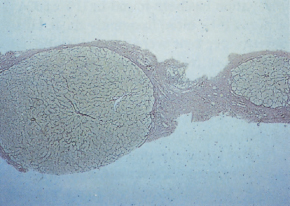
Liver biopsy is the gold standard for diagnosis [2]. Interpretation may be limited by small size and sampling error. This is particularly true if a suction needle rather than a cutting needle is employed to obtain the biopsy, resulting in many instances in fragmented liver tissue. Specialist liver histopathology is essential. Even with small biopsies the expert histopathologist may be able to make a diagnosis of cirrhosis in conjunction with the clinical situation or imaging findings, by recognizing a rim of fibrosis at the periphery of the fragments (Fig. 7.5), and the lack of normally related portal tracts and hepatic venules in the parenchyma, often with a widened reticulin pattern or architectural disruption. Conversely, a non-fragmented core of liver without definite nodules may be obtained from a macronodular cirrhotic liver. Helpful diagnostic points in these circumstances include absence of portal tracts, abnormal vascular arrangements, hepatic arterioles not accompanied by portal veins, the presence of nodules with fibrous septa and variability in cell size and appearance in different areas of the biopsy [3].
Fig. 7.5. Liver biopsy in cirrhosis: the specimen is small but nodules are shown outlined by reticulin. (Reticulin stain, ×40.)
Liver biopsy contributes to the diagnosis of the aetiology of cirrhosis by identifying features such as alpha-1 antitrypsin globules. The biopsy may help in the reclassification of cryptogenic cirrhosis by identifying histological markers of aetiology, such as steatosis indicating NASH, or inflammation suggesting autoimmune hepatitis (Table 7.2).
Table 7.2. Histopathology and aetiology of cirrhosis
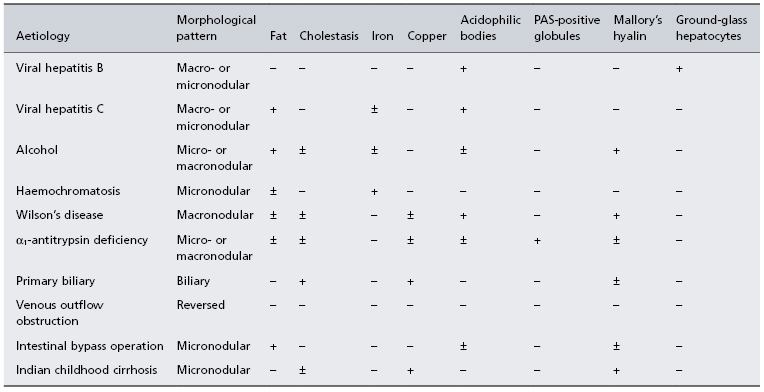
− Usually absent; ± may be present; + usually present.
Liver biopsy is not without risk (see Chapter 3). If there are contraindications, such as ascites or a coagulation defect, the transjugular approach should be used. In many cases a diagnosis of cirrhosis can be made on the basis of a combination of clinical features and liver imaging. Ultrasound, CT or MRI may identify cirrhosis if nodular change or alterations to the shape and size of the liver can be appreciated. Imaging may miss early cirrhosis. Non-invasive fibroscanning is increasingly being used to assess liver fibrosis, as are combinations of serum markers (see Chapter 6).
Transient elastography (fibroscan) is a non-invasive method of evaluating liver fibrosis/ cirrhosis. It appears to be particularly useful in patients with chronic hepatitis C and is superior to laboratory based tests [4]. Fibroscan is technically difficult in obese subjects and this may limit its usefulness in the diagnosis of NASH cirrhosis.
Ultrasound is not reliable for the diagnosis of cirrhosis but is useful for screening for hepatocellular carcinoma in patients with known cirrhosis, and for evaluating patency of the portal vein and the presence of ascites. Contrast-enhanced ultrasound is helpful in distinguishing benign and malignant liver nodules.
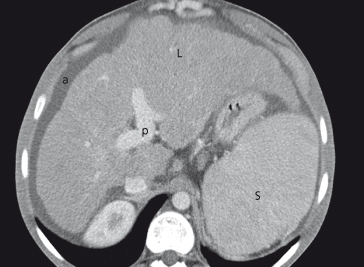
CT scan can assess liver size and shape and identify liver nodules (Fig. 7.6). It provides an objective, permanent record for evaluating changes over time. Fatty change and space-occupying lesions can be recognized. After intravenous contrast, the portal vein and hepatic veins can be identified, and a collateral circulation with splenomegaly may confirm the diagnosis of portal hypertension. Ascites can be seen. Multiphase CT is useful in the evaluation of focal liver lesions and directed biopsy of a selected area can be performed safely. However the radiation dose with repeated multislice CT scans is substantial and may be an issue, particularly in younger patients.
Fig. 7.6. CT scan, after intravenous contrast, in cirrhosis shows ascites (a), liver with irregular surface (L), patent portal vein (p) and splenomegaly (S).
MRI may identify cirrhosis of the liver but is expensive and many patients find the procedure claustrophobic. It is most useful for evaluating the biliary tree (MR cholangiography) or for evaluating possible malignancy in liver nodules (contrast enhanced MRI) (see Chapter 33).
Reversible Cirrhosis
Cirrhosis is usually believed to be irreversible. However fibrosis may regress if the initiating insult is removed, for example hepatitis C, biliary obstruction, obesity or iron overload. Reversal of cirrhosis has been demonstrated in some patients [5–7]. In most cases repeat liver biopsies have shown a lesser degree of fibrosis rather than a reversion to normal liver. Nevertheless, they support the concept that irreversibility is not an absolute rule.
Clinical Cirrhosis: Compensated Versus Decompensated
Patients may present with complications of liver disease or the presence of liver disease may be picked up when they are seen for other reasons. Cirrhosis can be symptomatic or asymptomatic. Many patients are found to have abnormal liver tests during routine medical or preoperative examinations. These liver test abnormalities may be relatively minor. On physical examination the detection of unexpected hepatomegaly or splenomegaly may trigger further investigation. The finding of an enlarged, smooth, palpable left lobe in the epigastrium is a particularly useful clinical sign [8]. Investigations useful in the work up of a patient with suspected cirrhosis are summarized in Table 7.3.
Table 7.3. General investigations in the patient with cirrhosis (see also Table 9.1)
| Occupation, age, sex, domicile |
| Clinical history |
| Fatigue and weight loss |
| Anorexia and flatulent dyspepsia |
| Abdominal pain |
| Jaundice. Itching. Colour of urine and faeces |
| Swelling of legs or abdomen |
| Haemorrhage—nose, gums, skin, alimentary tract |
| Loss of libido; menstrual history |
| Past health: jaundice, hepatitis, drugs ingested, blood transfusion |
| Social: alcohol consumption |
| Family history: liver disease, autoimmune disease |
| Examination |
| Nutrition, fever, fetor hepaticus, jaundice, pigmentation, purpura, finger clubbing, white nails, vascular spiders, palmar erythema, gynaecomastia, testicular atrophy, distribution of body hair, parotid enlargement, Dupuytren’s contracture, blood pressure |
| Abdomen: ascites, abdominal wall veins, liver, spleen |
| Peripheral oedema |
| Neurological changes: mental functions, stupor, tremor |
| Investigations |
| Haematology |
| haemoglobin, leucocyte and platelet count, prothrombin time (INR) |
| Serum biochemistry |
| bilirubin |
| transaminase |
| alkaline phosphatase |
| γ-glutamyl-transpeptidase |
| albumin and globulin |
| immunoglobulins |
| transferrin saturation and serum ferritin |
| serum caeruloplasmin and copper |
| α-1-antitrypsin phenotype |
| If ascites present |
| serum sodium, potassium, bicarbonate, chloride, urea and creatinine levels |
| weigh daily |
| 24-h urine volume and sodium excretion |
| Serum immunological |
| smooth muscle, mitochondrial, nuclear, LKM1 antibodies, and ANCA |
| hepatitis B antigen (HBsAg), anti-HCV (other markers of hepatitis, see Chapters 18 and 20) |
| α-fetoprotein |
| Endoscopy |
| Hepatic ultrasound, CT or MRI scan |
| Needle liver biopsy if blood coagulation permits |
| EEG if neuropsychiatric changes |
In clinical terms, cirrhosis is described as are either ‘compensated’ or ‘decompensated’. Decompensation means cirrhosis complicated by one or more of the following features: jaundice, ascites, hepatic encephalopathy or bleeding varices. Ascites is the usual first sign. Hepatorenal syndrome, hyponatraemia and spontaneous bacterial peritonitis are also features of decompensation but in these patients ascites invariably occurs first. Compensated cirrhotic patients have none of these features.
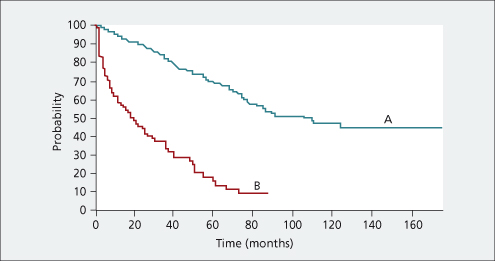
This is a very important clinical distinction and has major implications for prognosis and treatment. Compensated cirrhotic patients have a 50% 10-year survival as compared to 50% survival at 18 months for decompensated patients [9] (Fig. 7.7). Cirrhotic patients become decompensated at the rate of approximately 10% per year. Decompensated patients can improve and become compensated with an associated improvement in prognosis. In general, patients with decompensated cirrhosis should be considered for liver transplantation.
Fig. 7.7. Patients with decompensated cirrhosis (B) have much reduced probability of survival compared to patients with compensated cirrhosis (A).
Reprinted with permission of John Wiley and Sons Inc. [9].
Compensated Cirrhosis
The disease may be discovered at a routine examination or biochemical screen, or at operation undertaken for some other condition. Cirrhosis may be suspected if the patient has vascular spiders, palmar erythema, unexplained epistaxis or oedema of the ankles. Firm enlargement of the liver, particularly in the epigastrium, and splenomegaly are helpful diagnostic signs. Confirmation should be sought by biochemical tests, scanning and, if necessary, liver biopsy.
Biochemical tests may be quite normal in this group. The most frequent changes are a slight increase in the serum transaminase or γ-glutamyl transpeptidase concentration. Portal hypertension may be present even with normal liver function tests. Diagnosis is confirmed by liver imaging or needle liver biopsy.
These patients may remain compensated until they die from another cause. Hepatocellular carcinoma occurs at a rate of 1–3% per year and appropriate screening is recommended (see Chapter 35). Decompensation may be precipitated by bacterial infection, surgery, trauma or medication.
Decompensated Cirrhosis
The patient usually seeks medical advice because of ascites, jaundice or gastrointestinal bleeding. General health fails with weakness, muscle wasting and weight loss. Continuous mild fever (37.5–38°C) is often due to Gram-negative bacteraemia, to continuing hepatic cell necrosis, ongoing alcoholic hepatitis or to a complicating hepatocellular carcinoma. A liver flap may be present. Cirrhosis is the commonest cause of hepatic encephalopathy.
Jaundice implies that liver cell destruction exceeds the capacity for regeneration and is always serious. The deeper the jaundice the greater the inadequacy of liver cell function.
The skin may be pigmented. Clubbing of the fingers is occasionally seen. Purpura over the arms, shoulders and shins may be associated with a low platelet count. The circulation is over-active. The blood pressure is low. Sparse body hair, vascular spiders, palmar erythema, white nails and gonadal atrophy are common.
Ascites is usually preceded by abdominal distension. Oedema of the legs is frequently associated.
The liver may be enlarged, with a firm regular edge, or contracted and impalpable. The spleen may be palpable.
Vasodilatation and Hyperdynamic Circulation
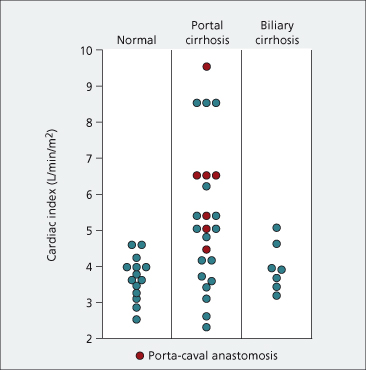
Many of the complications seen in decompensated cirrhotic patients are believed to be due to vasodilatation and the hyperdynamic circulation (Fig. 7.8). Decompensated cirrhosis can be viewed as a circulatory or a haemodynamic disease. Vasodilatation is shown by flushed extremities, bounding pulses, capillary pulsations and relative arterial hypotension. Peripheral arterial blood flow and portal venous blood flow are increased. Cardiac output is raised [10] (Fig. 7.9) and evidenced by tachycardia, an active precordial impulse and frequently an ejection systolic murmur. Renal blood flow, and particularly cortical perfusion, is reduced.
Fig. 7.8. Many of the complications of cirrhosis are due to arterial dilatation and the hyperdynamic circulation.
Reprinted with permission of Elsevier from McCormick PA, Donnelly C. Pharmacol. Ther. 2008; 119: 106.
Fig. 7.9. The cardiac output is raised in many patients with hepatic cirrhosis but within normal limits in biliary cirrhosis. Mean normal cardiac index is 3.68 ± 0.60 L/min per m2. Mean in hepatic cirrhosis is 5.36 ± 1.98 L/min per m2.
From Lunzer MR et al. [11].
Systemic vascular peripheral resistance is reduced as is the arteriovenous oxygen difference. In patients with cirrhosis, whole body oxygen consumption is decreased and tissue oxidation is abnormal. This has been related to the hyperdynamic circulation and to arteriovenous shunting. Thus, the vasodilator state of liver failure may contribute to general tissue hypoxia.
Vasomotor tone is decreased as shown by reduced vasoconstriction in response to mental exercise, the Valsalva manoeuvre and tilting from horizontal to vertical [11,12]. The cirrhotic patient shows arterial hyporeactivity to endogenous vasoconstrictors. Autonomic neuropathy is a poor prognostic indicator [13]. The effective arterial blood volume falls as a consequence of an increase in the arterial vascular compartment induced by arterial vasodilatation. This activates the sympathetic and renin–angiotensin systems and is important in sodium and water retention and ascites formation. The hyperdynamic splanchnic circulation is related to portal hypertension.
A large number of arteriovenous anastomoses, which are normally present but functionally closed, may have opened under the influence of a vasodilator. The nature of the vasodilators concerned—there are likely to be many—remains speculative. They might be formed by the sick hepatocyte, fail to be inactivated by hepatocytes or bypass the liver through intra- or extrahepatic portal–systemic shunts. The vasodilators are likely to be of intestinal origin. In cirrhosis, increased permeability of the intestinal mucosa and portosystemic shunting allow endotoxin and cytokines to reach the systemic circulation and these could contribute [14,15].
Nitric oxide (NO), a potent endothelium-derived vasodilator, may be involved in the hyperdynamic circulation [16]. It is released from L-arginine by a family of NO synthase enzymes encoded by different genes (Fig. 7.10). The endothelial constituent, NO synthase (NOS3), plays an important part in regulating normal vasoconstrictor tone [17].
Fig. 7.10. Nitric oxide (NO) is a general vasodilator. It is produced from L-arginine, NO synthase being the responsible enzyme. This is induced by endotoxin and inhibited by L-NMMA.
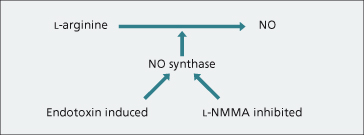
L-arginine analogues such as NG-monomethyl-L-arginine (L-NMMA) inhibit NO release. Inhibitors have been shown to reverse the hyperdynamic circulation in portal-hypertensive rats [18]. Cirrhotic rats show increased sensitivity to the pressor effect of NO inhibition and portal pressure rises [19]. NO synthase is inducible after stimulation with bacterial endotoxin or cytokines.
Various gastrointestinal peptides, such as vasoactive intestinal polypeptide (VIP) type II, have little effect on the portal circulation. Glucagon is unlikely to be the sole vasodilator responsible.
Prostaglandins (E1, E2 and E12) have vasodilatory actions and prostanoids are released into the portal vein in patients with chronic liver disease [20]. They may play a part in vasodilatation.
After hepatic transplantation, portal pressure becomes normal. The cardiac index and splanchnic flow remain high due to the persistence of portal–systemic collateral flow [21]. These gradually return to normal over time.
Prognosis (Child–Pugh Score, MELD, UKELD)
Poor prognosis is associated with a prolonged prothrombin time, marked ascites, gastrointestinal bleeding, advanced age, high daily alcohol consumption, high serum bilirubin and alkaline phosphatase, low albumin values and poor nutrition. The availability of liver transplantation has emphasized the need for an accurate prognosis so that surgery may be performed at the right time.
Child’s classification (grades A–C)—which depends on jaundice, ascites, encephalopathy, serum albumin concentration and nutrition (see Table 9.4)—gives a good short-term prognostic guide. Prothrombin time can be used rather than nutritional status (Child–Pugh modification) and individual features scored by severity. The total score classifies patients into grade A, B or C [22], although published studies often differ in their choice of numerical boundary between one grade and another.
The MELD score was developed to determine prognosis in patients undergoing TIPS insertion. MELD stands for Model for End-stage Liver Disease. It is calculated from serum creatinine, prothrombin time (INR) and serum bilirubin. MELD was applied to liver transplantation and found to accurately predict waiting list mortality in cirrhotic patients. It is now widely used as a criterion for liver transplant listing and to determine priority for organ allocation. Use of the MELD score for organ allocation appears to have reduced mortality on the waiting list in the USA [23]. The addition of serum sodium to the calculation may further improve its predictive ability—MELD-Na [24]. A similar scoring system has been developed in the UK (UKELD). This also uses INR, serum creatinine, serum bilirubin and serum sodium and has similar predictive abilities to the MELD-Na. These scores can be calculated on-line using a number of websites.
Disease-specific scoring systems may be useful. Maddrey’s discriminant function (DF) is helpful in alcoholic hepatitis [25]. It is calculated as follows:
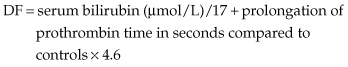
A DF greater than 32 is associated with a very high in-hospital mortality (30–50%).
The following clinical points may be useful prognostically:
1 Aetiology: if the initiating factor can be removed the prognosis is better. Thus abstinence in alcoholic cirrhosis and antiviral treatment in viral cirrhosis may improve prognosis.
2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree