Fig. 20.1. Estimated HCV prevalence by region.
(Source: Perz J et al., unpublished data. Centers for Disease Control, 2002.)
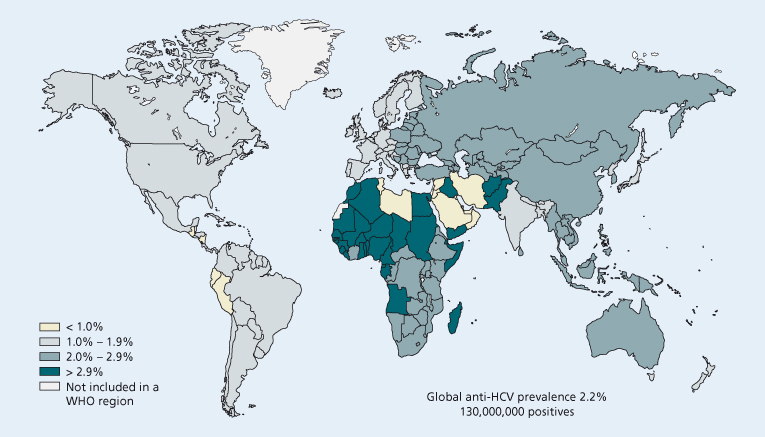
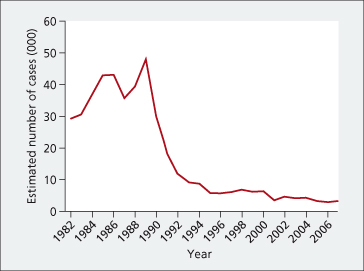
Fig. 20.2. Estimated incidence of acute hepatitis C in the USA, 1980–2007.
(Source: Centers for Disease Control.)
HCV can be transmitted via three routes: parenterally (usually by intravenous drug use or blood product transfusion), permucosally (usually sexually) or vertically. Parenteral transmission via intravenous drug use is the most common route, following the introduction of blood product screening for HCV. It is now estimated that intravenous drug use accounts for 80% of acute HCV infections. Acute HCV in HIV-positive individuals differs significantly from acute HCV monoinfection in its epidemiology, natural history, immunology and virology and is becoming an increasingly significant problem in HIV-infected persons. In contrast to the usual transmission of HCV, the majority of individuals in the reported HIV-positive cohorts describe possible permucosal transmission related to high-risk traumatic sexual practices, and perhaps parenteral transmission. In the past, in countries such as Italy, intrafamilial and sexual transmission of HCV undoubtedly contributed to the pool of infection. Nosocomial outbreaks of hepatitis C have been reported after lapses in infection control practices.
Modes of infection and groups at risk differ in developed versus developing countries (Table 20.2).
Table 20.2. Groups at risk of HCV infection
| In developed and in developing countries, individuals at risk of HCV infection include: |
| Injecting drug users (including past users) |
| Health care workers with needlestick injury (0–10%) |
| Individuals on haemodialysis, and after nosocomial outbreaks |
| Those who engage in high-risk sexual practices |
| Persons who received blood transfusion or infusion of factor concentrates before 1992 |
| HIV positive men who have sex with men |
| Infants born to HCV-infected mothers (1–5%) particularly in mothers with high HCV RNA or HIV–HCV coinfection |
| In developing countries, additional sources of HCV infection include: |
| Transfusions of unscreened blood |
| Unsafe injections (including in health care settings) or other parenteral exposure to blood |
| Use of blood-contaminated instruments for circumcision or surgery |
| Traditional scarification |
| Acupuncture |
| Tattooing and ear piercing |
Virology
HCV is a small, enveloped RNA virus and a member of the family Flaviviridae [5]. The genome of HCV resembles those of Flaviviridae, the pestiviruses and flaviviruses. The RNA genome contains around 9400 nucleotides of positive-sense RNA, comprising one long open reading frame encoding a polyprotein of 3010 to 3033 amino acids which is cleaved into functionally distinct polypeptides during or after translation. HCV has been designated the prototype of a third genus in the family Flaviviridae, hepacivirus. The nucleocapsid and envelope structural proteins are encoded at the 5′ end of the genome; the non-structural elements are downstream of this region (Fig. 20.3). Translation is mediated by an internal ribosome entry site in the 5′ untranslated region. The non-glycosylated capsid protein, C, complexes with the genomic RNA to form the nucleocapsid. Two glycoprotein products, E1 or gp35 and E2 or gp70, are found in the viral envelope. There are hypervariable regions, particularly in the E1 and E2 domains; these regions (particularly those of the envelope glycoproteins) may be important antigenic sites and their variability may be important in persistence of infection and immunopathogenesis. Hepatitis C replicates via an error-prone RNA-dependent RNA polymerase. The rapid replication rate and turnover of HCV combined with the poor fidelity of the HCV RNA-dependent RNA polymerases, which lacks a proof-reading function, generates a population of viruses with closely related, but different, nucleotide sequences, or a ‘quasispecies’, in infected persons. Divergence may be enhanced by the induction of neutralizing antibodies targeted to the envelope proteins.
Fig. 20.3. HCV genome organization and polyprotein processing. S and NS correspond to regions coding for structural and non-structural proteins.
(Reproduced from Penin F, Dubuisson J, Rey FA et al. Hepatology 2004; 39: 5–19.)
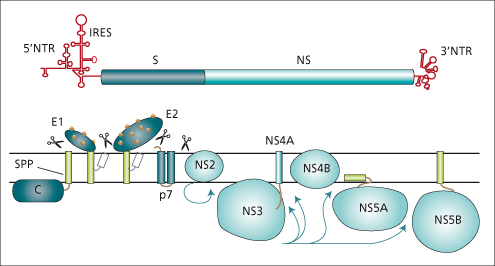
Cleavage at the carboxyl terminus of E2 generates a small protein, p7. The remainder of the non-structural region of the HCV genome is divided into regions NS2 to NS5 (Fig. 20.3). HCV encodes a protease at the NS2/NS3 junction which cleaves this site. After cleavage, non-structural proteins remain associated with cellular membranes (the membranous web), forming a replication complex. NS3 has two functional domains, a protease, which is involved in cleavage of the remainder of the non-structural region of the polyprotein, and a helicase, which is assumed to be involved in RNA replication. The HCV protease, using NS4a as a cofactor, is a major target of specific protease inhibitor antiviral agents. HCV NS5 is cleaved to yield NS5a and NS5b. NS5b is a viral RNA-dependent RNA polymerase; NS5a also may be involved in genome replication [6].
HCV comprises six genotypes and hundreds of subtypes. Detailed investigation of HCV was previously hampered by the lack of an appropriate viral culture system, but this hurdle has been overcome with the development of the subgenomic HCV replicon system; these genomes, coupled to the non-structural region of the HCV genome, are able to replicate when transfected into Huh7 cells (derived from a human HCC) [7]. The production of infectious virus has been reported from full-length HCV RNA transfection into Huh-7 cells, using a JFH-1 strain isolated from a patient with fulminant hepatitis due to HCV genotype 2a [6,8].
Candidate receptors have been proposed; these include the tetraspannin CD81, the low density lipoprotein receptor, scavenger receptor class B type I (SR-BI) and heparin. Claudin-1 (a tight junction component) has been shown to be an important coreceptor [9].
Pathology and Pathogenesis
In 15–40% of individuals, the acute disease resolves completely, with clearance of HCV RNA from serum, within 4 months. Thus the majority of patients infected with HCV progress to chronic infection. The pathogenic mechanisms that result in hepatitis are unknown. Lymphocytes are typically observed within the hepatic parenchyma, but the functional characteristics of these cells have not been fully defined.
Given the heterogeneous nature of the viral population that infects individuals, it is likely that a variety of strategies enable evasion of the host innate and adaptive immune response. Interferon-α (IFN-α) is induced by double-stranded RNA (present during viral replication); signalling pathways leading to type I IFN production are a first line of defence of the host to eradicate viruses. IFNs exert their antiviral function by binding to the IFN-α and -β receptors on the cell surface. The interaction triggers the JAK-STAT signalling cascade and induces expression of IFN-stimulated genes. The resulting innate antiviral response is a first line of immune defence against virus infection. HCV may inhibit the antiviral action of IFN-α and has evolved a mechanism to interfere with type 1 IFN induction and signalling. For example HCV proteins E2 and NS3 may inhibit the action of the IFN-induced double-stranded-RNA-activated protein kinase, enabling the virus to abrogate the development of the adaptive immune response [10]. The retinoic-acid-inducible gene I and IFN regulatory factor 3 pathway of the innate antiviral response within infected hepatocytes are abrogated, thus enabling the evasion of innate immune defences within the infected cell [11].
HCV-specific HLA class I-restricted cytotoxic T lymphocytes that recognize epitopes in variable regions of the envelope or of non-structural proteins have been identified. A CD4+ proliferative T lymphocyte response to recombinant viral antigens has been found in infected individuals. There may be a correlation between the presence of CD4+ T-cell responses to HCV core and a benign course of infection in viraemic carriers with minimal hepatitis. Patients with self-limited hepatitis C display stronger virus-specific CD4+ IFN-γ cell reactivity. HCV-specific CD8+ cytotoxic T lymphocytes (CTL) are thought to play a key role in the elimination of HCV since the vigour of the HCV-specific CTL response during the incubation phase of acute HCV infection is greater in those who resolve infection than in those who progress to chronic infection.
There is an effect of viral mutation on T-cell recognition so that escape mutations leading to immune escape may favour persistence of the virus. The role of cell surface inhibitory receptors in recognition of HCV, and the development of antibodies that neutralize HCV infection require further study. A down-regulated cytotoxic activity of natural killer (NK) cells against HCV-infected liver cells has been postulated. Understanding the constitution of an effective immune response in the control of HCV may enable improved immunomodulatory therapies. The existence of multiple genotypes that differ by up to 20% at the amino acid level represents a major obstacle for immunological control and the development of prophylactic and therapeutic vaccines for HCV.
Once chronicity is established, HCV-specific CTL remain weakly detectable implying that the virus can persist despite the presence of these CTL and that it may be resistant to the effects of antiviral cytokines. T-cell exhaustion may be present. Owing to the lack of a proof-reading mechanism of the viral polymerase, mutations occur frequently in HCV and, if CTL are unable to clear the virus rapidly during early infection, the CTL response may select for T-cell escape mutants. Later, when the HCV-specific CTL response is weaker, there may be less selection pressure [12].
Fibrosis in chronic hepatitis C infection occurs as a result of the activation of hepatic stellate cells by cytokines and other signalling molecules induced by the inflammatory process. These produce and deposit extracellular matrix proteins. Fibrosis begins around the portal tracts and gradually extends out into the lobules towards the central veins. Factors shown to accelerate the progression to cirrhosis include older age at HCV acquisition, male gender, heavy alcohol intake and coinfection with either HBV or HIV. Steatosis may lead to advancing fibrosis.
There is no DNA intermediate in the replication of the HCV genome or integration of viral nucleic acid, and viral pathology may contribute to oncogenesis through cirrhosis and regeneration of liver cells. HCV infection rarely seems to cause acute liver failure.
Diagnostic Tests for Hepatitis C
Anti-HCV
Because detection of the viral antigen is difficult, measurement of antibodies to HCV by enzyme-linked immunosorbent assay has become important as an indication of past or present infection. Anti-HCV antibody titres may decline or disappear over time after resolved infection. Third-generation immunoassays include antigens from the nucleocapsid (C22) and other non-structural regions of the genome. The sequence of the HCV core protein is relatively highly conserved. Most patients with acute HCV infection are anti-HCV positive by the time serum alanine aminotransferase (ALT) levels peak but anti-HCV may be undetectable at presentation. IgM anti-HCV is not a reliable test for acute disease and is not readily available. Diagnosis of acute hepatitis C may require documentation of seroconversion to anti-HCV in an at-risk population.
Routine testing of blood donors occurs in most industrialized countries, and has greatly reduced the risk of transmission of HCV. Supplemental tests for antibody to HCV are required to confirm the specificity of an anti-HCV test in low-risk populations, including blood donors. The most widely used method is the recombinant immunoblot assay (RIBA) in which antibodies are sought to recombinant antigens of HCV coated on nitrocellulose strips. Samples are confirmed positive if antibodies to two or more HCV proteins are present, and are indeterminate if antibody to only one antigen is found. However, HCV RNA testing offers greater clinical utility to confirm or quantitate viraemia. A confirmed positive anti-HCV result in a patient negative for HCV RNA usually indicates past, resolved infection. Any unexplained raised serum aminotransferases, or a risk factor for hepatitis C, should prompt a test for anti-HCV.
New tests for HCV core antigen are in development. These tests may provide a more direct test for antigenaemia.
Serum HCV RNA
HCV RNA testing enables direct testing and quantitation for the HCV viral genome. Polymerase chain reaction (PCR) analysis provides a sensitive and specific assay for HCV RNA in blood and other tissues. PCR testing utilizes primers of known nucleotide sequence of HCV. Currently, the limit of detection for plasma HCV RNA by real time PCR is 10–15 IU/mL. HCV RNA has been detected within 1–2 weeks of transfusion in patients with hepatitis C. HCV RNA usually persists for decades in patients who develop chronic disease and there is little advantage in repeatedly quantitating HCV RNA in untreated patients, as there is generally no direct correlation with activity or progression of the disease. Accurate methods to quantify HCV RNA in serum have been developed and are of value in estimating viral concentrations before and during antiviral therapy. Several commercially available assays are in wide use. Individuals who test positive for anti-HCV should be tested for HCV RNA.
Genotyping
Variation in the genome sequence of HCV isolates has enabled classification into types and subtypes [13,14]. Several isolates of hepatitis C have been cloned. The sequence divergence of these isolates indicates that there are several major genotypes of hepatitis C, and component subtypes [15]. Six major genotypes and about 100 subtypes can be differentiated by restriction fragment length polymorphism, type-specific primers in PCR reactions or hybridization with type-specific probes. The definitive method for viral genotype is sequencing [16]. Geographic localization of these genotypes has been reported. Infections with types 1b and 1a are relatively common in Europe and the United States; infection with type 1b is frequent in Southern Europe. Epidemiological differences in age distribution of major types and the risk factors associated with particular genotypes have become apparent. In Europe, type 3a and 1a are relatively more common in young individuals with a history of intravenous drug use. Type 1b accounts for most infections in those aged 50 or more. Type 4 infection is the most prevalent infection in Egypt, and many parts of the Middle East and Africa. Genotype 5 was thought to be confined to South Africa but pockets of have been found in France, Spain, Syria and Belgium. Type 6 is prevalent in South East Asia, Asian Americans and Asian Australians.
Importantly, response to treatment is influenced by the infecting genotype. No inherently greater pathogenicity of type 1 HCV has been documented and several clinical investigations have documented severe and progressive liver disease after infection with each of the well-characterized genotypes.
Approaches to Resistance Testing After Virological Failure
Population and clonal sequencing are utilized. Other technologies are evolving, such as ultra deep pyrosequencing. The initial genetic characterization of resistance mutations following treatment with direct antiviral agents is population sequencing; sequencing of the coding region of the antiviral target is required.
Acute Hepatitis C
The acute course of HCV infection is clinically mild, and typically unrecognized. Acute hepatitis C is thus only infrequently diagnosed. The mean incubation period of hepatitis C, defined as the time from exposure to the onset of symptoms, ranges from 2–12 weeks (average 7). It is shorter with large inoculae (e.g. following administration of factor VIII concentrate). The incidence of acute hepatitis C is falling in several industrialized countries as a result of blood screening, and perhaps the prevention of transmission of HCV during drug use. The diagnosis of acute hepatitis C is made by testing for anti-HCV and HCV RNA. HCV RNA becomes positive within 2 weeks of exposure. Most patients are anti-HCV positive within 8–10 weeks of exposure. Symptoms during the acute phase are non-specific and may include fatigue, lethargy, anorexia and right hypochondrial discomfort. Perhaps 25% of cases are icteric; patients with jaundice are more likely to clear the virus. Female gender and acute severe hepatitis associated with jaundice are associated with a higher chance of viral clearance. HIV coinfection is associated with viral persistence. Fulminant hepatitis is rare following hepatitis C infection, but has been reported, particularly following chemotherapy or withdrawal of chemotherapy. About 25% of patients have mildly elevated serum bilirubin. Peak serum ALT elevations are less than those encountered in acute hepatitis A or B. During the early clinical phase, serum ALT levels may fluctuate, and may become normal or near normal; HCV RNA may be intermittently negative during the acute phase, making determination of true convalescence somewhat difficult. Chronic hepatitis C infection is the major complication of acute hepatitis C. Higher rates of spontaneous recovery from acute hepatitis C have been observed in individuals with identified single nucleotide polymorphisms that lie in or near the IL28B gene on chromosome 19, which encodes IFN-λ3 [17]. The C/C genotype at rs12979860 strongly enhances resolution of HCV infection among individuals of both European and African descent, suggesting a primary role for IL28B in resolution of HCV infection.
Pathology
Most patients with well-documented acute hepatitis C do not require a liver biopsy. The pathological features that are constant in all types of acute viral hepatitis consist of parenchymal cell necrosis and histiocytic periportal inflammation [18]. The reticulin framework of the liver is usually well preserved, except in some cases of massive and submassive necrosis. The liver cells show necrotic changes that vary in form and intensity. The necrotic areas are usually multifocal, but necrosis tends to be frequently zonal, with the most severe changes occurring in the centrilobular areas. Individual hepatocytes often are swollen and may show ballooning but they can also shrink, giving rise to acidophilic bodies.
Dead or dying, rounded liver cells are extruded into the perisinusoidal space. There are variations in the size and staining quality of the nuclei. A monocellular infiltration, which is particularly marked in the portal zones, is the characteristic mesenchymal reaction. This is also accompanied by some proliferation of bile ductules.
Kupffer cells and endothelial cells proliferate and the Kupffer cells often contain excess lipofuscin pigment. In the icteric phase of typical acute hepatitis, the walls of the hepatic vein tributaries may be thickened and frequently are infiltrated, with proliferation of the lining cells in the terminal hepatic veins. Cholestasis may occur in the early stages of viral hepatitis, and plugs of bile thrombi may be found in the bile canaliculi.
Management
Early identification of acute hepatitis C is important, but may be difficult as the disease may be relatively silent; 75% of patients are not jaundiced and have non-specific symptoms. Management of acute sporadic hepatitis C includes conventional supportive treatment or specific antiviral therapy. Early spontaneous convalescence can be difficult to confirm; patients should be retested if HCV RNA becomes negative to differentiate transient versus sustained virus clearance [19]. Therapeutic trials of IFN-α have been undertaken. Recent studies have indicated that treatment benefits those patients who have been treated early. Early treatment of these individuals with IFN or combination pegylated IFN (PEG-IFN) and ribavirin (RBV) is usually advisable, but no consensus exists. In those who do not appear to be convalescing 2–4 months after onset of the disease, antiviral treatment (see below) should be considered, as a high percentage of patients (>80–90%) may respond, and the risk of chronic disease is high. Acute HCV monoinfection is far more responsive to treatment than chronic disease, and even IFN monotherapy has yielded very high (>90%) sustained virological responses (SVR), defined as HCV RNA negativity 6 months post-HCV treatment. In contrast, without specific HCV treatment only 20–50% of HIV-negative individuals will clear acute HCV infection.
The optimal timing and form of treatment for acute hepatitis C is not yet determined [20]. Studies are in progress to determine whether a wait and see strategy is detrimental, compared to immediate treatment. However, asymptomatic patients could be treated immediately as they appear to have a higher risk of chronic disease than those who present with acute symptomatic disease.
There needs to be increased surveillance for HCV, both to identify cases and assess the scope of the problem in at risk individuals. Generally, treatment should be given if the serum aminotransferases are elevated, and HCV RNA remains detected 2–4 months after first ascertainment, in a patient with apparent recent onset, to minimize the risk of chronicity. It is reasonable medical practice to recommend PEG-IFN and RBV although the need for RBV in this setting remains unclear. Treatment after an onset of 1 year is unsatisfactory, with results tantamount to the treatment of chronic infection. Immediate prophylactic intervention with IFN after a needlestick exposure, before the appearance of HCV RNA, is not justified. However, larger studies comparing immediate intervention after diagnosis versus delayed intervention, to avoid unnecessary treatment in those resolving the infection, have yet to be reported [21–26].
Chronic Hepatitis C
Natural History
The acute phase and progression to chronic hepatitis C is usually silent. Thus the onset of chronic hepatitis C is often insidious and usually has gone unnoticed by the patient. The diagnosis of chronic hepatitis C is arbitrarily defined as the persistence of HCV RNA in serum for 6 months or longer. Self-limited infections may be associated with a delayed clearance of HCV RNA, albeit this is relatively uncommon [27]. Epidemiological risk factors, for example a past blood transfusion, intravenous drug use or sexual risk, can indicate a possible aetiology. Serum aminotransferase levels remain abnormal after 12 months in 60 to 85% of patients with type C post-transfusion or sporadic hepatitis; these patients have chronic hepatitis histologically. Serum aminotransferases decline from the peak values encountered in the acute phase of the disease, but typically remain abnormal by twofold to eightfold. Serum ALT concentrations may fluctuate over time, and may even be intermittently or consistently normal.
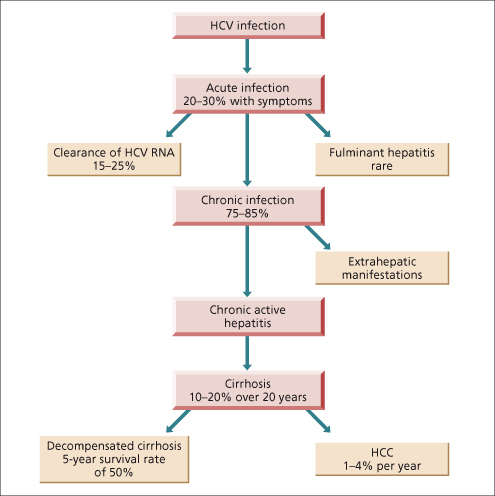
The chronic disease is generally slowly progressive; cirrhosis develops within 20 years in about 10–20% of patients with chronic disease, but can remain indolent for a prolonged period of time (Fig. 20.4) [28–30]. Variability in rates of progression of the disease makes the prediction of ultimate outcome difficult. The disease is not necessarily benign, however, and rapidly progressive cirrhosis can occur. HCV causes an estimated 8000 to 10 000 deaths annually in the USA [31].
Fig. 20.4. Natural history of hepatitis C infection.
(Reproduced from Chen SL, Morgan TR. The natural history of hepatitis C virus (HCV) infection. Int. J. Med. Sci. 2006; 3; 47–52.)
Several interactive factors, including the age of acquisition, concomitant alcohol abuse, gender, coexisting viral disease particularly concurrent HBV or HIV infection and the host immune response, are aggravating factors that affect the morbidity of the disease (Table 20.3) [31–33]. A study of 2313 untreated patients reported that increasing age at infection was independently associated with disease progression. Two per cent of those infected before the age of 20 developed cirrhosis over a 20-year period compared to 6% of those infected between 31 and 40 years, 37% infected between the age of 41 and 50 years and 63% infected after the age of 50 [29]. HCV genotypes and HCV RNA viral load, although important determinants of the effectiveness of treatment, are not thought to influence the natural history of infection.
Table 20.3. Factors adversely determining response to IFN
| Baseline host and virus factors: |
| Genetic polymorphism: IL28b rs12979860 TT |
| African American ethnicity |
| Age greater than 50 years |
| High body mass index |
| Hepatic steatosis |
| High homeostasis model assessment index (HOMA) |
| Advanced fibrosis or cirrhosis or advanced liver disease |
| High baseline viral load |
| Genotype 1 versus genotype 2 or 3 |
| Genotype 4, and probably 5 and 6 |
| HIV and HCV coinfection |
| On treatment: |
| Poor adherence to therapy |
| Excess alcohol |
| Failure to achieve RVR |
| Failure to achieve cEVR |
Older patients may present with complications of cirrhosis or HCC. HCC has been rarely observed in the absence of cirrhosis. During progressive disease, laboratory values become progressively more abnormal. Aspartate aminotransferase (AST) concentrations greater than ALT, a low serum albumin, a prolonged prothrombin time and low platelet counts suggest cirrhosis. Low levels of autoantibodies may be detected. A subset of patients with chronic hepatitis C infection may test positive for LKM1 antibody.
Anti-HCV persists for decades in chronic hepatitis C. HCV RNA is usually detectable in patients with abnormal serum aminotransferases and anti-HCV antibodies. Although most patients with raised serum ALT are HCV RNA-positive, the converse is not always true. Isolates of HCV in individual patients may show nucleotide substitutions over time, suggesting that HCV RNA mutates at a rate similar to that of other RNA viruses. A proportion of patients, perhaps 25–50% may have persistently normal serum ALT. However, ‘normal serum aminotransferases’ in patients with hepatitis C are frequently actually high relative to healthy individuals [34,35]. Low-grade hepatitis, and even low-grade fibrosis, may be present but fibrosis progression may be less rapid in females with low or normal ALT. In patients with ‘normal ALT’ who received antiviral therapy, further decline in ALT is observed in virological responders.
Extrahepatic Manifestations
The infection can cause systemic disease, and may be associated with various systemic complications; these include a form of autoimmune hepatitis, cryoglobulinaemia, a vasculitis, lichen planus, porphyria cutanea tarda, lymphocytic sialoadenitis and membranous glomerulonephritis. There is an association between non-Hodgkin’s lymphoma and hepatitis C infection [36–39].
Hepatitis C appears to induce insulin resistance; patients with hepatitis C have an increased prevalence of insulin resistance and type 2 diabetes mellitus. Molecular studies have shown that the HCV core protein can directly inhibit the insulin signalling pathway and increase reactive oxygen species production. There may be a direct genotype association (steatosis is more common in patients with genotype 3) that influences this metabolic effect, although this remains a contentious issue. Hepatitis C is commonly associated with hepatic steatosis. Replication of the virus is linked to lipid metabolism. The metabolic syndrome, that is obesity, insulin resistance or overt diabetes is associated with a poorer prognosis in patients with chronic hepatitis C. The clinical relevance is yet imperfectly understood but the interaction between steatosis, inflammatory processes, insulin resistance and impaired response to IFN may lead to an increased risk of hepatic fibrosis. The role of adjuvant therapies to improve insulin sensitivity or lipid-lowering agents to decrease cholesterol or triglyceride level is uncertain.
Pathology
The pathological features of HCV infection are quite characteristic, albeit not pathognomonic [18,40]. The presence of HCV RNA in serum tends to correlate with some degree of hepatitis and disappearance of HCV RNA, for example following successful IFN-α treatment, is followed by histological improvement. Typically, patients with chronic hepatitis C have mild portal tract inflammation with lymphoid aggregates or follicles and mild periportal piecemeal necrosis. Parenchymal steatosis, apoptosis and mild lobular inflammation are present and portal fibrosis or portal–central fibrosis may be present in later stages of disease. Bridging necrosis is not common. Rarely, granulomas can be observed. Although many of the lymphoid follicles are associated with bile ducts, ductopenia is not observed. Advanced disease, with cirrhosis or HCC is not generally associated with distinguishing features.
HCV antigens have been detected in scattered groups of cells, with granular cytoplasmic staining. The periportal lymphocytes around lymphoid follicles are mixed, but contain relatively large numbers of CD4 lymphocytes. A characteristic histological pattern of mild chronic hepatitis with portal lymphoid follicles and varying degrees of lobular activity is found in many patients with persistent hepatitis C infection. Fatty changes in the liver are usually not marked, but some steatosis can be observed in chronic HCV infection. Iron homeostasis may affect the outcome of hepatitis C. Hepcidin is the central regulator of systemic iron homeostasis. A positive correlation has been documented between serum hepcidin levels and both necroinflammation and fibrosis [41]. Others have found hepcidin levels to be lower in patients with chronic hepatitis C, suggesting that HCV may suppress this hormone, leading to liver iron accumulation [42].
Minor histological abnormalities, or degrees of hepatic fibrosis, in anti-HCV-positive, HCV RNA-negative individuals with normal serum ALT has been reported. The absence of HCV RNA in serum is evidence for, but not proof of, the absence of ongoing chronic hepatitis C as some reports have documented mild histological changes in some patients. The explanation and clinical significance of these abnormalities, and their prognosis, remains debated [43].
Management
Evaluation of Liver Disease
Treatment of hepatitis C has improved considerably over the last 10 years. A substantial proportion of patients with chronic hepatitis C can be cured although current treatments have limitations [44–46]. HCV RNA should be quantitated in all patients to confirm viraemia. If the test is reproducibly positive, serum aminotransferases, and bilirubin, alkaline phosphatase and prothrombin time should be measured to assess hepatic function. The HCV genotype should be ascertained. In patients with risk factors for other forms of viral hepatitis, HBsAg and HIV infection must also be tested. Because autoimmune hepatitis is treated differently and because IFN can unmask or exacerbate autoimmune hepatitis, it is advisable to exclude this diagnosis by measuring titres of antismooth muscle and antiliver–kidney microsomal antibodies, even in patients with a positive anti-HCV test.
Liver Biopsy and Assessment of Fibrosis
A liver biopsy is helpful in grading the degree of inflammation and staging the degree of fibrosis. Earlier guidelines recommended antiviral therapy for those patients with chronic hepatitis C who were deemed to be at highest risk of developing cirrhosis, that is patients with chronic hepatitis C who had persistently increased serum ALT levels, detectable levels of HCV RNA and histological evidence of portal or bridging fibrosis or inflammation and necrosis. However, recent guidelines suggest that potentially all patients with hepatitis C are candidates for treatment; a liver biopsy can be informative and provides unique clinical information but is not now considered mandatory for all patients. Liver biopsy has inherent sampling variability, but can be minimized by the inclusion of samples more than 25 mm in length. Interobserver variability has been found to occur.
Several non-invasive methods can be used to assess the stage of disease. Patients with hepatitis C can be appropriately studied by these markers of fibrosis, as these tests have been validated in this group. A calculation of aspartate aminotransferases to platelet ratio index (APRI) (aspartate aminotransferases (U/L)/ upper limit of normal × 100 divided by the platelet count (109
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








