Fig. 36.1
Recipient’s incision
3.
Exposure: Satisfactory exposure is achieved using retractors (Fig. 36.2). Attention should be given to exposing the second hepatic hilum and right hepatic area so that the suprahepatic IVC can be easily exposed and reconstructed and the retro-hepatic IVC area can be easily dissected.
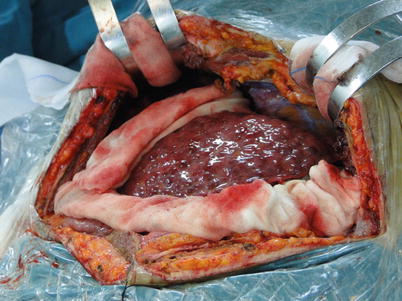

Fig. 36.2
Exposure using retractors
4.
Intra-abdominal exploration: Extrahepatic metastases and cancerous emboli in the portal vein and IVC should be carefully inspected in recipients undergoing LT for hepatic malignancy. A splenectomy that is indicated in patients with splenomegaly and hypersplenism should be performed prior to liver removal, which improves coagulation function and facilitates liver resection.
5.
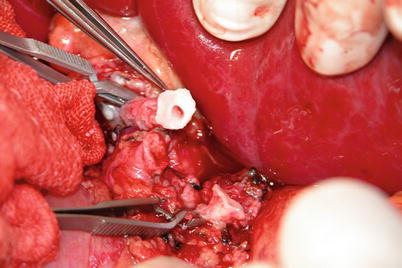
Exposure of hepatic artery and portal vein: The hepatic artery proper can be located on the left border of hepatoduodenal ligament and freed up a short distance beyond the area of arterial bifurcation from its point of origin. Hepatic arteries should not be clamped or tracted by suture (Fig. 36.3). Periarterial tissue instead of the arterial sheath tissue is dissected to expose the hepatic artery and preserve its adventitia intact. The left and right hepatic arteries can be ligated and cut close to the liver to facilitate later procedures (Fig. 36.4). The portal vein can be perfectly exposed when the surrounding tissue and lymph nodes are removed after both the hepatic artery and common bile duct are freed. The portal vein should be exposed from its bifurcation to the superior surface of the pancreas. Sutures are placed at the root of the left and right branches of the portal vein, respectively, for later ligature and division (Figs. 36.5 and 36.6).
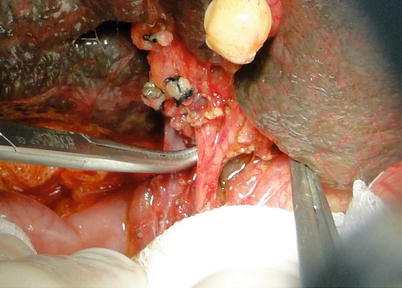
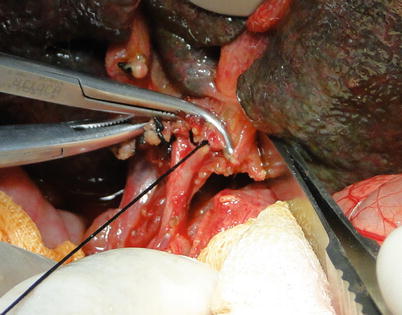
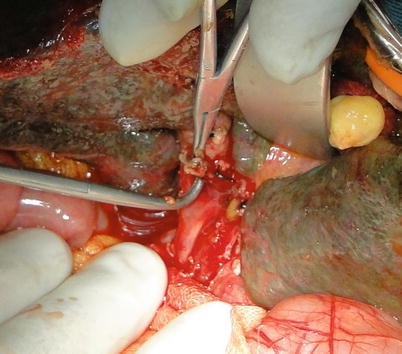
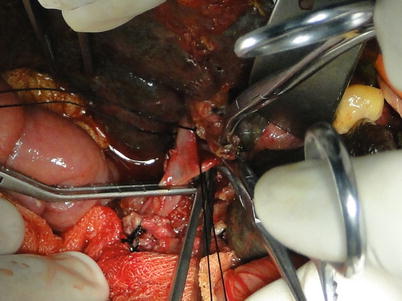

Fig. 36.3
Dissection of bifurcation of the hepatic artery

Fig. 36.4
Ligature of the left and right hepatic arteries

Fig. 36.5
Portal vein mobilization

Fig. 36.6
Exposure of portal vein and its branches
6.
Suprahepatic IVC exposure: Extensive adhesions and abundant collaterals can be found around the liver in patients with portal hypertension. As a result, the periodontal ligaments of the liver should be cut off after dissection of the first liver hilum to prevent severe bleeding. The left triangular ligament is cut off subsequent to hepatic falciform ligament division using an electrotome. Both ends of the left triangular ligament should be ligated or sutured because collaterals commonly form in this ligament. The hepatogastric ligament can be exposed and cut off when the left lateral segment is turned over to the right after the left coronary ligament is cut off. The accessory left hepatic artery originating from the left gastric artery can be found in the hepatogastric ligament and hence requires double ligation before division. The right triangular ligament and right coronary ligament are cauterized. The ligamenta hepatocolicum and hepatorenal ligament can be cauterized after the right lobe is lifted gently to the left. The right venas suprarrenales should be ligated and detached close to the retro-hepatic IVC after exposure. The right posterior border of the retro-hepatic IVC should also be exposed. The left posterior border of the IVC can be exposed by longitudinal opening peritoneal reflection on the left side of the retro-hepatic IVC after lifting the left lobe and caudate lobe to the right. The suprahepatic IVC can be freed using an index finger and a right-angle clamp. The infrahepatic IVC can be reached by opening the lateral peritoneum along the duodenum. The pleural short hepatic veins joining the IVC should be carefully handled and ligated. A blood vessel loop should be placed around the infrahepatic IVC at the converging point of the right renal vein. Delicacy and patience is required in blunt dissection of the suprahepatic IVC so as to avert injury. Dissection of the ligaments around the liver should be performed under direct vision as close to the liver surface as possible. To avoid retroperitoneal hemorrhage, complete mobilization of the retro-hepatic IVC can be neglected in patients with severe portal hypertension or those with difficulties in exposing the IVC. At this time, the supra- and infrahepatic IVC can be exposed, and the area behind the vena cava can be handled when the liver is removed (Figs. 36.7 and 36.8).
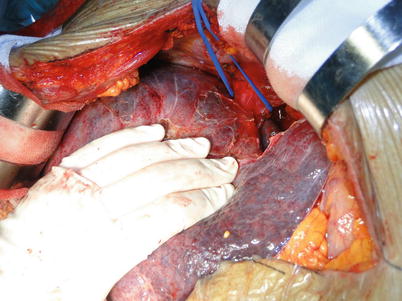
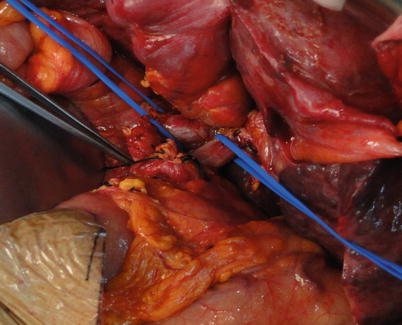

Fig. 36.7
Exposure of the suprahepatic IVC

Fig. 36.8
Exposure of the infrahepatic IVC
7.
Management of the portal vein and infrahepatic IVC at liver removal: Blockage of supra- and infrahepatic IVC is achieved using harmless vascular clamps (Satinsky’s vascular clamps). The liver should be maintained at its anatomical position, and the clamps should be placed in a horizontal manner. The left and right renal veins should not be clamped. Partial diaphragmatic tissue can be included when the suprahepatic IVC is clamped to prevent accidental slip; however, the phrenic nerve should be protected. The opening of the clamp can be further coalesced using coarse thread. The posterior hepatic plane can be detached from the IVC by lifting the liver after cutting off the portal vein and infrahepatic IVC. At this time, diaphragmatic tissue posterior to the liver and retroperitoneal collaterals can be clamped. A long posterior wall of suprahepatic IVC should be spared for reconstruction when it is cut apart from the point close to the liver surface. Injury to the liver bed and the volume of bleeding can be minimized by removing the liver as close to the back of the retro-hepatic IVC as possible.
8.
36.2 Liver Removal and Vessel Exposure in Living-Donor Liver Transplantation (LDLT)
Steps
2.
Exposure of the hepatic artery and portal vein: See Sect. 36.1. To shorten the hepatic duration, the portal vein should not be occluded until the dissection of the second and third liver hilar is finished because venous bypass is absent in LDLT.
3.
Exposure of supra- and infrahepatic IVC: Abundant collaterals can be found in the perihepatic ligaments in cirrhotic patients. Therefore, the perihepatic ligaments need to be severed using an electrotome and sutured when there is bleeding. The perihepatic ligaments are generally severed in the following order: left triangular ligament, coronary ligament, right triangular ligament, hepatocolic ligament, hepatorenal ligament, right suprarenal vein, left hepatogastric ligament, and the peritoneal reflection beneath the IVC. Then, blood vessel loops are placed around the supra- and infrahepatic IVC after mobilization (Fig. 36.9).
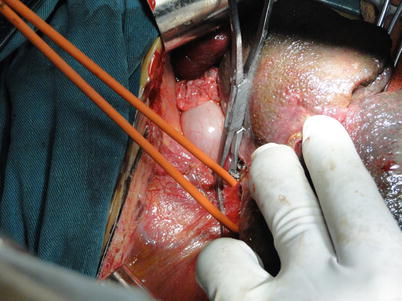

Fig. 36.9
Traction and occlusion of the suprahepatic IVC
4.
Exposure of the second liver hilum and hepatic veins: The tissue about the right hepatic vein (RHV) is carefully divided to provide sufficient exposure of the RHV for a right-angle clamp to be inserted under the vein to permit the placement of a blood vessel loop for traction. The RHV is doubly clamped with harmless vascular clamps. Both ends of the RHV are oversewn with 5-0 Prolene sutures. The left and middle hepatic veins are freed of liver substance until a sufficient distance is gained to permit the application of a pair of long curved Cooley vascular clamps. The confluence of the left and middle hepatic veins should be expanded and spared for anastomosis. In cases with difficulty in exposing the RHV, the RHV can be attained subsequently to division of the left hepatic vein or to understanding the relationship between hepatic veins and retro-hepatic IVC by dividing the portal vein and lifting of the liver (Figs. 36.10, 36.11, and 36.12).
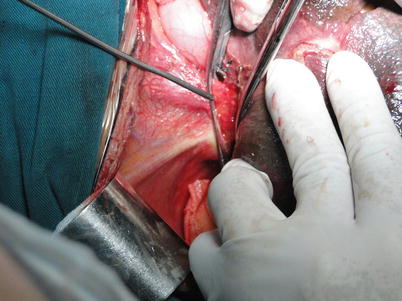
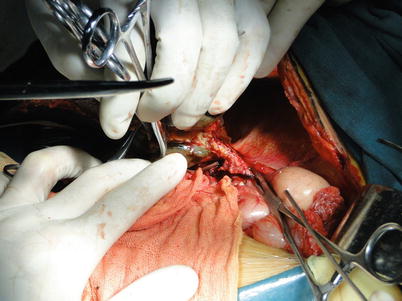
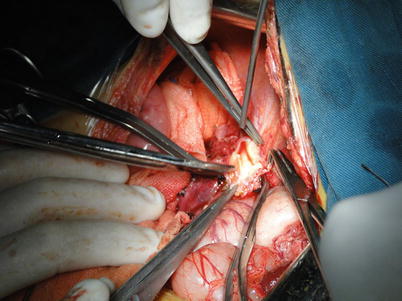

Fig. 36.10
Exposure of the hepatic veins

Fig. 36.11
Dissection and division of the hepatic veins

Fig. 36.12
Hepatic vein trimming
5.
Third hepatic hilum and retro-hepatic IVC exposure: This is the most challenging part of liver removal in LDLT and hence requires patience, meticulousness, and accuracy in handling. The caval ligament can be exposed and then severed after lifting the right lobe to the left. The caval ligament is a fibrous tissue that bridges each side of the caudate lobe behind the IVC. The number of short hepatic veins varies among patients and can be as many as ten. Hepatic veins, which are short fragile, tend to be torn, resulting in IVC wall bleeding and air embolism. Short hepatic veins can be cut off when each end is closed using metal clips to avoid slippage. The ends of short hepatic veins joining the IVC can be closed using harmless suture after liver removal. Caution must be executed as an inferior RHV can be encountered in many cases. The right lobe is placed back and the left lateral and caudate lobe are rotated medially. The caudate lobe and posterior peritoneal transferring portion are cut open using an electrotome. Short hepatic veins can be handled from the left side of the IVC toward the major hepatic veins cranially after the caudate lobe is freed from the IVC (Fig. 36.13).
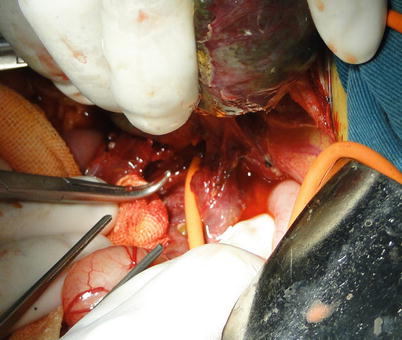

Fig. 36.13
Exposure of the third hepatic hilum and retro-hepatic IVC
6.
Management of the portal vein and hepatic veins: At this time, the liver is connected to the body only by the portal vein, hepatic artery, and hepatic veins. The liver is removed by cutting off the portal vein, hepatic artery, and hepatic veins when there is 15–30 mins left to finish repairing the donated liver. The left, middle, and right hepatic veins are clamped individually using curved Cooley vascular clamps and then divided. Compared to cadaveric livers, living-donor livers provide shorter stumps of hepatic arteries, portal vein, and hepatic veins. As a result, it is critically important to preserve the stumps of hepatic arteries, portal vein, and hepatic veins of the recipient as long as possible. Recipient’s hepatic arteries and portal veins should be dissected as close to the first hepatic hilum as possible. The portal vein should be freed up a short distance beyond the area of arterial bifurcation. The point of division of the recipient’s hepatic artery depends on its own size and length, which can be as far as the bifurcation of the hepatic artery proper, or the point of gastroduodenal artery leaving the common hepatic artery. The middle and left hepatic veins should be severed as close to the liver as possible to spare a long enough venous remnant for repair and anastomosis. When the liver is removed, the liver bed should be checked carefully for bleeding, and the openings of the recipient’s hepatic veins need to be trimmed to match those of the donated liver. LDLT is not suitable in patients with hepatic malignancy adjacent to hepatic veins and retro-hepatic IVC because it is hard to achieve complete liver removal [8–11].
36.3 Vascular Reconstruction in OLT
Steps
1.
Reconstruction of the suprahepatic IVC: The suprahepatic IVC reconstruction begins in the midline anteriorly and posteriorly with 3-0 Prolene sutures, and knots are tied when the donated liver is implanted. Two corner stitches are placed using 4-0 Prolene sutures for suture traction when the IVC is anastomosed. A back row anastomosis is completed first. Over-and-over suturing is then carried from the endothelial surface outward, ensuring the endothelium is tacked down as well as avoiding the involvement of the contralateral wall. At the end, it is tied in the midline anteriorly. Key points: (1) The suprahepatic IVC from either the donated liver or the recipient should be trimmed to an appropriate length before anastomosis. Otherwise, when it is too long, the suprahepatic IVC can overlap or obstruct following the anastomosis. (2) The anastomosis can twist if involution of the suprahepatic IVCs from the donor and the recipient is not satisfactory. (3) Anastomotic stenosis or thrombosis can be avoided when sutures are carried from the endothelial surface outward. (4) The large-sized end of IVC can be readjusted in a parallel mattress suture fashion. (5) The final knot should be made 1 cm far from the IVC wall in order to avert anastomotic stenosis when blood fills the IVC (Fig. 36.14).
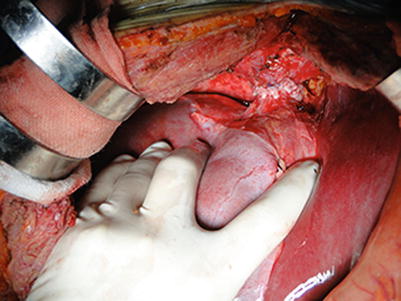

Fig. 36.14
Completed reconstruction of the suprahepatic IVC
2.
Reconstruction of the infrahepatic IVC: The infrahepatic IVC is reconstructed using 3-0 Prolene suture in a similar fashion as for the suprahepatic IVC. A number 8 urinary catheter is passed from the anastomosis into the retro-hepatic IVC, facilitating blood flush out when the portal vein is patent (Fig. 36.15).
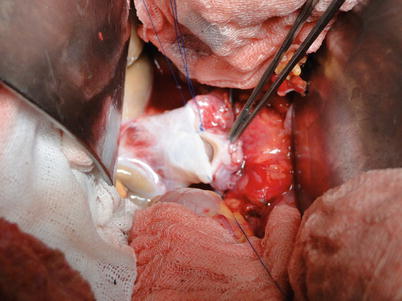

Fig. 36.15
Reconstruction of the infrahepatic IVC
3.
Reconstruction of the portal vein: The portal vein of the recipient is clamped proximally, while that of the donor is cut off close to the hilum. Reconstruction begins in the midline anteriorly and posteriorly with 5-0 Prolene sutures. Continuous suturing running bilaterally is then carried from the endothelial surface outward, ensuring the endothelium is tacked down. The contralateral wall of the portal vein should not be involved when the other side is being anastomosed. A continuous flush of heparinized saline is used to decontaminate the anastomosis. The vein is irrigated with heparinized saline to flush out any clot and air once anastomosis is nearly completed. A knot is made on the anterior wall of the portal vein when blood fills the lumen. The clamp at the donor’s side of the portal vein is released prior to that at the recipient’s end, so potassium-rich and acidic metabolite can flow out along with 100–200 ml of blood from the infrahepatic IVC. The suture is tightened and knotted when the urinary catheter is withdrawn, with portal vein re-clamping. When the anesthetist is informed that hepatic reperfusion is occurring, the suprahepatic IVC, portal vein, and infrahepatic IVC regain patency in that order (Figs. 36.16, 36.17, 36.18, and 36.19).
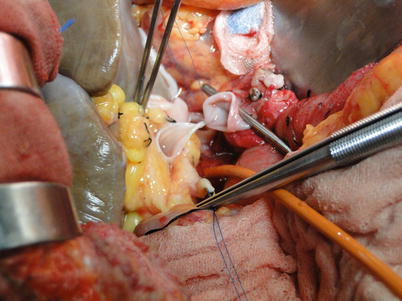
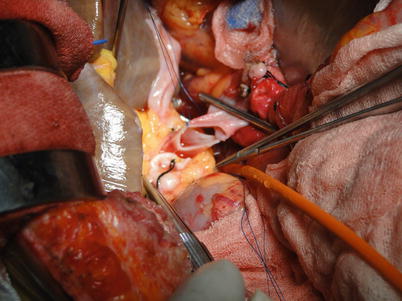
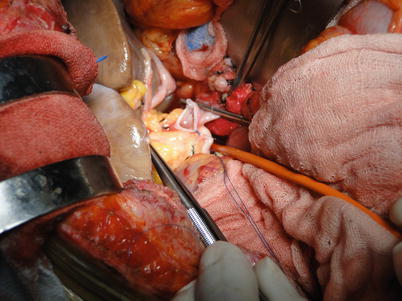
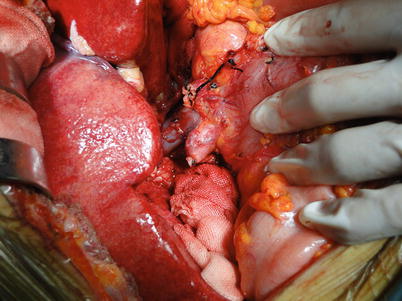

Fig. 36.16
Placement of one stitch on the posterior wall of the portal vein

Fig. 36.17
Placement of one stitch on the anterior wall of the portal vein

Fig. 36.18
Anastomosis of the bilateral walls of the portal vein

Fig. 36.19
Completed reconstruction of the portal vein
In patients with portal thrombosis or cavernous transformation, an interpositional vascular graft can be used for reconstruction because the length of the normal portal vein might not be sufficient. The donor’s iliac vein, which is an optimal substitution graft source, is anastomosed to the donor’s portal vein at the back table. When the portal thromboses extend to the origin of the superior mesenteric vein, the substituted vessel can be anastomosed to the anterior wall of the vessel at the root of the mesocolon transversum running behind the stomach and in front of the pancreas [5–7].
4.
Reconstruction of the hepatic artery: Hepatic arterial reconstruction is an important determinant of successful liver transplantation. Arterial stenosis, flow insufficiency, and thrombosis can result in primary non-function, severe infection, and biliary complications, leading to a mortality rate of 75 %. These complications commonly require re-transplantation. Hepatic arteries from the donor and the recipient require careful appraisal and cautious management. Autologous vessels can be used for interposition when the recipient’s hepatic artery is unsuitable for reconstruction [12–16].
Get Clinical Tree app for offline access

(i)
End-to-end anastomosis: This method should be tailored to cases where the hepatic arteries from the donor and the recipients are similarly sized. The hepatic artery proper, the bifurcation where the gastroduodenal artery leaves the hepatic artery proper, the common hepatic artery, and the splenic artery from the recipient can be used for reconstruction. End-to-end arterial reconstruction can be performed in a continuous or interrupted fashion using 8-0 or 7-0 Prolene suture (Figs. 36.20, 36.21, and 36.22).