- The hepatitis C virus (HCV) is a small, parenterally transmitted RNA virus; today it is acquired almost exclusively by the use of unsterile needles.
- It occurs in six distinct genotypes, genotype 1 being the most prevalent in North America, Europe and Japan.
- HCV causes an acute hepatitis that is clinically silent in most cases and persists in the majority (80%) of patients, leading to chronic hepatitis.
- Chronic hepatitis C remains clinically silent and progresses to cirrhosis in about 10% of patients within 20 years. Once cirrhosis is established, it causes significant morbidity and mortality from hepatic decompensation and hepatocellular carcinoma.
- Non-hepatic manifestations of chronic hepatitis C infection are relatively common, especially asymptomatic or symptomatic mixed cryoglobulinemia, which may cause renal failure.
- Concomitant alcohol consumption, male gender, coinfection with HIV or HBV, and older age at time of infection accelerate the progression to cirrhosis.
- Every new patient should be asked for risk factors of HCV infection including: a history of transfusion of blood and blood products prior to the early 1990s, a history of past or present i.v. drug use (or snorting cocaine with a straw), a history of piercings and tattoos and medical procedures performed without appropriate sterile techniques, coming from a high prevalence region of the world, or being born to a mother with HCV infection.
- Patients with any of the above risk factors should be screened by anti-HCV antibody testing; positive test results need to be confirmed by direct detection of circulating virus using an HCV RNA PCR assay.
- Antiviral therapy should be considered in every patient with confirmed HCV infection. Patients with HCV infection should be referred for consideration/conduct of antiviral treatment to physicians with expertise and up-to-date knowledge in the field.
- Therapy of hepatitis C virus infection is rapidly evolving; the current standards consist of pegylated interferon-α and ribavirin. The addition of new, directly acting antiviral agents improve cure rates, but not without cost (both in terms of side effects and financial).
- Efficacy of therapy depends on genotype, with cure rates (i.e. sustained virological response (SVR)) being achieved with current standard therapy in 50% of genotype 1 and 4, and 80% of genotype 2 and 3 infected patients. Side effects of therapy are common and can be severe.
- Infants of mothers who have chronic hepatitis C should be tested for hepatitis C infection by checking anti-HCV antibodies—but only after 18 months old when a positive test signifies actual infection. Since many parents are unwilling to be uncertain about HCV transmission for such a long time, testing for serum HCV RNA plus ALT can also be performed when the infant is 2–6 months old. If the test result is negative, this indicates that HCV infection of the infant has not occurred.
Introduction
Chronic hepatitis due to hepatitis C virus (HCV) infection is the single most important infectious cause of death in Ontario, the most populous province of Canada.1 It is also the most frequent indication for liver transplantation worldwide. HCV was first identified in 1989 using molecular cloning techniques and the serum of patients with post-transfusion non-A/non-B hepatitis.2 A diagnostic test allowing detection of circulating anti-HCV antibodies in the serum of HCV infected patients was reported simultaneously.3 It has since become clear that HCV was responsible for almost all cases of post-transfusion non-A/non-B hepatitis, which has all but vanished since mandatory testing of all blood products was introduced in the early 90s. During the last 20 years, much has been learned about the virus, such as its epidemiology, diagnosis, natural history, and its ability to avoid immune clearance by the host and cause chronic hepatitis in the majority of infected patients. Last but not least, effective therapies have been developed and continue to be improved at the time of this writing.4 This chapter reviews aspects of chronic hepatitis that are relevant for the clinician not trained in hepatology.
The Virus
HCV is a small (around 50 nm), enveloped, positive sense, single-stranded RNA virus that belongs to the Flaviviridae family (Fig. 14.1). Its genome consists of 9600 base pairs and encodes for three structural and seven non-structural proteins. The former consist of the nucleocapsid (core) and two envelope proteins (E1 and E2); the latter includes a protease/helicase (NS3-4A) and an RNA-dependent RNA polymerase (NS5B). The life cycle of the virus proceeds from viral attachment and entry into the host cell, to viral RNA translation into a single polyprotein and its post-translational processing into specific structural and non-structural viral proteins, to viral RNA replication, and, finally, virion assembly and release (for review see reference 4). Processes involved in all of these steps are currently under investigation as potential targets for new antiviral drugs. Prime targets are the viral protease and RNA polymerase, since they are indispensable for HCV replication. Thus, several HCV protease and HCV polymerase inhibitors are currently in late clinical development.
Fig. 14.1 Schematic of the hepatitis C virus. (A) Schematic depiction of the hepatitis C virus (HCV). The envelope contains the E1 and E2 viral proteins. The core is the viral core protein and harbors the viral genome, a 9.6-kD positive-sense (+), single stranded (ss) RNA. (B) Schematic depiction of the genomic organization of HCV. The genome is translated into a single precursor protein that is post-translationally processed and cleaved into the individual viral proteins. This process is initiated by host cell enzymes and, upon its liberation, completed by the HCV protease. HCV RNA polymerase and protease are essential for viral replication and therefore form prime targets for the new directly acting antiviral drugs.
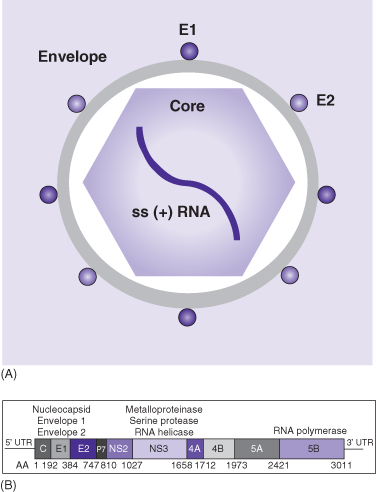
HCV exists in six major, genetically distinct subtypes (genotypes 1–6) with distinct geographical distribution. Genotype 1 is the predominant genotype in North America and Europe (50–80%) with genotypes 2 and 3 accounting almost entirely for the remainder cases, whereas in Egypt almost 100% of cases are genotype 4 infections, and genotype 3 predominates in Pakistan.
Unlike DNA polymerases, RNA polymerases do not have a proof-reading mechanism and are therefore prone to mistakes. HCV’s RNA-dependent RNA polymerase is no exception and numerous mutations occur during HCV replication. Based on HCV’s small size (9600 base pairs) and its very high replications rate—it has been estimated that each day 1012 new virions are produced in an infected individual—every possible mutation likely occurs daily in a patient with chronic HCV infection. HCV therefore never occurs as a single genotype entity, but rather as a family of closely related, slightly different variants, so called quasispecies, which still belong to the same genotype. It is thought that HCV’s high replication rate paired with its high mutation rate facilitates viral escape from host defenses. It is intuitively understandable that this also facilitates appearance and selection of resistant HCV species during antiviral therapies and forms a major obstacle to vaccine development.
Epidemiology
HCV infection has been reported from all over the world and is a major global health problem. The WHO estimates that about 3% of the world’s population or worldwide approximately 170 million people are chronically infected with HCV. The prevalence of chronic HCV infection varies in different regions of the world and, according to WHO estimates, ranges from less than 1% in Northern Europe and Canada to 10% or above in some countries such as Egypt (Table 14.1). In Canada, an estimated 250 000 people are infected with HCV.5
Table 14.1 Global prevalence of hepatitis C virus infection according to WHO6
| Prevalence | Geographic regions/countries |
| <1% | Canada, Mexico, Argentina, Chile, Germany, Scandinavia, UK, Australia |
| 1–2.4% | USA, France, Italy, Russia, Middle East, South Africa, India, Pakistan, Japan |
| 2.5–4.9% | Brazil, Central Africa, China |
| 5–9.9% | Tunisia, parts of Central Africa, South East Asia |
| ≥10% | Peru, Egypt, Mongolia |
HCV is a classic parenterally transmitted virus. Thus, HCV infection worldwide is highly prevalent in hemophiliacs (50 to >90%) and i.v. drug users. HCV was responsible for more than 90% of transfusion-associated non-A/non-B hepatitis; since introduction of mandatory screening of all blood products in the early 1990s, this route of transmission has virtually disappeared. Today, new infections occur almost exclusively through illicit drug use (sharing needles or snorting of cocaine with a straw) and medical procedures performed without adequate sterile precautions taken. In the absence of appropriate sterile technique, HCV can be transmitted through piercing and tattooing. Sexual transmission is possible in patients with high-risk behavior such that mucosal membranes are damaged, but rare without and can be virtually eliminated by safe sex practices (use of condoms). However, no health authority worldwide feels that the low risk (in the absence of high-risk behavior) would justify recommending safe sex practices in all monogamous relationships involving an HCV infected partner. HCV is not transmitted by breast feeding, kissing, sneezing/coughing, sharing eating utensils or drinking glasses, or other normal social contact, including hand shaking or hugging, food, or water. Vertical transmission from mother to child during pregnancy or perinatally is well documented and has become the main route of infection for HCV in children. Unfortunately, there is no “protective” antibody to HCV, that is, no effective postexposure prophylaxis.
In 1995, there were 2600 hospitalizations for HCV-related complications in the USA, leading to charges of US$514 million. Costs related to in-patient and out-patient care of HCV-infected patients were estimated to amount to almost US$5.5 billion.6 Modeling studies from several Western countries using local epidemiological information and natural history data (see below) predict that the disease burden and the costs related to complications of chronic hepatitis C, that is, the morbidity and the need for liver transplantation due to decompensated cirrhosis and hepatocellular carcinoma, will continue to increase at least until around 2020, while the incidence of cirrhosis will start to decline around 2005–2010.7
Natural History
Once transmitted, HCV infects and replicates in liver cells, causing an acute hepatitis that only infrequently becomes clinically manifest (Fig. 14.2). While HCV has also been shown to be able to infect selected cell types outside the liver, including some white blood cells, the clinical significance of such potential extrahepatic infections remains unclear. HCV is not cytopathic; that is, it does not kill its host cell during replication. Instead it stimulates a host reaction that attempts to clear the virus and causes inflammation and collateral liver damage. Both innate and adaptive immune mechanisms are involved; however, innate immunity, in particular the type I interferon system (interferon-α and interferon-β), plays a pivotal role. This is illustrated by the facts that (1) HCV has evolved to undercut the type of interferon response at several levels, which is thought to facilitate its persistence and (2) high-dose exogenous interferon-α is effective in treating clinical HCV infection (see below). During infection, the host mounts an antibody response against HCV. Although these anti-HCV antibodies are useful for diagnosis (see below), clinically they have little relevance as they neither neutralize the virus nor convey immunity and thus if re-exposed, reinfection may occur.
Fig. 14.2 Schematic of the natural history of hepatitis C virus infection and potential interventions. Upon (parenteral) transmission, the hepatitis C virus (HCV) homes to the liver and leads to an acute hepatitis, which rarely becomes clinically manifest. Currently, there is no vaccination available to protect against HCV infection. The ensuing innate and adaptive host immune response is able to clear the virus in a minority of patients (around 20%). If detected in the acute phase, HCV infection can be effectively treated and cured in more than 90% of patients with interferon-α-based monotherapy. In the majority of patients (about 80%), HCV is able to persist and leads to chronic hepatitis, which remains asymptomatic until late in its course. The inflammatory reaction associated with chronic hepatitis C triggers a wound-healing response leading to fibrosis and progression to cirrhosis over decades. Thus, 10% of patients with chronic hepatitis C develop established cirrhosis within 20 years. Treatment of chronic hepatitis C with pegylated interferon-α and ribavirin can cure the infection and prevent progression to cirrhosis in 50% of genotype 1 and 4, and 80% of genotype 2 and 3 infected patients, respectively. Once cirrhosis is established, chronic HCV infection leads to morbidity and mortality secondary to decompensation and hepatocellular carcinoma (the latter develops in 2–4% of patients per year). Factors accelerating fibrosis progression and development of cirrhosis include older age at time of infection, any concomitant alcohol consumption, male gender, and coinfection with HIV or HBV.
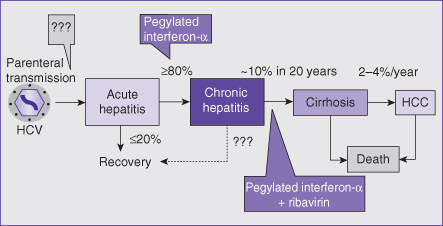
Upon acute infection, only about 20% of patients are able to eliminate the virus spontaneously (Fig. 14.2). In the remaining 80%, HCV will escape the host defenses, persist, and cause chronic infection with ongoing liver inflammation. Chronic hepatitis C does not cause symptoms and remains undetected if not specifically sought. If spontaneous clearance of HCV in a chronically infected individual is at all possible, it is a rare event, at least in adults.
As with any chronic inflammation, chronic hepatitis (of any cause) triggers a wound-healing response with activation of hepatic stellate cells that synthesize and deposit collagen, thus leading to progressive liver fibrosis and, finally, cirrhosis. In chronic hepatitis C, this process takes decades; about 10% of chronically infected patients develop cirrhosis within 20 years (Fig. 14.2). Only once cirrhosis is established, morbidity and mortality ensue. Patients decompensate with ascites, portal hypertensive bleeding, jaundice (due to liver dysfunction), and/or hepatic encephalopathy. Per year, about 2–4% of HCV-infected cirrhotic patients develop hepatocellular carcinoma. The life expectancy of patients with HCV infection is shorter than that of a normal population, and the difference is attributable solely to an increased mortality from cirrhosis and its complications, including HCC. Fibrosis progression and cirrhosis formation is accelerated by any amount of concomitant alcohol consumption, concomitant HIV or HBV infection (or other chronic liver disease), male gender, and older age at the time of infection.
Extrahepatic Complications of Hepatitis C
It is not unusual for individuals infected with hepatitis C to present not with symptoms of liver disease but rather with an extrahepatic manifestation. Perhaps the most common is cryoglobulinemia, which often goes undiagnosed as a non-organ-specific complication of an undiagnosed hepatitis C. Lesions typically associated with cryoglobulinemia may be asymptomatic or cause variably sized red skin blotches due to a small vessel vasculitis, which may affect internal organs such as kidneys. This can affect any part of the body but most often lower legs and arms. Another common non-hepatic complication is lymphoproliferative disease (B-cell lymphoma).8
Diagnosis
Since HCV infection typically remains clinically silent in the acute hepatitis phase and through most of the chronic hepatitis phase, that is until complications of cirrhosis occur, it is of prime importance to ask about risk factors for acquiring hepatitis C in any patient with a “hepatitis” (Table 14.2). In addition, patients with known risk factors should be tested for HCV infection.
Table 14.2 Risk factors for hepatitis C virus infection that mandate screening, including pregnant women
| History of or current illicit drug use (i.v. or cocaine snorting sharing a straw) |
| Transfusion of blood or blood products before mandatory testing for HCV was introduced (1992 in Canada) |
| Immigration from a country with high HCV prevalence |
| History of tattoos, piercings, or medical procedures performed without adequate sterile precautions |
| Traumatic sexual behavior, including current or previous sexual partners known to have used illicit i.v. drugs and in vitro fertilization from anonymous donors |
| History of incarceration |
| Known HIV positivity |
| Children of HCV infected mothers |
A highly sensitive and specific, commercially available enzyme linked immunoassay (EIA) is the screening test of choice. This test becomes positive as short as 2 weeks after acquiring HCV infection, but this may take longer, especially in immunocompromised individuals. Of note, IgG antibodies can cross the placenta. In babies born to HCV-infected mothers, such passive mother-to-child antibody transfer may lead to a positive anti-HCV antibody test in the absence of true infection of the baby. Thus, children should not be tested for anti-HCV antibodies prior to age 18 months. In an immunocompetent individual, a negative HCV antibody rules out chronic HCV infection. A positive antibody test indicates that the patient has had contact with the virus, but it does not prove current infection; as with many antibodies, anti-HCV can persist for years beyond clearance of the virus. Thus, the presence of ongoing infection needs to be confirmed in all anti-HCV-positive individuals by direct detection of the viral genome using a sensitive RNA assay, usually a commercially available RT-PCR assay. HCV RNA in serum becomes detectable by RT-PCR as early as 1 week after infection.
Determination of HCV genotype and HCV viremia level using a commercially available line-probe assay and quantitative RT-PCR test is only required prior to initiating therapy. Genotype and level of pretreatment viremia are important prognostic markers to predict outcome and treatment duration in adults (see below).
Therapy
All patients with HCV infection should be considered for antiviral therapy. Hence all individuals with risk factors for HCV infection should be considered for screening. Treatment of chronic hepatitis C remains a rapidly evolving field and should therefore be undertaken only by physicians with appropriate up-to-date knowledge and expertise. Referring patients for an expert opinion regarding indication for and conduct of treatment is often the best plan. Therefore, the following focuses on the overarching principles of current antiviral therapy, rather than providing detailed treatment schemes that will be outdated almost as fast as they are written down.
As with any treatment, the decision to treat has to balance a number of factors, including the likelihood of chronic hepatitis C reaching a stage associated with significant morbidity and mortality during the lifetime of the patient (e.g. age, comorbidities, severity of HCV-related liver disease), the chance of being cured by the therapy (efficacy), and the risk of experiencing side effects during treatment (safety and tolerability). If treatment were highly effective, safe, and well tolerated, HCV infection per se would likely be an indication for treatment in all patients with chronic hepatitis C. It should be emphasized that the extent of aminotransferase elevation correlates poorly with the hepatic inflammatory activity and fibrosis stage in hepatitis C; in fact, patients may have completely normal aminotransferases at the time of liver biopsy despite a chronic hepatitis being present. Other laboratory tests and technologies to assess fibrosis stage, such as FibroTest and FibroScan, may be able to discriminate the extremes, namely cirrhosis from minimal fibrosis. However, the overlap of test results in the mild to moderate fibrosis stages is too large to make these tests clinically useful in decision making regarding antiviral therapy. A liver biopsy therefore continues to play an important role in assessing the immediate need for antiviral therapy, especially in patients with genotype 1 (and 4) infection that respond suboptimally to current treatment regimens (see below).
The current standard antiviral therapy for hepatitis C consists of a combination of pegylated interferon-α and ribavirin. While interferon-α has numerous direct and indirect antiviral effects, the mechanism(s) of action of ribavirin are less clear, but includes immunomodulation.9 Treatment aims at durable elimination of HCV, that is a sustained virological response (SVR), defined as undetectable HCV RNA in serum 24 weeks after cessation of therapy. Patients who reach an SVR almost never relapse later on and are regarded as cured. However, they do not have a protective immunity and HCV re-infection can occur with repeat risk behavior.
The standard of care for treatment of chronic hepatitis C is a combination of pegylated recombinant interferon-α plus ribavirin. Genotypes 2 and 3 respond much better to therapy (SVR 80% after 24 weeks of therapy) than genotypes 1 and 4 (SVR 50% after 48 weeks of therapy).10,11 High baseline levels of HCV viremia, a slow decline of viremia during therapy, advanced fibrosis/cirrhosis, inability to adhere to full dose therapy (tolerability/side effects), and African American ethnicity are associated with impaired SVR rates. While the latter has recently been linked to a genetic polymorphism (IL-28B) upstream of the interferon-λ gene,12 the role of interferon-λ and of the polymorphism in HCV infection and response to interferon-based antiviral therapy remains to be elucidated. If viremia levels decline very rapidly leading to undetectable HCV in serum after 4 weeks of treatment, there is a very high (90%) chance of reaching an SVR with continuing treatment, irrespective of genotype. Conversely, adult patients who do not respond with a decline in viremia levels of at least 2 logs at week 12 of treatment have a very low (<5%) probability of reaching an SVR with continuing therapy, but remain at risk for side effects; in this situation, continuing treatment does not carry a favorable risk–benefit ratio and so it should be stopped.
Side effects of pegylated interferon-α include flu-like symptoms lasting 1–2 months in the vast majority of patients, mood changes ranging from a frequent mild irritability to rare severe depression (including suicidal ideation), a decrease in neutrophil (and platelet) counts requiring dose reduction (and/or treatment with granulocyte colony stimulation factor [GCSF] in up to one-third of patients), and, last but not least, triggering of autoimmune diseases including autoimmune thyroiditis. While most side effects are reversible upon cessation of treatment, generally within 2–4 weeks, autoimmune diseases may persist and require lifelong therapy (e.g. thyroid hormone replacement). Pre-existing autoimmune conditions such as psoriasis are frequently aggravated during therapy. Ribavirin is concentrated in red blood cells and leads to a hemolytic anemia and in combination with pegylated interferon-α the hemoglobin levels typically drop by 30–40 g/L during the first 4–8 weeks of treatment. This may require dose reductions and/or treatment with erythropoietin in some patients. Ribavirin is excreted by the kidneys and is not dialyzable. Patients with impaired renal function are therefore at risk of accumulating ribavirin and require dose adjustments from the start. Ribavirin is fetotoxic and has a risk for teratogenicity when either the mother or father is taking ribavirin at the time of conception/pregnancy. In some age groups this hazard imposes special problems for managing treatment.
Populations that are difficult to treat because of decreased efficacy and/or increased risk for side effects includes patients with decompensated cirrhosis, renal failure, HIV coinfection, transplant recipients, and other patients who are immunocompromised/on immunosuppressive drugs, and i.v. drug users. Treating such patients requires special experience and caution.
Several new antiviral drugs designed to act directly on viral (but also host) targets essential for HCV replication are currently in clinical development. The HCV protease inhibitors telaprevir and boceprevir are the first of these compounds to be licensed in 2011. These drugs will be used in conjunction with the standard pegylated interferon-α plus ribavirin regimen to avoid resistance selection that rapidly occurs with monotherapy. Based on currently available data from the phase III studies,13–16 these drugs will permit shorter duration of treatment and also increase SVR rates in genotype 1 infection (by 20%). Their side effects may be severe, particularly in those with cirrhosis. The most effective and tolerable treatment regimens are currently still being determined. There is a possibility of IFN and ribavirin-free treatments becoming available in the future!17,18
For patients with HCV-related cirrhosis, especially if decompensated or complicated by HCC, a liver transplant offers the only chance for long-term survival although, once transplanted, the graft is universally reinfected by HCV, antiviral therapy tolerance is suboptimal, and is less likely to result in cure. Thus it is best to treat all those with cirrhosis before they develop liver failure—as an SVR pretransplant means no recurrence post transplant. Should an SVR be achieved, liver failure is unlikely to ensue although the risk of HCC is not entirely eliminated.
Special Considerations in Pediatric Patients
Any discussion of hepatitis C infection in children has to include aspects of maternal infection because nowadays almost all chronic hepatitis C occurring in children is due to infection by mother-to-infant transmission (also known as vertical transmission). In North America, children who developed transfusion-associated hepatitis C are now young adults. Among an immigrant population, however, from countries where hepatitis C is endemic and standards of blood unit screening for transfusion and sometimes even medical procedures are suboptimal, some children or teenagers with transfusion-associated chronic hepatitis C will still be found. Often they have some other important medical problem such as a congenital anemia. In general, chronic hepatitis C in children is slowly progressive and seemingly mild. The therapeutic approach used in adult patients cannot be transferred uncritically to the pediatric age bracket, and early treatment of children with chronic hepatitis C is often appropriate and justified. Extended natural history studies suggest that most patients with chronic HCV infection from childhood onwards will develop hepatic fibrosis and possible cirrhosis over the first 2–4 decades of life and thus are at risk for developing clinically important chronic liver disease at a age which most would regard as the “prime of life”. Cirrhosis has been reported in children with chronic hepatitis C and more than 100 children have already been transplanted in North America for end-stage liver disease related to chronic hepatitis C; however, hepatocellular carcinoma is rare, though reported in early adolescence.
In general, mother-to-infant transmission of HCV infection occurs with mothers who are viremic. In order to get a handle on the scope of this problem, studies need to be very large; a review of those involving more than 15 000 pregnant women, indicated that the percent of viremic women in such cohorts is approximately 0.8%. Universal screening of pregnant women for HCV infection may not be cost effective; however, risk factors (Table 14.2) should be sought by direct questioning. With respect to the risk of transmission of HCV from mother to child, an exhaustive analysis of the literature up to 2001 showed that the risk was 1.7% if the mother was only known to be positive for anti-HCV antibodies but 4.3% if the HCV RNA testing was positive once. If the HCV RNA was detected twice, then the risk was 7.1%. Further experience reporting transmission rates with viremic mothers suggests that this latter rate is most relevant, and the overall risk for the HCV-monoinfected woman appears to be 5–7% per pregnancy. Transmission risk is increased if the mother is coinfected with HCV and HIV, probably two- to threefold overall. High viral load (>105–106 viral copies/mL) also seems to be associated with increased rates of transmission of infection; however, viral load fluctuates during pregnancy and thus is not a reliable predictor. The use of fetal internal (“scalp-vein”) monitoring and prolonged rupture of membranes also appear associated with increased risk of transmission. The data regarding amniocentesis are so incomplete that its risk cannot be assessed. There is no advantage to elective caesarean section in mothers with chronic hepatitis C in terms of reducing risk of transmission of HCV. With vaginal delivery, major tears should be avoided. Breast feeding is generally recognized as permissible, although care must be taken to ensure that the nipples do not get cracked and bleed (e.g. mastitis) and the mother does not have a clinical flare of hepatitis postpartum. Even with the best precautions some transmission of HCV to the infant may take place because approximately 30% of this infection may occur in utero instead of around the time of birth.
Determining whether or not the infant gets HCV infection by mother-to-infant transmission is unfortunately less straightforward. The transplacental transfer of anti-HCV antibodies makes it impossible to test the infant for anti-HCV antibodies before 18 months of age. Testing cord blood is useless as the results are impossible to interpret; direct testing for viral RNA is not sufficiently informative when the infant is 1 month-old to warrant testing so early. It is also quite obvious that some infants get an acute infection and clear it in the first year of life. The current standard recommendation is not to test for anti-HCV before the child is 18 months old. If it is negative at 18 months, then the child does not have chronic hepatitis C. In situations where diagnostic information is needed earlier, testing for HCV RNA at 2 months is reasonable. If it is negative, then this is highly reassuring. If it is positive, it should be tested again when the infant is 6 months old, when it may be negative with spontaneously resolved infection. Serum alanine aminotransferase (ALT) should be checked each time (recognizing that the upper limit of normal for ALT is higher in the first year of life than in adults).
The natural history of chronic hepatitis C in childhood is somewhat different from that in adults. In addition to resolution of acute infection in very early infancy, there is a tendency for young children to attain a spontaneous resolution of chronic HCV infection in the first few years of life. Studies have shown that this tendency to spontaneous resolution is the same whether the infection is acquired by mother-to-infant transmission or through some sort of transfusion. A large Canadian natural history study showed that 25% of the cohort achieved spontaneous resolution of chronic HCV infection at approximately 7 years old for those infected at or around birth, and at approximately 12 years old for those where the infective episode was identified.19 Several recent European retrospective studies indicate that the general rate of spontaneous resolution is rather low in children, on the order of 6–15%. Most children with chronic hepatitis C are asymptomatic; extrahepatic manifestations of chronic hepatitis C appear rare in children. Nevertheless, it is clear that chronic hepatitis C in children is a progressive disease, even if it is indolent. Some children have no distinct abnormality on liver biopsy; most have mild inflammation and comparatively little fibrosis. Cirrhosis is uncommon, occurring in approximately 1% of children. Fatty liver, either due to the HCV infection or associated with childhood obesity, may increase the rate of disease progression.20,21 An important but under-recognized aspect of chronic hepatitis C in childhood is that it entails significant social stigmatization for the child and family, and thus it can have a broad negative impact on the child’s social growth and development. Practical issues like going to day-care, playing sports, participating in school trips, dating, and other routine aspects of adolescence loom large for parents; restrictions are problematic for their HCV-infected children.
Accordingly, the threshold for treating children is somewhat more relaxed than for treating adults. There is quite a lot of available research data regarding treatment in children. Two recent pediatric randomized controlled trials have established that combination therapy with pegylated interferon-α plus ribavirin is currently the best standard treatment.22 No child should be treated before the age of 3 years because of the potential for severe neurotoxicity associated with interferon-α. Ribavirin is given using a weight-based regimen, as is customary with most drug treatment regimens in the pediatric age bracket. Children tolerate this regimen very well, although the usual side effects of anemia, neutropenia, transient flu-like syndrome, and weight loss because of anorexia may occur. SVR is on the same order as with adults. Optimal length of treatment in relation to HCV genotype is still being determined. Teenage sexual behavior may be problematic when ribavirin is used, and pregnancy tests must be done frequently. Likewise depression and suicidal ideation, impulsive behaviors, or change in general emotional outlook all need to be monitored closely. Suicidal ideation is an indication for stopping treatment. Practitioners need to be aware that the use of complementary and alternative medicine is highly prevalent in families where a child has chronic hepatitis C.23
The decision to treat a child with chronic hepatitis C is complex, but in principle any child over 3 years old might be at least considered for treatment. For the child with chronic hepatitis C due to mother-to-infant transmission, it may make sense to monitor the disease for another 3–4 years in case later spontaneous resolution occurs. For the child with genotype 2–3 infection, pre-emptive treatment may make sense even with relatively mild liver disease, especially if the family is not coping well or if the child has other chronic health problems whose treatment will be hampered in the face of chronic hepatitis C. For children with chronic hepatitis C due to genotype 1 or 4–6, waiting for even better therapeutic regimens while the child has trivial disease may be the better choice. These issues are important for the generalist who is providing continuity of care to the family or teenager because the decision about treatment and the treatment itself are stressful and require increased medical support.
Likewise it is not really clear how to manage the child who has had spontaneous remission of chronic HCV infection. Most will not have cirrhosis, and the prevailing inclination is to regard them as cured. However, giving in to this definite sense of relief is not necessarily the best plan. They probably need some ongoing surveillance and counseling about lifestyle choices, notably about alcohol use and maintenance of healthy body weight. They may have questions about child bearing. As we accumulate more children who have been treated successfully and achieved SVR, similar considerations will apply to them. Finally, displaced homeless “street kids” are children/teenagers who are at high risk for chronic hepatitis C and may not have the opportunity for adequate treatment; they pose an important challenge for ongoing management of pediatric chronic hepatitis C.








