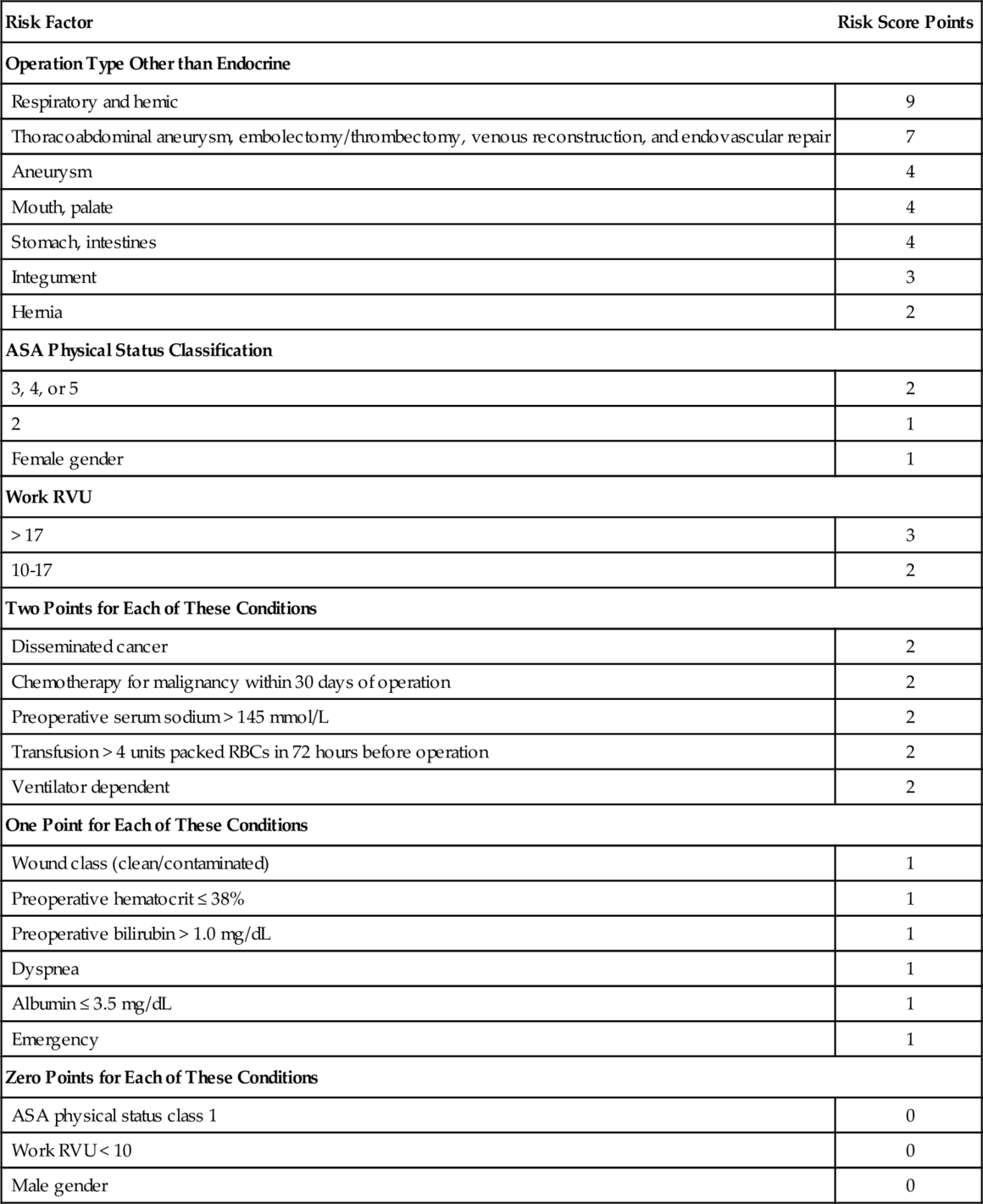
Chapter 32 Todd E.H. Hecht, MD, FACP, SFHM Venous thromboembolism (VTE) is common in the United States, particularly in surgical populations. The incidence of VTE in the United States has been estimated at approximately 70 to 113/100,000/year, with the consequent estimation of upwards of 200,000 new cases annually. Further, VTE historically has been strongly associated with mortality, with studies estimating a 1-month all-cause mortality rate of 11% after VTE and a 3-month all-cause mortality rate of 17.4% after pulmonary embolism (PE). The rate of VTE in urological surgery has been variably estimated in the literature. One recent guideline estimated that, without the use of VTE prophylaxis, the rate of deep venous thrombosis (DVT) and PE was 10% and 1%, respectively, in open urological surgery patients. Another guideline estimated the rate of symptomatic VTE to be 1% to 5%. In general, the risk of symptomatic VTE depends on the nature of the procedure, with transurethral procedures carrying a lower risk of VTE than open procedures. In light of this, the most recent American College of Chest Physicians (ACCP) Guidelines on VTE Prevention advocates the use of two risk-scoring systems to quantify the risk of VTE; these scoring systems will be further explored later in this chapter. A number of options are available for VTE prophylaxis, the supporting evidence for which will be discussed later in this chapter. Pharmacologic options will be discussed first, followed by mechanical forms of prophylaxis. Unfractionated heparin (UFH) has been used for decades for the prevention of VTE in a variety of surgical and medical populations. UFH acts through several mechanisms, including the binding and activation of antithrombin (AT) with inactivation of various clotting factors. The standard dosing regimen is 5000 units administered subcutaneously three or two times daily. Low molecular-weight heparin (LMWH) has been available clinically for the past 2 to 3 decades; the two formulations available in the United States are enoxaparin (Lovenox) and dalteparin (Fragmin). LMWH exerts its effect predominantly through the inhibition of Factor Xa, and to a lesser degree through the inhibition of Factor IIa, via AT. LMWH has been associated with a lower incidence of heparin-induced thrombocytopenia (HIT) than with UFH,10 but should be used with caution and only at lower doses in patients with a creatinine clearance less than 30 mL/min or on dialysis. Enoxaparin is typically dosed at 40 mg subcutaneously once daily in nonorthopedic populations; dalteparin is dosed at 5000 units subcutaneously once daily. Fondaparinux (Arixtra) is a synthetic inhibitor of factor Xa which has been studied as a form of VTE prophylaxis in a number of surgical and medical patients. The dose used for prophylaxis is typically 2.5 mg subcutaneously once daily. While a variety of novel oral anticoagulants have recently been approved for either the prevention of embolic complications from atrial fibrillation or the prevention and treatment of VTE, none of these agents has been studied as a form of VTE prophylaxis in nonorthopedic surgical populations, so these agents should not be considered options for urologic patients. The two major mechanical prophylaxis strategies are intermittent pneumatic compression (IPC) devices and elastic stockings (ES). IPC sleeves are typically applied to the calves and require a power source to operate, though portable battery-operated devices have been developed over the past decade and may be associated with better outcomes than nonportable versions. ES come in both thigh-high and knee-high varieties. Primary evidence for VTE prophylaxis in urological surgery is fairly scant. Most trials have either been retrospective or nonrandomized. As a consequence, most guidelines extend their recommendations from other comparable surgical populations such as general surgery and gynecology. Although certain unique urologic populations (e.g., patients undergoing cystoscopic and transurethral procedures) may not be addressed properly using this approach, the paucity of primary urological data compels most guidelines to proceed in this fashion. Several guidelines exist to inform the approach for providing VTE prophylaxis in urological surgery patients. The most widely recognized VTE prophylaxis guidelines are the ACCP Guidelines, which published its ninth edition in 2012. The most extensive guidance for urologic patients in particular is provided by the American Urological Association (AUA) Best Practice Statement for the Prevention of Deep Vein Thrombosis in Patients Undergoing Urologic Surgery. Lastly, the National Clinical Guideline Centre in the UK published the NICE Guidelines on VTE Prophylaxis for all populations in 2010. The ACCP Guidelines made a number of new recommendations in their ninth edition that differed somewhat from their prior versions. In the eighth edition, published in 2008, the authors gave a number of grade 1 recommendations. For patients undergoing transurethral or other low-risk urological procedures, they recommended only early and frequent ambulation without routine use of thromboprophylaxis (grade 1A recommendation). For patients undergoing major, open urologic surgery, they gave a grade 1A recommendation for routine use of thromboprophylaxis. UFH (1B recommendation), ES and/or IPC (1B recommendation), LMWH (1C recommendation), fondaparinux (1C recommendation), and combination pharmacologic and mechanical prophylaxis (1C recommendation) were given fairly strong support. The authors also recommended that mechanical methods be used in patients with active bleeding or at very high risk of bleeding (1A recommendation), with pharmacologic prophylaxis either substituted or added when the bleeding risk subsided (1C recommendation). In the ninth edition, the ACCP authors recommended instead that a VTE risk assessment should be performed first to guide whether and what type of prophylaxis should be given. They advocated for two scoring models: the Rogers score derived from data from the Patient Safety in Surgery Study and an adaptation of the Caprini score. The Rogers score was developed retrospectively from a database based upon patients who underwent general and vascular surgery (the Patient Safety in Surgery Study). The risk scoring system is presented in Table 32-e1. Based on their derivation data set, the Rogers score authors recommended separating patients into three groups: low VTE risk (score less than 7), medium risk (score 7 to 10), and high risk (score greater than 10). They then validated this scoring system retrospectively in the other half of the database that they used. The risk of VTE found in each data set broken down by Rogers score is shown in Table 32-e2. Table 32-e2 Risk of VTE Stratified by Rogers Score The Caprini score is obtained by tallying the number of points based upon an inventory of VTE risk factors, which was modified by the ACCP to that shown in Table 32-e3. The ACCP Guidelines authors then advocated for classifying patients’ VTE risk as presented in Table 32-e4
Venous Thromboembolism in Urology Surgery
Risk of venous thromboembolism in urology
VTE prophylaxis methods
VTE prophylaxis strategies
Derivation VTE Rate
Validation VTE Rate
Low VTE risk (score < 7)
0.103%
0.110%
Medium VTE risk (score = 7-10)
0.436%
0.474%
High VTE risk (score > 10)
1.456%
1.315%
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree