CHAPTER 36 Vascular Lesions of the Gastrointestinal Tract
Through the widespread use of endoscopy and angiography, as well as advances in imaging techniques such as computed tomographic angiography (CTA) and magnetic resonance angiography (MRA), vascular lesions of the gastrointestinal (GI) tract are being increasingly characterized. Vascular lesions are a common cause of GI hemorrhage and may be solitary or multiple, benign or malignant, isolated or part of a syndrome or systemic disorder (Table 36-1). It is important at the outset to understand the nomenclature for the commonest lesions. Vas and its derivative vascular are Latin words meaning vessel; the Greek equivalent is angeion. Ectasia is a word of Greek derivation that refers to the process whereby a blood vessel becomes dilated or lengthened; the resulting lesion also can be referred to as an ectasia. Telangiectasia is the lesion resulting from dilation of the terminal aspect (tele) of a vessel. Angiodysplasia is used as a general term to describe the lesion or process whereby a badly formed (dys, “bad”; plasis, “molded”) vessel develops. An arteriovenous malformation is a congenital lesion, whereas an angioma is a neoplasm. This chapter discusses the more important vascular lesions that cause GI bleeding and that are representative of the spectrum of vascular lesions of the GI tract.
Table 36-1 Vascular Lesions of the Gastrointestinal Tract
| Primary Vascular Lesions |
| Diseases and Syndromes with Vascular Lesions |
| Systemic Disorders Associated with Vascular Lesions |
CREST, calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly, telangiectasia; GAVE, gastric antral vascular ectasia.
VASCULAR LESIONS
ANGIOECTASIA
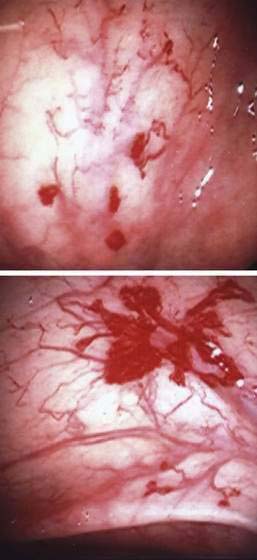
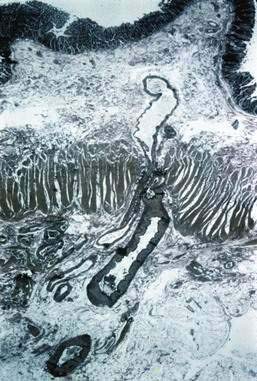
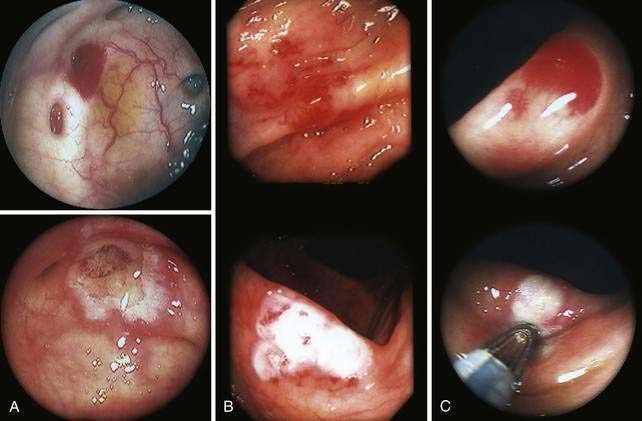
Angioectasia (AE) of the colon is a distinct clinical and pathologic entity.1–3 It is the most common vascular abnormality of the GI tract and probably the most frequent cause of recurrent or chronic lower intestinal bleeding in persons older than 60 years of age.4 AEs are probably acquired with aging, and there does not appear to be a gender predominance. In contrast to congenital or neoplastic vascular lesions of the GI tract, AEs are not associated with lesions of the skin or other viscera. However, when patients with vascular lesions of the colon are aggressively studied with angiography or enteroscopy, concomitant lesions may be seen in the small intestine in approximately 10% of patients.3,5,6 AEs almost always are confined to the cecum or ascending colon, usually are multiple rather than single, and usually are smaller than 10 mm in diameter. They are seldom identified by the surgeon at operation or by the pathologist using standard histologic techniques, but usually they can be diagnosed by angiography; colonoscopy (Figs. 36-1 and 36-2); or, as recently shown, helical CTA.7
The roles of computed tomography (CT) and magnetic resonance imaging (MRI) for vascular lesions of all types are evolving but are certain to increase as these sophisticated modes of diagnosis become more widely available; it is also clear that conventional angiography at present is more important for therapy than for diagnosis. To determine the precise nature of a vascular lesion, histologic examination, with or without injection studies of the vasculature, is necessary. In one report in which histologic confirmation of vascular lesions was not performed, AEs reportedly occurred distal to the hepatic flexure in 46% of patients8; review of tissue sections from supposed AEs in the small bowel or left colon revealed histologic changes different from those of AEs in the right colon (personal review by S. J. Boley and L. J. Brandt).
Bleeding from cecal AEs was first shown in 1961 by intraoperative angiography and has since become well recognized, especially after the introduction of selective angiography and colonoscopy for identifying the source of intestinal bleeding (see Chapter 19).8 In older literature, AEs and diverticulosis were considered the two most common causes of severe lower GI hemorrhage in older adults; however, more recent publications have cited AEs and diverticulosis to be responsible for 3% to 37% (mean: 10%) and 15% to 55% (mean: 30%) of major lower intestinal bleeding episodes, respectively (see Chapters 19 and 117).9 The problem of attributing bleeding to one or the other cause, when bleeding from the lesion is not demonstrated by endoscopy or by extravasation of contrast material on radiologic imaging studies, is compounded by the frequency and coexistence of these disorders without bleeding in people older than 60 years of age. The prevalence of diverticulosis is estimated to be as high as 50% in the population older than age 60; mucosal and submucosal AEs of the right colon can be found by injection studies of colons removed at surgery in more than 25% and 50%, respectively, of patients in this age range without evidence of bleeding.1,10 In large series of colonoscopic examinations, AEs have been seen in 0.2% to 2.9% of nonbleeding persons and 2.6% to 6.2% of patients evaluated specifically for occult blood in the stool, anemia, or hemorrhage.3,11–12 In a patient being studied for GI bleeding, in whom the site of active bleeding is unproven, the only basis for determining that an identified ectasia or diverticulosis is responsible for bleeding is the indirect evidence provided by the patient’s course after ablation or resection of the suspected lesion. It is unusual for AEs found incidentally to bleed, and an AE, even in a patient with a history of bleeding, cannot be assumed to be the cause.13
Bleeding from AEs typically is recurrent and low grade, although approximately 15% of patients present with massive hemorrhage. The nature and degree of bleeding frequently vary in the same patient with different episodes: Patients may have bright red blood, maroon stools, or melena on separate occasions. In 20% to 25% of episodes, only tarry stools are passed, and in 10% to 15% of patients, bleeding is evidenced solely by iron deficiency anemia, with stools that are intermittently positive for occult blood.4 This spectrum reflects the varied rate of bleeding from the ectatic capillaries, venules, and arteriovenous communications, depending on the developmental stage of the lesions (see later). In more than 90% of instances, bleeding stops spontaneously.
In 1958 E. C. Heyde described what is still a controversial association of AEs, GI bleeding, and aortic stenosis; aortic valve replacement had even been recommended for “Heyde’s syndrome” when bleeding could not be managed adequately. Numerous reports of Heyde’s syndrome appeared in the literature; subsequent analysis14 and many studies,15 however, failed to support the association. This association recently has been suggested again16 in a retrospective study in which the frequency of aortic stenosis was 31.7% in patients with “AVMs” compared with 14% in the general population; severe aortic stenosis was also more likely in the group with intestinal vascular lesions. The additional postulate has been offered that deficiencies of the largest forms of von Willebrand factor multimers (von Willebrand syndrome, type 2A) result in hemostatic abnormalities that may predispose preexisting AEs to bleed.17 Preoperative deficiency of these multimers reverses after aortic valve replacement,18 but the general recommendation to replace the aortic valve to control bleeding from AEs seems premature now that a variety of transendoscopic and angiographic means are available to ablate AEs.
Pathology
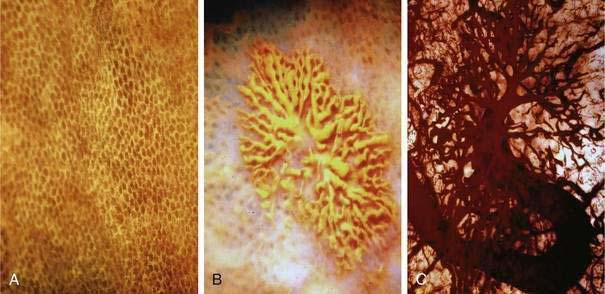
Histologic identification of AEs is difficult unless special techniques are used.1 Although usually less than one third of lesions are found by routine pathologic examination, almost all can be identified by injecting the colonic vasculature with silicone rubber, dehydrating the cells with increasing concentrations of ethyl alcohol, clearing the specimen by immersing it for 24 hours in a bath of methyl salicylate, and then viewing the specimen by dissecting stereomicroscopy (Fig. 36-3).1 In a study using these methods, surgically resected colons were analyzed and found to have one or more mucosal AEs measuring 1 mm to 1 cm in diameter. AEs were usually multiple, and in this study, all were located within the cecum and ascending colon; the most distal one was 23 cm beyond the ileocecal valve.1
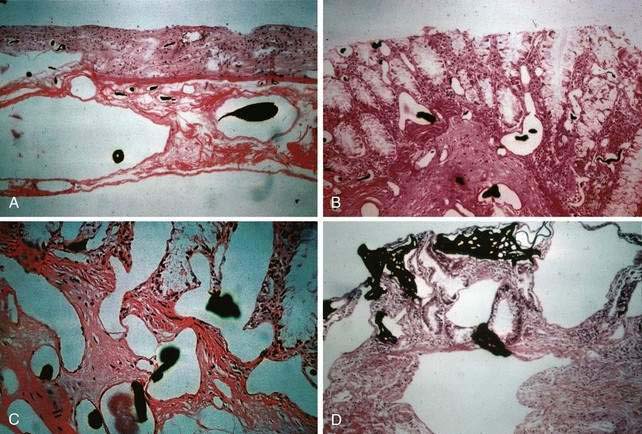
Microscopically, mucosal AEs consist of ectatic, distorted, thin-walled venules, capillaries, and arterioles, vessels that are lined by endothelium, and, infrequently, a small amount of smooth muscle. The earliest abnormality is the presence of dilated, tortuous, submucosal veins (Fig. 36-4A), often in areas where mucosal vessels appear normal. More extensive lesions show increasing numbers of dilated and deformed vessels traversing the muscularis mucosa and involving the mucosa (see Fig. 36-4B and C) until, in the most severe lesions, the mucosa is replaced by a maze of distorted, dilated vascular channels (see Fig. 36-4D). Enlarged arteries and thick-walled veins occasionally are seen in advanced lesions, in which the dilated arteriolar-capillary-venular unit has become a small arteriovenous fistula because of loss of prearteriolar sphincter function. Large thick-walled arteries are more typical of congenital arteriovenous malformations.
Pathogenesis
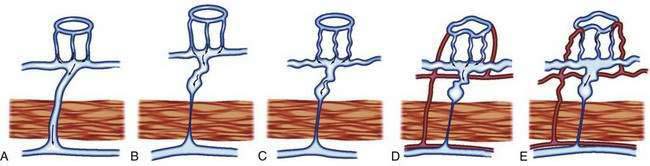
The previously described studies using injection and clearing techniques indicated that AEs are acquired lesions associated with aging and that they represent a unique clinical and pathologic entity.1 That AEs are common lesions associated with aging is supported by their frequent identification at colonoscopy in older adults and in injected colons resected from older patients with no history of bleeding.1,11 Boley postulated that the likely cause of AEs is partial, intermittent, low-grade obstruction of submucosal veins at the site where these vessels pierce the muscular layers of the colon1 (Figs. 36-5 and 36-6). He further suggested that repeated episodes of transiently elevated pressure during muscular contraction and distention of the cecum over many years conceivably result in dilation and tortuosity of the submucosal vein and, later, of the venules and capillaries of the mucosal units that drain into it. Finally, he postulated that the capillary rings dilate, the precapillary sphincters lose their competency, and a small arteriovenous fistula is produced. The latter is responsible for the “early-filling vein,” which was the original angiographic hallmark of this lesion (Fig. 36-7). Prolonged increased flow through the arteriovenous fistula can then produce alterations in the arteries supplying the area and in the extramural veins that drain it. This developmental concept of the cause of AEs was based on the finding of (1) a prominent submucosal vein, either in the absence of any mucosal lesion, or underlying only a minute mucosal AE supplied by a normal artery; (2) dilation of the veins, starting where they traverse the muscularis propria (see Fig. 36-5); and (3) previous studies showing that venous flow in the bowel may be diminished by increases in colon motility, intramural tension, and intraluminal pressure.19 Following this logic, the prevalence of AEs in the right colon can be attributed to the greater tension in the cecal wall compared with that in other parts of the colon, according to LaPlace’s principle: T ∝ pDP (where T is tension, D is diameter, and P is intraluminal pressure).
An alternative concept for the development of AEs is based on the demonstration that AEs have been shown to express vascular endothelial growth factor (VEGF) and its receptors along the endothelial lining in surgical specimens from patients who have undergone colectomy for recurrent bleeding20; this indicates a proliferative phase of angiogenesis. VEGF and VEGF receptor 1 have been shown to be up-regulated by hypoxia21 and therefore a role has been suggested for hypoxia in the pathogenesis of AEs. Further research still is needed to clarify the pathophysiology of AEs.
Diagnosis and Management
Management of bleeding AEs consists of three phases: (1) diagnosis; (2) conversion of an emergency situation to an elective one by control of acute bleeding; and (3) definitive treatment of the AE by colonoscopic ablation or surgical removal. The diagnostic approach to colonic AEs is essentially the same as that for lower intestinal bleeding in general and includes radionuclide bleeding scans; colonoscopy; angiography; and, to exclude the small intestine as a site of bleeding, push enteroscopy and wireless capsule endoscopy (WCE). WCE is a relatively new diagnostic technique that enables visualization of the entire small intestine, and rarely the very proximal colon where colonic AEs are found. WCE is particularly useful for evaluating patients with obscure and occult GI bleeding.22 WCE has been shown to be superior to push enteroscopy in the evaluation of patients with small intestinal bleeding, yielding a diagnosis in 55% to 75% of patients with obscure bleeding that required transfusions, most common of which was angiodysplasia.23,24 Radionuclide scans are used to determine whether a patient is actively bleeding and, if so, to localize the site (see Chapter 19). Although angiography previously had been the principal means of identifying AE as the source of bleeding, colonoscopy currently is the preferred method. Helical CTA is a relatively new, sensitive, specific, and well-tolerated technique to diagnose colonic AEs, although prospective studies comparing CTA with other imaging techniques are necessary.7
The endoscopist’s ability to diagnose the specific nature of a vascular lesion is limited by the similar appearance of different types of lesions. AEs, spider angiomas, hereditary hemorrhagic telangiectasia, angiomas, the focal hypervascularity of radiation colitis, ulcerative colitis, Crohn’s disease, ischemic colitis, certain infections (e.g., syphilis, Pneumocystis), hyperplastic and adenomatous polyps, and malignancies, including lymphoma and leukemic infiltrations, can all, on occasion, resemble each other (Table 36-2). Because traumatic and endoscopic suction artifacts may resemble vascular lesions, all lesions must be evaluated on insertion of the colonoscope, rather than during withdrawal. Pinch biopsy samples of vascular lesions obtained during endoscopy usually are nonspecific; therefore, the risk of performing biopsies of these abnormalities is not justified.
Table 36-2 Lesions That May Be Confused with Angioectasias on Endoscopy
| Vascular Lesions |
| Nonvascular Lesions |
| Neoplasms |
| Colitis |
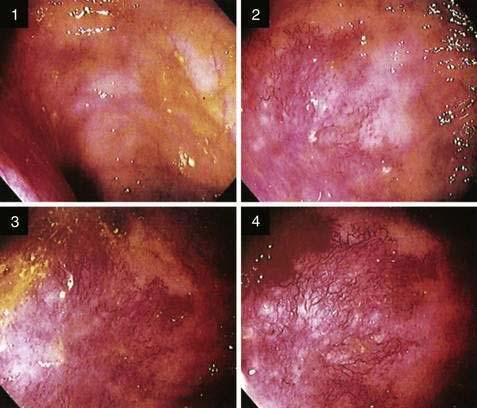
Because the appearance of vascular lesions is influenced by a patient’s blood pressure, blood volume, and state of hydration, such lesions may not be evident in those with severely reduced blood volumes or shock; thus accurate evaluation may not be possible until red cell and volume deficits are corrected. Meperidine also may diminish the prominence of some vascular abnormalities (e.g., AEs and the telangiectasias of hereditary hemorrhagic telangiectasia); use of meperidine, therefore, should be minimized and its effects reversed by naloxone so that vascular lesions can be detected accurately. Such a masking effect does not seem to occur with fentanyl. Naloxone has been shown to enhance the appearance of normal colonic vasculature in approximately 10% of patients and cause existing AEs to appear (2.7%) or increase in size (5.4%) (Fig. 36-8).25 For these reasons, naloxone is an important adjunctive medication for patients undergoing endoscopic evaluation for lower intestinal bleeding. Cold water lavage of the colon, as is sometimes done to cleanse the luminal surface of debris during colonoscopy, also may cause underlying AEs to disappear transiently.26
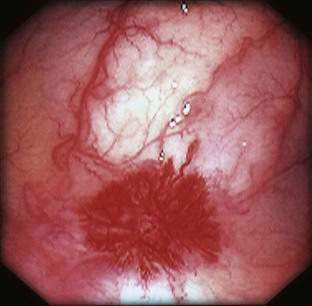
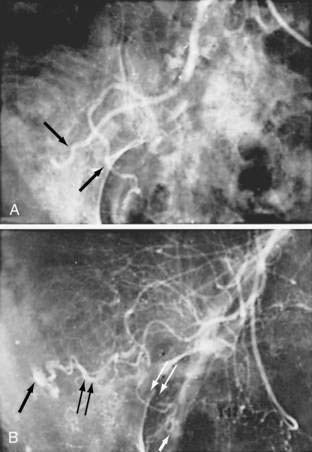
Angiography is used to determine the site and nature of lesions during active bleeding and can identify some vascular lesions even after bleeding has ceased. The three reliable angiographic signs of AEs are a densely opacified, slowly emptying, dilated, tortuous vein; a vascular tuft; and an early-filling vein (see Fig. 36-7).27 A fourth sign, extravasation of contrast material, identifies the site of bleeding when bleeding volume is at least 0.5 mL/minute but is not specific for AE. The slowly emptying vein (see Fig. 36-7A) persists late into the venous phase, after the other mesenteric veins have emptied. Vascular tufts (see Fig. 36-7B) are created by the ectatic venules that join the mucosal AE and the submucosal vein. They are seen best in the arterial phase; are usually located at the termination of a branch of the ileocolic artery; appear as small candelabra-like or oval clusters of vessels; and still are seen in the venous phase communicating with a dilated, tortuous, intramural vein. The early-filling vein is seen in the arterial phase within four or five seconds of injection (see Fig. 36-7B); it is not a valid sign of AE if vasodilators such as papaverine or tolazoline (Priscoline) have been used to enhance the study. When the lesion is bleeding, intraluminal extravasation of contrast material usually appears during the arterial phase of angiography and persists throughout the study. Extravasation identifies the site of active bleeding, but in the absence of other signs of AEs, it suggests another cause for the bleeding.
Management of incidental (nonbleeding) AEs detected by colonoscopy is expectant. The natural history of colonic AE is benign in healthy, asymptomatic people, and the risk of bleeding is small.13,28,29 In such cases, endoscopic therapy is not warranted.30
Bleeding can be controlled endoscopically or angiographically in most patients, thereby avoiding the morbidity and mortality of emergency operation. In decades past, intra-arterial embolization and vasopressin were used to control upper and lower GI bleeding, respectively. Vasopressin, given via an angiographic catheter placed into the feeding splanchnic vessel, arrested hemorrhage successfully from AE in more than 80% of patients in whom extravasation was demonstrated. Now, superselective microcoil embolization has largely replaced intra-arterial vasopressin infusion for the treatment of lower intestinal hemorrhage.31 Such embolization is highly effective and safe but complicated by ischemic events in approximately 5% of cases.32 Vasopressin still is recommended, however, when intestinal lesions are diffuse throughout the bowel or when superselective catheterization is not possible.32
Hormonal therapy, using estrogens in combination with progestins, has been used to treat patients with a variety of vascular lesions of the GI tract, in an attempt to reduce or terminate bleeding. The mechanisms by which such agents work are not known, although procoagulant effects and endothelial injury are popular theories. Although one long-term observational study33 showed that combination hormonal therapy stopped bleeding in patients with occult GI bleeding of obscure origin (likely to have resulted from small bowel angiodysplasia), current studies do not support the use of these agents to prevent rebleeding from GI angiodysplasia.34 It is likely that hormonal therapy affects different vascular lesions differently and that vascular lesions in the small intestine may respond differently to such treatment than the same lesions in the colon; no study of hormonal therapy has been done for known colonic AEs.
A novel therapy for AEs, and perhaps other vascular lesions in the gastrointestinal tract, is the use of antiangiogenic factors. Thalidomide was developed in the 1950s as a sedative, sleeping pill, and antiemetic for pregnant women, but it soon became notorious for causing phocomelia and other malformations in the newborn.34 In 1994, D’Amato and colleagues reported that thalidomide inhibited VEGF and basic fibroblast growth factor-mediated angiogenesis, which led to further characterization and subsequent clinical applications of its antiangiogenic activity.35,36 Recent data suggest the mechanism for its antiangiogenic effect is related to reduced expression of integrin genes and resulting decreased cell-cell surface interactions and response to angiogenic cytokines.37 Several case reports and case series have described the successful use of thalidomide to treat life-threatening or refractory bleeding from intestinal AEs and Crohn’s disease with refractory bleeding.38–42 After treatment with thalidomide for three months, substantial reductions in the number, size, and color intensity of AEs were observed by WCE.39
Of the available antiangiogenic biologic therapies, most information regarding clinical efficacy and toxicity is available for bevacizumab (Avastatin), a humanized monoclonal antibody against VEGF that is effective against colon and renal cancers and that also has a strong antiangiogenic activity.43 Curiously, dose-dependent nasal and GI bleeding is observed in up to 59% of patients during treatment, possibly caused by a loss of vascular integrity as a result of bevacizumab-induced endothelial-cell shedding in highly regenerative mucosal tissues with active angiogenesis. It is unclear why some antiangiogenic substances like bevacizumab cause mucosal bleeding and others like thalidomide do not; this disparity effect may be related to the phase of angiogenesis that is antagonized, or might reflect a particular strong antiangiogenic activity. Although VEGF-based antiangiogenic therapy is a promising therapy, the issue of aggravation of bleeding from vascular lesions needs further study. A more detailed understanding of the angiogenic cascade and how antiangiogenic substances act within it will be needed to resolve this issue.
Neodymium:yttrium-aluminum-garnet (Nd:YAG) laser3,6,44,45; endoscopic sclerosis11; monopolar46 and bipolar47 electrocoagulation; heater probe47; and, recently, hemoclips in combination with cautery,48 endoscopic band ligation,49 and argon plasma coagulation (APC)50 have been used to ablate vascular lesions throughout the GI tract and can be used to control active bleeding (Fig. 36-9). Control of bleeding has been obtained with a variety of endoscopic thermal means in 47% to 88% of cases,3 and no technique has been established as superior to the next.11 Severe delayed bleeding occurs in 5% of patients with colonic AEs after thermal therapy.46 Recurrent bleeding from colonic AEs appears to be reduced after these therapies, but more than one treatment session is usually necessary.47 Rebleeding can be expected to increase with time after the procedure and has been seen in 28% to 52% of patients over a follow-up period ranging from 15 to 36 months.3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree