The incidence of intrahepatic cholangiocarcinoma is increasing. Studies from England and Wales [13,14] and the USA [15] show a 10-fold increase in incidence and mortality between the early 1970s and the mid-1990s. The explanation is unclear. Although improved diagnostic techniques for cholangiocarcinoma and primary sclerosing cholangitis (PSC) may have played a part, they do not alone explain the marked increase. Mortality from extrahepatic cholangiocarcinoma fell over the same period.
Suspicion of cholangiocarcinoma, for example after ultrasound scan, should lead to direct referral to a specialist unit. This is to co-ordinate the work-up to evaluate resectability of the tumour. Modern CT techniques and MRI with MR cholangiography allow a high degree of non-invasive evaluation [16] and the accuracy of tumour staging is reduced by prior biliary stent insertion.
The necessity and timing of ERCP and percutaneous drainage depends on clinical circumstances. They should not be done inappropriately since they may introduce sepsis into the biliary tract which may compromise later treatment [17,18].
These aspects emphasize the importance of a multidisciplinary approach. Staging and formulation of a surgical treatment plan should be the first step. Those patients who have unresectable tumour undergo a planned non-surgical biliary drainage procedure [19].
Associations
PSC predisposes to cholangiocarcinoma with a incidence of up to 40% [20]. The majority of patients with PSC who develop cholangiocarcinoma also have ulcerative colitis [21]. Colonic neoplasia on the background of ulcerative colitis and PSC increases the risk [21]. In a group of 70 patients with PSC followed prospectively for a mean of 30 months, 15 patients died of liver failure. Five of 12 patients having an autopsy had cholangiocarcinoma—7% of the total group [22].
Biliary malignancy is not necessarily a late complication of PSC. In one series, 30% of patients with PSC had a diagnosis of cholangiocarcinoma made within 1 year of the first evidence of underlying liver disease [23]. Clinical features associated with malignancy were epigastric pain, weight loss and raised CA 19-9 and carcinoembryonic antigen (CEA) [23].
Patients with congenital fibrocystic liver disease are predisposed to cholangiocarcinoma. These include congenital hepatic fibrosis, cystic dilatation (Caroli’s syndrome), choledochal cyst and von Meyenburg complexes.
Cholangiocarcinoma may be associated with biliary cirrhosis secondary to biliary atresia.
Liver fluke infestations of the Orient may be complicated by intrahepatic cholangiocarcinoma. In the Far East (China, Hong Kong, Korea, Japan), where Clonorchis sinensis is prevalent, cholangiocarcinoma accounts for 20% of primary liver tumours. These arise in the heavily parasitized bile ducts near the hilum. Opisthorchis viverrini infestation, prevalent in Thailand, Laos and western Malaysia, has a strong association with cholangiocarcinoma [20].
Intrahepatic bile duct stones (hepatolithiasis) is also a risk factor for cholangiocarcinoma [20].
The risk of extrahepatic bile duct carcinoma is significantly lower 10 years or more after cholecystectomy, suggesting a link with gallstones [24] or concentrated gallbladder bile.
Pathology
The common sites of origin are the confluence of the cystic duct with the main hepatic duct and the junction of the main right and left hepatic ducts at the porta hepatis (Fig. 13.1). Tumours of the hepatic duct confluence extend into the liver. They cause complete biliary obstruction with intrahepatic duct dilatation and enlargement of the liver. The gallbladder is collapsed. If the tumour is restricted to one hepatic duct, biliary obstruction is incomplete and jaundice absent. The lobe of the liver drained by this duct atrophies and the other hypertrophies.
In the common bile duct the tumour presents as a firm nodule or plaque which causes an annular stricture which may ulcerate. It spreads along the bile duct and through its wall.
In autopsy studies local and distant metastases are found in about 50% of patients. They involve peritoneum, abdominal lymph nodes, diaphragm, liver or gallbladder. Blood vessel invasion is rare and extra-abdominal spread is unusual.
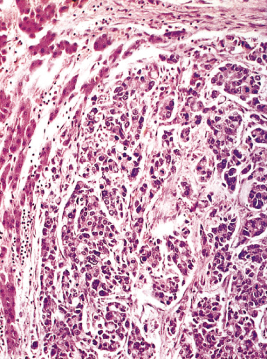
Histologically the tumour is usually a mucin-secreting adenocarcinoma with cuboidal or columnar epithelium (Fig. 13.2). Spread along neural sheaths may be noted. Tumours around the hilum are sclerosing with an abundant fibrous stroma. Distal biliary tract cholangiocarcinoma are often nodular or papillary.
Molecular Changes
Point mutations in codon 12 of the K-ras oncogene are found in cholangiocarcinoma [25]. p53 protein is synthesized particularly in distal duct cholangiocarcinomas [26]. Aneuploidy (divergence from the normal chromosome content) is found in hilar cholangiocarcinoma [26] and is associated with shorter survival. Many other tumour suppressor genes and oncogenes are altered. Cholangiocarcinoma cells contain somatostatin receptor RNA and cell lines have specific receptors. Cell growth is inhibited both in vitro and in vivo by somatostatin analogues [27].
Clinical Features
This tumour tends to occur in the older age group, patients being about 60 years old. Slightly more males than females are affected. Jaundice is the usual presenting feature, followed by pruritus—a point of distinction from primary biliary cirrhosis where itching usually precedes jaundice.
Jaundice is not usually present in those with intrahepatic cholangiocarcinoma or unilateral main duct involvement.
In those presenting with jaundice, the bilirubin rise is progressive but periods of temporary improvement occur in up to 50% [28].
Pain, usually epigastric and mild, is present in about one-third of patients. Diarrhoea may be related to steatorrhoea or underlying ulcerative colitis. Weakness and weight loss are marked.
Examination.
Jaundice is deep. Cholangitis is unusual unless there has been prior cannulation of the biliary tree by ERCP or PTC.
The liver is large and smooth, extending 5–12 cm below the costal margin. The spleen is not felt. Ascites is unusual.
Investigations
Serum biochemical findings are those of cholestatic jaundice. The serum bilirubin, alkaline phosphatase and γ-glutamyl transpeptidase levels may be very high. Fluctuations may reflect incomplete obstruction.
The serum antimitochondrial antibody test is negative and α-fetoprotein is not increased.
The faeces are pale and fatty. Glycosuria is absent.
Anaemia may be greater than that seen with ampullary carcinoma; the explanation is unknown—it is not due to blood loss. The leucocyte count is high normal with increased polymorphs.
Liver biopsy shows features of large duct obstruction. However, it must be emphasized that liver biopsy on the background of biliary obstruction has a risk of serious complications such as biliary peritonitis, and should be performed only when there is doubt about the diagnosis. In PSC, biliary dysplasia raises the possibility of coexistent cholangiocarcinoma [29].
Cytology taken at the time of ERCP or percutaneous drainage is worthwhile, but requires cytological expertise for interpretation. Brush cytology has a higher sensitivity than analysis of aspirated bile [30].
Percutaneous biopsy is contraindicated in patients with resectable disease due to the risk of needle tract seeding [31]. A tissue diagnosis is required in those with unresectable or metastatic disease prior to palliative therapy. Approaches include biopsy or fine-needle aspiration cytology from the suspected tumour guided by fluoroscopy, transabdominal or endoscopic ultrasound, or cholangioscopy with endobiliary biopsy.
In PSC, brush cytology of dominant strictures at ERCP has a sensitivity of 60% for diagnosis of cholangiocarcinoma [32]. p53 and K-ras mutation analysis does not increase sensitivity but K-ras mutations may be found ahead of the diagnosis of cholangiocarcinoma in patients with PSC [33].
The serum concentration of the tumour marker CA 19-9 is often increased in patients with biliary tract malignancy. Extremely high levels are also reported with benign biliary obstruction [34]. The sensitivity for detecting cholangiocarcinoma in PSC is 50–60% [35,36].
Imaging [16]
Ultrasound is usually the initial imaging modality done for patients with cholestasis (see Chapter 12). With a typical hilar cholangiocarcinoma ultrasound shows dilated intrahepatic ducts with a normal extrahepatic biliary tree. A tumour mass may be shown in up to 80% of cases [37]. Ultrasound (real-time together with Doppler) accurately detects neoplastic involvement of the portal vein, both occlusion and wall infiltration, but is less good in showing hepatic arterial involvement [38]. Intraduct ultrasound is still experimental but can provide information on tumour extension in and around the bile duct [39].
Modern helical CT (chest and abdomen) can detect cholangiocarcinoma in more than 90% of patients (as small as 15 mm diameter). It also provides information on parenchymal infiltration and bile duct, portal vein and hepatic artery involvement [40]. Local and distant metastases can also be detected.
However, CT underestimates the extent of bile duct involvement, hepatic arterial invasion, lymph node spread and rarely detects peritoneal disease.
MRI is currently the best imaging modality when ultrasound suggests hilar cholangiocarcinoma [13]. MRCP detects over 90% of biliary strictures [40]. In cholangiocarcinoma (Fig. 13.3) MRI and MRCP correctly delineate duct obstruction and the extent of hilar tumour extension in more than 80% of patients [41]. The accuracy of MRCP in evaluating the level and extent of bile duct involvement is comparable to direct cholangiography by ERCP or PTC [42]. MRI with MRCP is an important technique for planning the treatment of malignant hilar strictures but does not replace invasive cholangiography which also allows brushings to be taken for cytology and bile drainage if indicated. MRA provides information on vascular involvement.
Fig. 13.3. A 75-year-old woman presenting with cholestatic jaundice. Ultrasound showed dilated intrahepatic ducts, a hilar mass and a normal common bile duct. (a) MRCP shows dilated intrahepatic ducts with at least three segments obstructed in the right lobe and the left hepatic system obstructed at the hilum. If non-surgical drainage is to be done, these appearances favour drainage of the left- rather than the right-sided system (D, duodenum). (b) MRI scan shows a mass in the liver (arrow) above the hilum. (c) Non-operative drainage was chosen since the patient was considered inoperable due to inadequate future remnant liver volume. ERCP shows a normal common duct with a hilar stricture. A stent could not be placed. (d) Following on the MRCP appearances, the left-sided duct system was chosen for percutaneous cholangiography and a stent inserted.
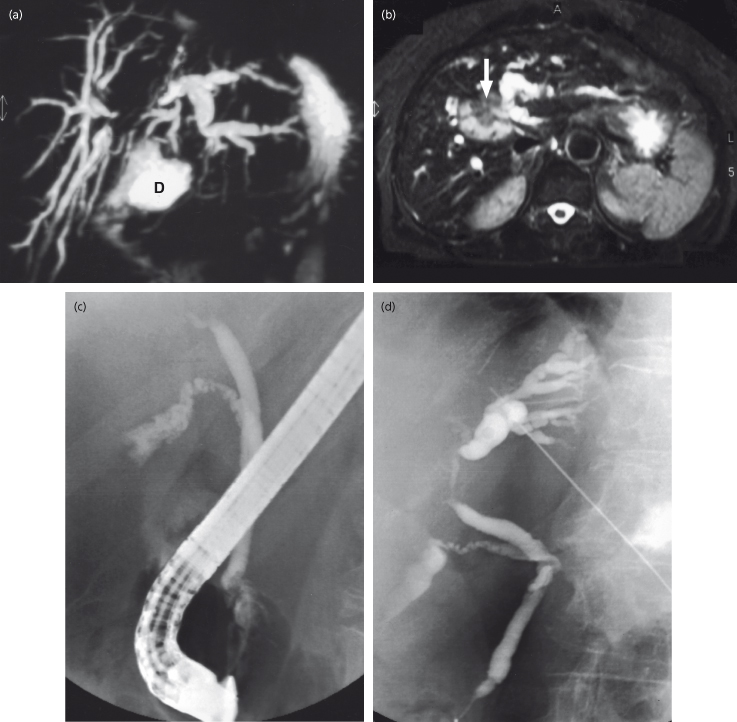
Thus CT and MRI usually demonstrate bile duct obstruction and often a mass but cannot reliably differentiate between inflammatory and malignant biliary strictures. If surgical resection is not possible then invasive techniques are used to obtain cytology or biopsy.
Detecting cholangiocarcinoma in patients with PSC is difficult. PET scanning using [18F] fluoro-2-deoxy-D-glucose (FDG) can detect cholangiocarcinomas in patients with and without PSC with a sensitivity of 90% [43,44]. However, false-positive scans have been seen in normal individuals [44]. Most biliary cancers are FDG avid and PET or integrated PET-CT may have a role in the detection of unsuspected metastases or lymph node spread which can be underestimated by CT. In a prospective study [45] of 123 patients, PET-CT showed significantly higher accuracy over CT in the diagnosis of regional lymph node metastases and distant metastases. However, PET-CT demonstrated no significant advantage over CT and MRI/MRCP in the diagnosis of the primary tumour [45].
Direct Cholangiography
Endoscopic or percutaneous cholangiography (Fig. 13.3) prior to staging is only indicated in the septic patient or where there is renal failure associated with severe cholestasis. It is prudent to investigate the patient non-invasively with CT and MRI/MRCP to judge the nature and extent of the hilar lesion, and then consider direct cholangiography, cytology and drainage when the management plan is clear. MRCP is useful to select the duct system for endoscopic or percutaneous drainage.
In hilar cholangiocarcinoma, ERCP shows a normal common bile duct and gallbladder with obstruction at the hilum (Fig. 13.3c). Contrast usually passes through the stricture(s) into dilated bile ducts above. The stricture is passed with a guide-wire, brushings taken for cytology and a stent placed.
Percutaneous cholangiography shows the dilated intrahepatic ducts down to the stricture (Fig. 13.3d). A drain is inserted. When right and left hepatic ducts are individually obstructed, puncture of both systems may be necessary for planning surgical intervention but drainage of one lobe or sector is usually sufficient to relieve jaundice.
Percutaneous cholangiography usually provides more information on tumour extension within the liver and biliary tree compared with endoscopic cholangiography.
Angiography
Hepatic artery and portal vein involvement by tumour often dictates whether resection is feasible. MRA and CT angiography are useful in the assessment of vascular involvement and their diagnostic accuracy is comparable to conventional angiography which is invasive.
New Techniques
These include endoscopic ultrasound, intraductal ultrasound and direct cholangioscopy [13,16]. Their use is evolving but as yet their precise role for cholangiocarcinoma is not defined. They are best used within the context of clinical trials [13].
EUS provides good views of the extrahepatic biliary tree and regional lymph nodes. The sensitivity and specificity of EUS in the diagnosis of malignancy is comparable to ERCP [46]. In addition, EUS can facilitate fine-needle aspiration of lesions and lymph nodes. However, the availability and expertise of EUS is limited and poor views are often obtained for hilar strictures.
Cholangioscopy directly views bile duct mucosa and allows endobiliary biopsy. It can be performed perorally via an endoscope or percutaneously. However, this technique is expensive and invasive.
Intraductal ultrasound may have a role in differentiating benign and malignant biliary strictures.
Staging and Preparation for Surgery
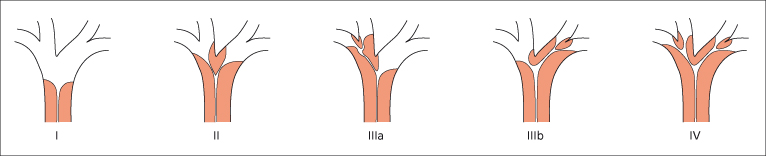
If the clinical state of the patient does not rule out surgery the resectability and stage of tumour must first be assessed as outlined above. If cholangiography shows involvement of the secondary hepatic ducts in both hepatic lobes (Fig. 13.4, type IV) then hilar cholangiocarcinoma would be considered unresectable. Bilateral involvement of hepatic arterial or portal vein branches or both is also considered unresectable disease. Encasement of the main portal vein or common hepatic artery is a relative contraindication to surgery. If the tumour is limited to the hepatic duct bifurcation with or without unilobar portal vein or hepatic artery involvement then the lesion is usually resectable with an extended right or left hepatectomy.
Fig. 13.4. Classification of hilar cholangiocarcinoma according to the involvement of bile ducts. Resectability of type I to III depends on angiographic findings. Type IV (bilateral involvement of secondary hepatic ducts) indicates incurable disease. In inoperable patients median survival after stent insertion depends upon the extent of tumour.
Portal Vein Embolization
Extended right or left hepatectomy may result in postoperative hepatic insufficiency if the volume of the remnant liver is not adequate. The volume of the future liver remnant can be reliably quantified by MRI or CT. Preoperative portal vein embolization is a safe and effective method of increasing the volume of the future liver remnant and should be considered when the future liver remnant is less than 20% in patients with normal liver function, or less than 40% in patients with compromised liver function [47].
Preoperative Biliary Drainage
The role of preoperative biliary drainage prior to resection for hilar cholangiocarcinoma is controversial, and there are no prospective randomized trials. The primary concern with preoperative biliary drainage is the associated risk of sepsis. However, malignant obstructive jaundice is associated with liver and kidney failure, and coagulopathy, and many patients will benefit from preoperative biliary drainage. It should be considered in association with preoperative portal vein embolization when the future liver remnant is small.
Laparoscopy
The sensitivity of all the imaging modalities outlined above in detecting peritoneal disease is poor. Laparoscopy is invasive, but can reliably detect peritoneal disease. There are no randomized trials reporting the role of laparoscopy in the staging of cholangiocarcinoma. However, it is widely accepted that staging laparoscopy should be performed before proceeding with definitive surgery as this will prevent unnecessary laparotomy in up to 30% of patients [48].
Treatment
Surgery
Surgical resection is the primary treatment for cholangiocarcinomas. Distal cholangiocarcinomas are resected with pancreatoduodenectomy, with a 5-year survival of about 30%. In patients with spread along the common bile duct, hepatic resection and pancreatoduodenectomy can be performed.
Mid-bile duct cancers are resected with excision of the biliary tree and hilar lymphadenectomy.
Typical hilar (Klatskin) tumours are resected by an extended right or left hepatectomy depending on the extent of involvement of the right or left duct system. The biliary tree is excised and the proximal bile duct(s) drained into a Roux-en-Y loop of intestine. Radical hilar lymphadenectomy is done because of the possibility of lymphatic dissemination. With Klatskin tumours a caudate lobectomy is usually performed since segment 1 ducts drain to the confluence of the hepatic ducts and are likely to be involved by tumour.
The proportion of cholangiocarcinomas being resected has increased from 5–20% of patients in the 1970 and 1980s to 30% or more in specialist centres currently. This is because of improved staging and resection techniques within specialist surgical units.
The challenge is to achieve resection with tumour-negative margins and aggressive, major hepatic resections may increase the likelihood of achieving this. Extended resection including the portal vein to achieve tumour clearance can be performed but at present the role of routine portal vein resection is not clear.
Median survival after aggressive resection of hilar cholangiocarcinoma is 18–40 months with good palliation for most of this time [49].
Local resection can be considered for Bismuth type I and II tumours (Fig. 13.4) with low perioperative mortality.
At present, there is no role for adjuvant chemotherapy or radiotherapy for patients with tumour-negative resection margins. Cancer Research UK is conducting a randomized phase III trial comparing surgery and capecitabine with surgery alone for cancer of the bile duct or gallbladder (BILCAP trial).
Liver Transplantation
In general cholangiocarcinoma is a contraindication for liver transplantation. The early experience for unresectable cholangiocarcinoma was disappointing because of early recurrence. In the last decade, however, the University of Nebraska and the Mayo Clinic have published reports where, in highly selected patients with early stage unresectable cholangiocarcinoma, liver transplantation in combination with preoperative chemoradiation was associated with prolonged disease-free and overall survival. These are single institution studies and it is not certain such results could be reproduced uniformly by other transplant centres. Overall, the role of liver transplantation for de novo cholangiocarcinoma is not clearly defined and this treatment should be carried out within a prospective trial.
Surgical Palliation
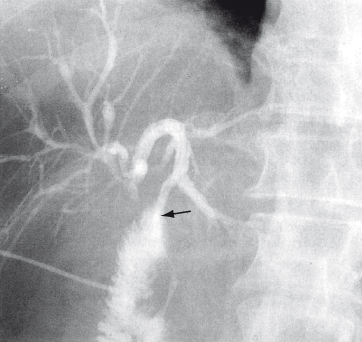
These include anastomosis of jejunum to the segment III duct in the left lobe, which is usually accessible above the hilar tumour (Fig. 13.5). Jaundice is relieved for at least 3 months in 75% of patients. If segment III bypass is not possible (atrophy, metastases), a right-sided intrahepatic anastomosis to the segment V duct can be done. Surgical approaches for palliation are rarely indicated in centres with specialists in ERCP and percutaneous biliary intervention.
Fig. 13.5. Check cholangiogram after surgical bypass for hilar cholangiocarcinoma. The anastomosis is between the jejunum and the third segment duct of the left lobe (arrow).
Non-Surgical Palliation
In those patients unfit for surgery or with irresectable tumours, jaundice and itching may be relieved by placing an endoprosthesis across the stricture either by the endoscopic or percutaneous route.
By the endoscopic route, stents can be inserted successfully in about 90% of patients. The major, early complication is cholangitis (7%). Thirty-day mortality is between 10 and 28% depending upon the extent of the tumour at the hilum and the mean survival is 20 weeks [50]. It is only necessary (initially) to stent one lobe [51]. Scoring systems to predict outcome in patients having palliative stenting are available and can aid decision making in patients with advanced disease [52].
Percutaneous transhepatic endoprosthesis insertion is also effective but carries a higher risk of complications such as bleeding and bile leakage. Metal mesh endoprostheses, which expand to 10 mm diameter in the stricture after insertion on a 5 or 7 French catheter, are more expensive than plastic types, but have longer patency [53,54].
There are no trials comparing surgical versus non-surgical palliation. Generally, non-operative techniques are appropriate for high-risk patients expected to have a shorter survival. If recurrent stent blockage occurs, a surgical bypass can be considered [55].
There is no conclusive evidence to support the role of chemotherapy or radiotherapy in patients with advanced or metastatic disease. The role of external radiotherapy or radiotherapy combined with biliary drainage is unproven. Cytotoxic drugs are ineffective. A UK phase II trial (the UK ABC-01 study) compared gemcitabine alone or in combination with cisplatin in patients with advanced biliary tract cancers [56]. The primary endpoint, 6 month progression-free survival, was 57.1% for the combination arm and 47.7% for the single agent arm [56]. This study has been extended into a phase III study (ABC-02) to determine the effect on overall survival and quality of life.
Intraduct photodynamic therapy combined with stenting in patients with unresectable cholangiocarcinoma has shown survival benefit in randomized, controlled trials [57,58]. The treatment is costly but appears to offer good palliation. However, further studies are required to confirm the benefits of photodynamic therapy.
Prognosis
Prognosis depends on the site, stage and treatment of the tumour. Distal cholangiocarcinomas are more likely to be resectable than those at the hilum. Polypoid cancers have the best prognosis.
If unresected, the 1-year survival for cholangiocarcinoma is 50%, with 20% surviving 2 years and 10% at 3 years [59]. This reflects the observation that some tumours are slow growing and metastasize late.
Intrahepatic Cholangiocarcinoma [60]
This intrahepatic bile duct-derived tumour is classified as a primary hepatic carcinoma (see Chapter 35). It becomes symptomatic as it enlarges, producing abdominal pain rather than jaundice. It grows rapidly with early metastasis and a particularly poor prognosis. Scanning shows an intrahepatic mass. Distinction from hepatocellular carcinoma may be difficult. Hepatic venous and portal vein involvement is rare. Surgery is the only chance for effective treatment. Resection is possible in 30–60% of cases [61]. One-year survival after resection is 50–60%. Five-year survival rates range from 4.1% [62] to 35% [63]. The results of liver transplantation for intrahepatic cholangiocarcinoma have been poor and at present there is no role for liver transplantation outside of a clinical trial [60].
Other Biliary Malignancies
Biliary cystadenocarcinoma and mixed hepatocellular and cholangiocarcinoma have been reported but are rare.
Metastases at the Hilum
Cholestatic jaundice developing following the diagnosis of carcinoma elsewhere (in particular the breast and colon) may be due to diffuse metastases within the liver or duct obstruction by nodes at the hilum. Differentiation between the two is by ultrasound. If dilated bile ducts are shown and the patient is symptomatic with itching, biliary obstruction can be relieved by insertion of an endoprosthesis by the endoscopic or percutaneous approach [64].
Periampullary Carcinoma
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree