These patients and those with MELD score >25 are re-certified every 7 days
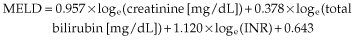
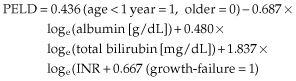
*If HAT ≤14 days not meeting above criteria are listed with the highest MELD score of 40.
† Hepatocellular carcinoma—a single lesion 2 cm diameter or more is given extra points
Regional Review Boards review exceptions to MELD.
Demand has exceeded supply of donor organs. The time spent awaiting transplant and deaths occurring before it can be performed have increased. The waiting time for low-risk patients is over 6–12 months in many countries. Although in general this may be longer for those of blood group B and AB, group O recipients may have the longest waiting time because group O is the universal donor type. Depending on the system of organ distribution, such livers can be given to recipients having any ABO group. Whole donor livers suitable for children are particularly rare and this has led to the split-liver technique (see Fig. 36.5), with use of the left lobe.
The equitable distribution of the available donor livers is difficult. Results (and costs) are much better if the patient is low risk (ambulatory) compared with high risk (in intensive care), and if the donor liver has optimal characteristics. Decisions are usually made by a multidisciplinary panel taking into account the wishes of the patient and their family.
There are three possible policies for liver organ allocation to prioritize recipients: medical urgency, utility and transplant benefit [3]. Medical urgency is based on the severity of cirrhosis. In the USA and in seven European countries (Eurotransplant zone), the Model for End-stage Liver Disease (MELD) is used [4]. MELD is a score based on serum bilirubin, creatinine and INR (Table 36.2). There are several limitations of the MELD score, including interlaboratory variations in measuring serum creatinine [5,6], gender differences in creatinine values [7] and variations in INR [8].
Despite more objectively measured variables than in the Child–Pugh system, which includes ascites and encephalopathy, and a greater spread of scores (up to 40 with MELD compared to between 5 and 15 with the Child–Pugh score), the MELD system does not have better prediction for survival than Child–Pugh score for cirrhosis in general [9], nor for mortality within 3 months on a transplant waiting list [10]. Indeed, known adverse prognostic factors in cirrhosis and/or heavily influencing quality of life, such as chronic encephalopathy, resistant ascites, recurrent cholangitis, difficult-to-treat variceal bleeding and low serum sodium, are not taken into account. Neither are metabolic conditions. These conditions are part of the ‘MELD exceptions’, assessed by special Regional Review Boards. Serum sodium is incorporated into the United Kingdom End Stage Liver Disease Score (UKELD), which determines minimal listing criteria [11]. MELD does not correlate with quality of life [12]. However, the diagnosis of hepatocellular carcinoma is catered for in the MELD allocation system by giving more points to these patients. The introduction of MELD in the USA has resulted in fewer new waiting list registrations, higher transplantation rates without increase in mortality after liver transplantation and a reduction of mortality on the waiting list [13], because time waiting on a list was removed as a major criterion. However, where the original allocation system did not have this policy, the introduction of MELD has led to a deterioration of outcomes after transplantation [14].
The second alternative for an allocation system can be based on utility, which considers outcome after liver transplantation as well as death on the waiting list. The MELD system ensures a sickest first principle, but if this by chance is combined with poor donor quality, the outcome is poor with less than a 50% chance of 1-year survival [15]. This can be considered an unacceptable outcome for the use of a scarce resource. The MELD system is a poor predictor of post-transplant survival [16]. Utility systems are becoming more important as the quality of donor organs is not improving, indeed it is worsening with increased use of suboptimal grafts (extended criteria grafts) [17]. Donor age is the most important risk factor [18,19], and also influences the severity of hepatitis C recurrence and increases the rate of fibrosis [20]. Utility models depend on validated models for outcome after liver transplantation. Only a few are available [18]. In the UK (Table 36.1), an estimated survival of 50% at 5 years is needed for selection onto the waiting list, with at least a 9% chance of dying within a year without transplantation [11].
Transplant benefit models represent the balance between waiting list and outcomes after liver transplant, that is the greatest difference between the two is the yard stick for prioritization. A virtual model has suggested most of the avoidable deaths occur on the waiting list [21]. However, the extent of survival benefit needs to be set by consensus, or according to the number of available donor organs. Thus, although survival benefit for HCV-related cirrhosis recipients with a MELD score between 9 and 20 is worse than those with alcoholic cirrhosis, it does not make clinical sense to wait for a MELD score of 30 (when there is no difference) before prioritizing for transplantation [22].
Candidates: Outcome (Table 36.3)
The major indications for liver transplantation in the USA and Europe are HCV-related cirrhosis, alcoholic cirrhosis and hepatocellular carcinoma (Fig. 36.1). More patients with acute and subacute hepatic failure are being included and fewer with chronic hepatitis B because of effective antiviral therapy (Table 36.4).
Table 36.3. Possible candidates for hepatic transplantation
| Cirrhosis |
| Cryptogenic/ NASH-associated cirrhosis |
| Autoimmune |
| Virus B (HBV DNA negative or under effective antiviral therapy) |
| Virus D |
| Virus C |
| Alcoholic (Chapter 25) |
| Cholestatic liver disease |
| Primary biliary cirrhosis |
| Biliary atresia |
| Primary sclerosing cholangitis |
| Secondary sclerosing cholangitis |
| Graft-versus-host disease |
| Chronic hepatic rejection |
| Cholestatic sarcoidosis (Chapter 31) |
| Chronic drug reactions (rare) |
| Primary metabolic disease (see Table 36.5) |
| Acute liver failure (Chapter 5) |
| Malignant disease (Chapter 35) |
| Hepatocellular carcinoma |
| Epithelioid haemangioendothelioma |
| Hepatoblastoma |
| Hepatic metastatic neuroendocrine tumours |
| Miscellaneous |
| Budd–Chiari syndrome (Chapter 9) |
| Short-bowel syndrome |
NASH, non-alcoholic steatohepatitis.
Table 36.4. Percentage survival of 47 651 patients transplanted between January 1988 and June 2006 according to diagnosis of cirrhosis, acute liver failure and cancer
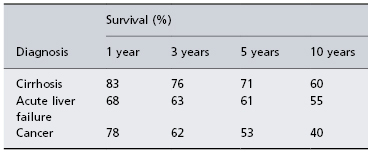
Data from European Liver Transplant Registry, 2008.
Fig. 36.1. Primary indications and diseases leading to liver transplantation in Europe between January 1988 and June 2006 (European Liver Transplant Registry).
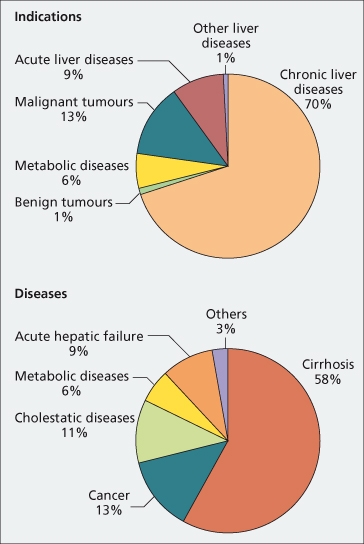
Cirrhosis
All patients with end-stage cirrhosis should be considered for liver transplantation. Selection of the right time is difficult. The patient must not be moribund, so that the transplant will fail, or be capable of leading a relatively normal life for a long period, so that transplant is unnecessary. In non-cholestatic cirrhosis, transplantation should be considered if prothrombin time is more than 5 s prolonged, or the serum albumin concentration is less than 30 g/L, as well as resistant ascites, chronic encephalopathy and previous spontaneous bacterial peritonitis, resolved or ongoing renal impairment or intractable variceal bleeding. In cholestatic cirrhosis an additional consideration is a serum bilirubin more than100 µmol/L. The cost of transplant is little different from that of long-term medical and surgical management of complications such as bleeding, coma and ascites.
The patients are poor operative risks if there is severely impaired blood coagulation and portal hypertension, so that blood loss is great. The technical difficulties are greater when cirrhosis is present, particularly when the liver is small and difficult to remove, or with previous abdominal surgery or extensive portal vein thrombosis. Survival at 1 year is much the same for all forms of cirrhosis, but long-term survival is dictated by disease recurrence.
Autoimmune Chronic Hepatitis
Post-transplant 5-year survival is 91% and graft survival 83% [23]. Despite triple immunosuppression, 33% develop recurrent chronic hepatitis of autoimmune type often related to insufficient immunosuppression. It mostly responds to changes in immunosuppression and is unrelated to the HLA status of the donor, but is associated with DR3 or DR4 in the recipient [24]. However, graft failure may occur [24].
Chronic Viral Hepatitis (Chapter 37)
Hepatic transplantation performed for acute fulminant viral hepatitis (A, B, D and most cases of E) is not followed by graft re-infection as the viral levels are very low. In the chronic situation, however, graft re-infection is very common, unless antiviral therapy is given as for hepatitis B, and currently is almost universal for HCV-related cirrhosis, unless there has been a sustained virological response before liver transplantation. Chronic hepatitis E has been described after liver transplantation.
Hepatitis B
Without antiviral therapy, post-transplant recurrence is usual and is related to viral replication in extrahepatic sites, particularly monocytes. A severe fibrosing cholestatic hepatitis may develop with ballooning of hepatocytes and ground-glass change [25]. This may be related to high cytoplasmic expression of viral antigens in the presence of immunosuppression [25]. HBV may sometimes be cytopathic.
Antiviral therapy in HBV DNA-positive patients selected for transplantation usually consists of dual therapy, lamivudine and tenofovir, or entecavir and tenofovir, which should be maintained after transplantation. If only lamivudine is available, hepatitis B immunoglobulin should be used intraoperatively, postoperatively on a daily basis, and then maintained life-long [26]. Initial titres of anti-HBs should be above 100 IU/L. However, despite hepatitis B-specific immunoglobulin with lamivudine, break-through mutants can occur, causing recurrent hepatitis [27], which then should be treated by adding tenofovir.
Immunoglobulin can be weaned down and stopped if HBV DNA was negative before liver transplantation, but validated schedules are not available. Adequate compliance, with patient education, must be ensured to prevent the development of viral escape mutants. Laboratory facilities to detect viral resistance must be available.
Hepatitis B infection can now be controlled completely, maintaining or rapidly resulting in HBV DNA negativity in serum, so that HBV DNA negativity or appropriately falling HBV DNA titres in patients requiring an urgent liver transplant is sufficient to list for transplantation. Currently, hepatitis B cirrhosis without hepatocellular carcinoma has the best survival after liver transplantation, comparable to primary biliary cirrhosis.
HBV vaccination, following discontinuation of HBIG, may be associated with the development of protective serum titres of anti-HBs [28], but this is rare. More immunogenic vaccines may make this strategy viable.
Hepatitis Delta
Without antiviral therapy for hepatitis B, transplantation is almost always followed by infection of the graft. HDV RNA and HDAg can be detected in the new liver and HDV RNA in the serum [29]. Hepatitis only develops if there is concomitant or superinfection with HBV, so that suppression of hepatitis B as described above prevents disease recurrence.
Hepatitis C Virus
Hepatitis C is the commonest indication for liver transplantation in most centres in the USA and Europe. All patients who are positive for HCV by PCR pretransplant will remain positive, and 97% will develop recurrent hepatitis C post-transplant (Fig. 36.2). Infection of the graft can come from infected mononuclear cells which contain negative-strand viral RNA—the replicative intermediate of the viral genome. The overall 5-year survival of patients with HCV is less than that with other liver diseases [30] and 10-year survival is significantly worse [31].
Fig. 36.2. Factors associated with recurrent HCV hepatitis after liver transplantation. BMI, body mass index.
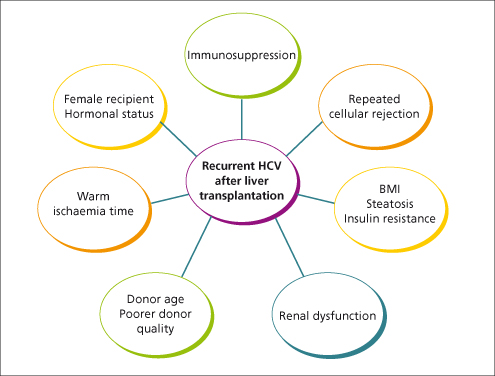
Treatment for recurrence is not very effective with pegylated interferon and ribavirin [32]. Use of low-dose tapering steroids and azathioprine may slow down the rate of fibrosis [33].
Neonatal Hepatitis
This disease of unknown aetiology is associated with jaundice, giant cell hepatitis and rarely liver failure necessitating liver transplant, which is curative [34].
Alcoholic Liver Disease
In Northern Europe, these patients provide the largest number of candidates for transplant. The selection and the results obtained are discussed in Chapter 25. Transplant benefit evaluation of survival is better than HCV-related cirrhosis with moderate severity of cirrhosis [22].
Cholestatic Liver Disease
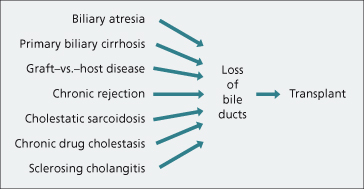
End-stage biliary disease, usually involving the small intrahepatic bile ducts, is an excellent indication for hepatic transplantation (Fig. 36.3). Hepatocellular function is usually preserved until late and the timing of the transplant is easy. In every case the liver shows an advanced biliary cirrhosis, often combined with loss of bile ducts (disappearing bile duct syndrome).
Primary Biliary Cirrhosis (Chapter 15)
One-year patient survival is over 90% [35]. Recurrence can occur, but there are only few reports of subsequent graft failure.
Extrahepatic Biliary Atresia (Chapter 29)
This indication comprises 35–67% of paediatric liver transplants and is always indicated if infants are diagnosed after 3 months. Calculated 1-year survival is 75%. Results are excellent and long-term survivors have good physical and mental development, although re-transplant and post-transplant surgery is often necessary.
A previous Kasai procedure increases the operative difficulty and the morbidity.
Alagille’s Syndrome
Transplant is required only in very severe sufferers [36]. Associated cardiopulmonary disease may be fatal and careful preoperative assessment is necessary.
Primary Sclerosing Cholangitis (Chapter 16)
Sepsis and previous biliary surgery provide technical problems. Nevertheless, the results for transplantation are good, 1-year survival being 70% and 5-year survival 57%. Disease recurrence is frequent [37]. Colectomy pretransplant is associated with less recurrence [37]. Cholangiocarcinoma is a complication that greatly reduces long-term survival. Inflammatory bowel disease must be monitored closely with annual surveillance colonoscopies. It can worsen after liver transplantation despite immunosuppressive drugs [38].
Other End-Stage Cholestatic Diseases
Hepatic transplantation has been performed for graft-versus-host cirrhosis in a bone marrow recipient. Other rare indications include cholestatic sarcoidosis (Chapter 31) and chronic drug reactions.
Primary Metabolic Disease
Liver homografts retain their original metabolic specificity. Consequently, liver transplantation is used for patients with inborn errors that result from defects in hepatic metabolism. Patients suffering from these conditions are good candidates. Selection depends on the prognosis and the likelihood of the later complication of primary liver tumours.
Liver transplantation for metabolic disorders is divided into those performed for end-stage liver disease or premalignant change and those performed for major extrahepatic features, in some cases associated with concomitant kidney transplantation (Table 36.5). Overall survival is over 85% at 5 years.
Table 36.5. Liver transplantation for metabolic disorders
| End-stage disease or premalignant change |
| α1-Antitrypsin deficiency |
| Genetic haemochromatosis |
| Wilson’s disease |
| Tyrosinaemia |
| Galactosaemia |
| Glycogen storage diseases |
| Protoporphyria |
| Neonatal haemochromatosis |
| β-thalassaemia |
| Cystic fibrosis |
| Byler’s disease |
| Major extrahepatic features |
| Primary oxaluria type 1 |
| Homozygous hypercholesterolaemia |
| Crigler–Najjar syndrome |
| Primary coagulation disorders (factor VIII, IX, protein C) |
| Urea cycle defects |
| Mitochondrial respiratory chain defects |
| Familial amyloidotic polyneuropathy |
End-Stage Liver Disease
Non-Alcoholic Fatty Liver Disease Associated Cirrhosis
The end stage of NAFLD is cirrhosis. Many cases of ‘cryptogenic’ cirrhosis are due to progressive non-alcoholic steatohepatitis. This is the fourth most common indication. Recurrence of liver disease is frequent [39], and there is excess cardiovascular morbidity due to features of the metabolic syndrome [40].
α1-Antitrypsin Deficiency
This is the most common metabolic disease leading to liver transplantation. Macronodular cirrhosis will develop in about 15% before the age of 20 years. Hepatocellular carcinoma is a complication. The plasma α1-antitrypsin deficiency is corrected and the lung disease stabilizes after the transplant. Advanced pulmonary disease is a contraindication unless both lungs and liver are transplanted.
Genetic Haemochromatosis (Chapter 26)
This is an uncommon indication for transplantation. Survival is lower than for other indications, because of infection and cardiac problems. Clear-cut recurrence of hepatic iron has not been reported but follow-ups are short [41].
Wilson’s Disease (Chapter 27)
Liver transplants have to be considered in patients presenting with fulminant hepatitis, in young cirrhotic patients with severe hepatic decompensation who have failed to improve after 3 months’ adequate D-penicillamine treatment, and in effectively treated patients who have developed severe hepatic decompensation following discontinuance of penicillamine.
The overall survival is 72% increasing to 90% when the indication is fulminant Wilson’s disease [42].
Neurological complications show significant improvement only if associated with liver disease [43].
Glycogen Storage Diseases (Chapter 29)
Liver transplantation has been successfully performed for types I and IV, with survival and continued growth into adult life.
Galactosaemia (Chapter 29)
A few patients diagnosed late develop advanced cirrhosis in childhood and early adult life and are candidates for transplantation.
Protoporphyria
This can lead to end-stage cirrhosis and so be an indication for liver transplantation [44]. Postoperatively, the high level of protoporphyrin in erythrocytes and faeces persists and the disease is not cured.
Tyrosinaemia
Hepatic transplantation is curative and should be considered early before the development of hepatocellular carcinoma [45].
β-Thalassaemia
Combined heart and liver transplantation has been reported for end-stage, iron-induced organ failure in an adult with homozygous β-thalassaemia [46].
Cystic Fibrosis (Chapter 29)
Hepatic transplantation is indicated for predominant liver involvement. Combined liver–lung transplant is often necessary. The 3-year survival of young patients with end-stage respiratory failure complicated by cirrhosis is 70% [47].
Byler’s Disease
Byler’s disease (progressive familial intrahepatic cholestasis type 1) results in death from cirrhosis or heart failure. The low serum apolipoprotein A1 concentration is corrected by transplant performed for cirrhosis [48].
Correction of Extrahepatic Features
Oxaluria
Primary oxaluria type I, due to deficiency of hepatic peroxisomal alanine-glyoxylate aminotransferase, is corrected by simultaneous hepatic and renal transplantation [49]. Cardiac dysfunction reverses. The hepatic transplantation should preferably be done before renal damage has developed.
Homozygous Hypercholesterolaemia
Liver transplant produces an 80% decrease in serum lipids. Cardiac transplant or coronary bypass are also usually necessary [50].
Crigler–Najjar Syndrome
Liver transplant is indicated to prevent neurological sequelae when the serum bilirubin level is very high and cannot be controlled by phototherapy.
Primary Coagulation Disorders
The usual indication is HCV cirrhosis. Transplant cures the haemophilia but the effects of HIV infection and recurrent viral hepatitis remain post-transplant complications [51].
Urea Cycle Enzyme Deficiencies
Transplantation has been performed for ornithine transcarbamylase deficiency as urea cycle enzymes are predominantly located in the liver [52]. The decision concerning the need for transplantation is difficult as some urea cycle disorders allow a normal lifestyle.
Mitochondrial Respiratory Chain Defects
These may cause liver disease in neonates associated with hypoglycaemia and postprandial hyperlacticacidaemia. They have been treated by liver transplant.
Primary Familial Amyloidosis
Transplant, often by the domino technique, should be performed before the onset of significant polyneuropathy and before autonomic bladder and rectal dysfunction. Neurological improvement is variable [53].
Acute Liver Failure (Chapter 5)
Indications include fulminant viral hepatitis, Wilson’s disease, acute fatty liver of pregnancy, drug overdose (for instance, paracetamol (acetaminophen)) and drug-related hepatitis [54].
Malignant Disease (Chapter 35)
Hepatic transplantation has been disappointing in patients with liver tumours despite preoperative attempts at identifying extrahepatic spread. Patients with cancer have a low operative mortality, but the worst long-term survival. Recurrence is frequent; carcinomatosis is the usual cause of death.
Hepatocellular Carcinoma (Chapter 35)
Patients with a single tumour 5 cm in diameter or less, and, if multifocal, only three tumours less than 3 cm in diameter [55] each, without macrovascular invasion have the lowest recurrence rate with a survival rate of over 70%. Vascular invasion, whether undetected macroscopically, or microscopic on examination of histological material, increases the recurrence rate and mortality [55,56]. Expansion of staging criteria is practiced in several centres, as well as down-staging with locoregional therapy with a period of observation to document control of tumour growth.
Fibrolamellar Carcinoma
The tumour is localized to the liver and cirrhosis is absent. This may be the best type of tumour for transplantation, and in certain cases transplantation is performed with localized and treatable metastases.
Epithelioid Haemangioendothelioma
This presents as multiple focal lesions in both lobes of an otherwise normal liver. The course is unpredictable and recurrence is likely in 50%. Metastatic spread does not always contraindicate surgery and this does not correlate with survival. It can be successfully treated by liver transplantation [57].
Hepatoblastoma
Transplantation results in a 50% survival at 24–70 months. Microscopic vascular invasion and anaplastic epithelium with extrahepatic spread are bad signs.
Neuroendocrine Tumours
When resection is not possible, worthwhile palliation can result from hepatic transplantation [58], especially if the primary tumour can be resected.
Abdominal Cluster Operations for Right Upper Quadrant Malignancy
Most of the organs derived from the embryonic foregut are removed including liver, duodenum, pancreas, stomach and intestine. With powerful immunosuppression, donor lymphoreticular cells circulate without causing clinical graft-versus-host disease and become those of the recipient without causing rejection [59]. The procedure is very radical and only few have survived without recurrent tumour.
Cholangiocarcinoma
Tumour recurrence is usual and 3-year survival is poor, being zero in some series, unless a preoperative regime of brachytherapy and chemotherapy with pretransplant staging laparotomy is instituted [60]. In most centres, patients with cholangiocarcinoma are not accepted as transplant candidates.
Budd–Chiari Syndrome (Chapter 9)
Hepatic transplantation is used in those who are too ill to perform decompressive shunting by TIPS, and where previous portal–systemic shunts have failed [61]. The 5-year survival is over 70% [61]. Recurrence of thrombosis is a risk, especially in those who have an underlying coagulopathy, and life-long anticoagulation is necessary.
Absolute and Relative Contraindications (Table 36.6)
Absolute
These include uncorrectable cardiopulmonary disease, ongoing infection, metastatic malignancy and severe brain damage.
Table 36.6. Absolute and relative contraindications to liver transplantation
| Absolute |
| Psychological, physical and social inability to tolerate the procedure |
| Active sepsis |
| Metastatic malignancy (except hepatic neuroendocrine tumours) |
| Cholangiocarcinoma (except trial protocols with neoadjuvant therapy and staging laparotomy) |
| AIDS |
| Advanced cardiopulmonary disease |
| Relative (higher risk) |
| Age more than 65 or less than 2 years |
| Prior-portacaval shunt |
| Prior complex hepatobiliary surgery |
| Portal vein thrombosis |
| Re-transplant |
| Multiorgan transplants |
| Obesity |
| HIV |
| Serum creatine more than 1.7 mg/dL (150 µmol/L) |
| Chronic renal failure (requires combined liver/ kidney transplantation) |
| Cytomegalovirus mismatch |
| Advanced liver disease |
Transplant should not be done if the patient cannot comprehend the magnitude of the undertaking and the exceptional physical and psychological commitment required [62].
Relative (Higher Risk)
Patients are at higher risk if they have advanced liver disease and are being treated in an intensive care unit and particularly if they are ventilation-dependent.
Children do particularly well but technical difficulties increase below the age of 2 years.
Risk increases with a body weight of more than 100 kg.
Multiorgan transplant adds to the risk.
A pretransplant serum creatinine level exceeding 1.7 mg/dL is the most accurate predictor of post-transplant death [63].
CMV mismatch (recipient negative, donor positive) adds to the risk.
Portal vein thrombosis makes the transplant more difficult and survival is reduced. However, the operation is usually possible [64]. An anastomosis is made between the donor portal vein and the recipient confluence of superior mesenteric vein and splenic vein, or a venous graft from the donor is used. Rarely, portacaval hemitransposition is performed [65].
Previous surgical portacaval shunts make the operation more difficult and a distal splenorenal shunt creates least problems. TIPS for variceal bleeding is the most satisfactory preliminary to transplantation [66]. Careful positioning of the stent is important, avoiding an excessive length down the portal vein, or protrusion into the vena cava.
Previous complex surgery in the upper abdomen also makes the transplant technically very difficult.
Re-Transplantation
The average re-transplantation rate is about 10%. Over half are due to primary non-function and hepatic arterial thrombosis, the remainder for chronic rejection and recurrent disease.
In Europe, primary transplant is associated with an 80% survival at 1 year. This is reduced to less than 50% for re-transplantation [18].
General Preparation of the Patient
The usual clinical, biochemical and serological investigation of any patient with liver disease is detailed.
Blood group, antibodies to cytomegalovirus and hepatitis C are measured and markers of hepatitis B infection noted. An assessment of renal function, preferably with radioisotope techniques of glomerular filtration rate measurement, should be made.
In patients with malignant disease, metastases must be sought by all possible techniques.
Cardiopulmonary assessment should be thorough, including the presence and severity of hepatopulmonary syndrome and severe pulmonary hypertension.
Imaging.
Splanchnic vasculature and particularly the hepatic artery and portal vein must be visualized as a guide to surgery. Doppler ultrasound is routine. The hepatic arterial tree is also shown in contrast-enhanced helical CT [67]. MRI may be used as an alternative, or together with CT to exclude vascular abnormalities and silent malignancy [67].
The bile ducts are visualized by MRI cholangiography [68] or, if cholangiocarcinoma is suspected, by endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic ultrasound (EUS).
The pretransplant medical ‘work-up’ takes about 5 days. It includes psychiatric counselling, nutritional assessment [69] and confirmation of the diagnosis. The patient may wait many months for a suitable donor liver and, during this period, intensive psychosocial support and close medical supervision is necessary.
Donor Selection and Operation
Donation may be informed with consent from the family, the clinician ensuring that the family have been consulted, or presumed consent including the patient having specifically indicated their wish to donate. Mandated consent requires a written confirmation during life to donate when one dies, which overcomes the reservations of relatives. In Spain, with the highest donation transplantation rate in Europe there is the custom of informed consent, with a very well-resourced programme of trained co-ordinators. Better education, support and advice is needed for all clinical staff who have contact with potential donors.
Donor shortage has encouraged the use of livers formerly regarded as unsatisfactory. These include livers from donors with abnormal liver tests, elderly donors, those with prolonged ICU stay receiving inotropes, or with moderate steatosis which was formerly an exclusion criterion [17]. Use of these marginal livers does not seem to have increased graft loss. There is an increasing use of controlled non-heart-beating donors [70].
Donors are considered between 2 months and 75–80 years of age, victims of brain injury that has resulted in brain death. For heart-beating donors, cardiovascular and respiratory functions are sustained by mechanical ventilation. The recovery of livers and other vital organs from heart-beating cadavers minimizes the ischaemia that occurs at normal body temperatures and is a major contribution to graft success.
Transplant across A, B and O blood groups may be followed by severe rejection and biliary complications. It should be avoided unless necessitated by an emergency situation [71], when appropriate adsorption and transfusion protocols should be used.
HLA matching is not practiced and indeed there is some evidence that selected HLA class II mismatches may be advantageous, particularly in preventing the vanishing bile duct syndrome [72].
Hepatitis B and C viral markers, CMV antibodies and HIV testing should be done.
The donor operation is as follows. The hepatic structures are dissected and the liver is precooled through the portal vein with Ringer’s lactate and 1000 mL of University of Wisconsin (UW) or other preservation solution perfused through the aorta and portal vein. A cannula in the distal inferior vena cava provides a vent for venous outflow. After removal, the cold liver is further flushed with an additional 1000 mL UW or other preservation solution through the hepatic artery and portal vein and stored in this solution in a plastic bag on ice in a portable cooler. This routine has extended the preservation time to at least 18 h so that the recipient operation may be semielective and not performed at unsocial hours. However, with non-heart-beating donors, and others with suboptimal quality, transplantation is performed with the shortest possible cold ischaemic time. Most centres now have designated multiorgan retrieval teams.
If possible, and particularly for elective procedures, the size of the donor liver should be matched to that of the recipient. This is based on a body weight within 10 kg of the recipient. Occasionally, a small-sized liver is transplanted into a larger patient. The donor liver increases in size at the rate of about 70 mL/day until it achieves the volume expected for the recipient’s size, age and sex [73].
The Recipient Operation (Fig. 36.4)
The average operative time is 8 h. Blood loss is variable, volumes being minimal or massive, but a proportion of transplants do not require any blood to be transfused. Cell savers have proven useful when high blood loss is anticipated; the blood is aspirated from the abdominal cavity, washed repeatedly, re-suspended and infused.
Fig. 36.4. Completed orthotopic liver transplantation. Biliary tract reconstruction is by duct-to-duct anastomosis.
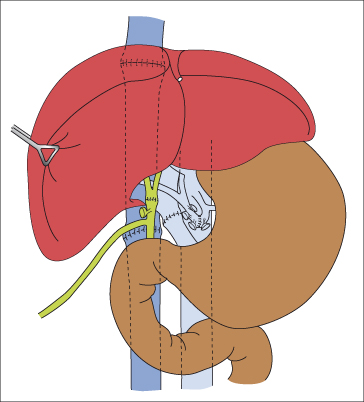
The hilar structures and vena cava above and below the liver are dissected. The various vessels are cross-clamped and divided to allow removal of the liver. The recipient vena cava can be left in situ to allow a piggy back technique in which single anastomosis is performed between the allograft supra hepatic inferior vena cava and the confluence of the hepatic veins.
During the implantation of the new liver, it is necessary to occlude the splanchnic and vena caval circulations. During this anhepatic phase, veno-venous bypass may be used to prevent pooling in the lower part of the body and splanchnic congestion allowing greater haemodynamic stability. The cannulae are placed in the inferior vena cava (via the femoral vein) and the portal vein, and run to the subclavian vein.
Once all vascular anastomoses are completed, the preservation fluid is flushed out of the graft before opening the blood supply to the liver. Portal vein thrombosis must be excluded. Hepatic arterial anomalies are frequent, and vessel grafts from the donor should be available for arterial reconstructions.
The usual order of anastomoses is: (a) suprahepatic vena cava; (b) intrahepatic vena cava; (c) portal vein; (d) hepatic artery; and (e) biliary system. The bile duct is usually reconstructed by direct anastomosis with external bile drainage through a T-tube in selected cases. If the recipient bile duct is diseased or absent, end-to-side Roux-en-Y choledochojejunostomy is chosen. Haemostasis is essential before closing the abdomen; perihepatic drains are placed.
Segmental (Split) Liver Transplantation
Because of the difficulty in obtaining small donor livers for young children, segments of adult cadaveric livers have been used (Fig. 36.5, Table 36.7). Two viable grafts can be obtained from a single donor liver [74]; with experience, results are nearly as satisfactory or similar to full liver grafts (93% 1-year survival) [75]. There are more complications, including increased intraoperative blood loss and biliary problems [76].
Table 36.7. Strategies to overcome shortage of heart-beating, brain-stem-dead liver donors
| Better clinician and public education |
| Presumed consent |
| Split livers |
| Live-related donors |
| Partial auxiliary grafts |
| Non-heart-beating donors |
| Hepatocyte transplantation |
Fig. 36.5. Diagram of the two grafts prepared from one donor liver. In this example the main vascular and biliary structures are attached to the right lobe. CBD, common bile duct; CT, coeliac trunk; HV, hepatic vein; IVC, inferior vena cava; LBD, left bile duct; LHA, left branch of hepatic artery; LHV, left hepatic vein; LPV, left branch of portal vein; MHV, middle hepatic vein; PV, portal vein; RHA, right branch of hepatic artery; RHV, right hepatic vein; RL, round ligament; RPV, right branch of portal vein. Numbers indicate hepatic segments [74].
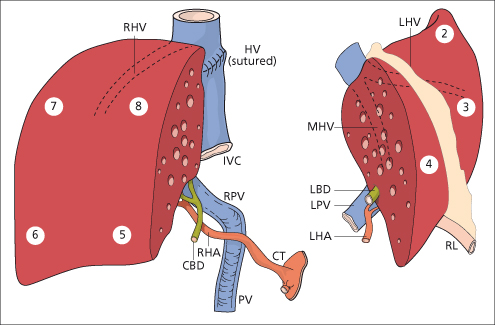
Cadaveric split liver grafts are also being used in the adult [77,78]. The split may be done ex vivo on the bench. Alternatively, the split may be done in situ
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree