CHAPTER 101 Maldigestion and Malabsorption
Malabsorption can be caused by many diseases of the small intestine and also by diseases of the pancreas, liver, biliary tract, and stomach (Table 101-1). Whereas in some of these diseases, malabsorption may be the presenting feature, in others malabsorption may be only a minor clinical problem or may be detected only as a laboratory abnormality.
Table 101-1 Diseases That Cause Nutrient Malabsorption
| Gastric Diseases |
| Pancreatic Diseases |
| Liver Diseases |
| Obstructive Biliary Diseases |
| Intestinal Diseases |
| Lymphatic Diseases |
| Neuroendocrine Tumors |
| Cardiac and Vascular Diseases |
| Endocrine Causes |
| Systemic Diseases |
AIDS, acquired immunodeficiency syndrome; HIV, human immunodeficiency virus.
ETIOLOGY AND PATHOPHYSIOLOGY
Normal uptake of nutrients, vitamins, and minerals by the gastrointestinal tract requires several steps, each of which can be compromised in disease. (Normal digestion and absorption are discussed in Chapter 100.)
Digestion of macromolecular compounds, such as polysaccharides, triglycerides, and proteins, to their molecular components—monosaccharides, fatty acids, and amino acids, respectively—is achieved by soluble or membrane-bound digestive enzymes. Absorption of undigested or partially digested macromolecular compounds occurs to a very minor degree in health and may be increased slightly in various intestinal diseases. Although such absorption does not play a nutritive role, it may be important for the normal function of the immune system and for the pathogenesis of diseases such as food allergy (see Chapter 9).
Chemical changes to nutrients may be required for absorption, such as changing the charge of iron.
Intestinal sensory and motor function permits detection of the presence of nutrients, facilitates adequate mixing of nutrients with intestinal secretions and delivery to absorptive sites, and provides adequate time for nutrient absorption (see Chapter 97).
An overview of pathophysiologic mechanisms of maldigestion and malabsorption is provided in Table 101-2. This table also shows the ingested substrates primarily affected by the individual pathophysiologic mechanisms and lists examples of etiologic disorders for these mechanisms.
Table 101-2 Mechanisms of Malabsorption, Malabsorbed Substrates, and Representative Causes
| MECHANISM | MALABSORBED SUBSTRATE(S) | REPRESENTATIVE CAUSES |
|---|---|---|
| Maldigestion | ||
| Conjugated bile acid deficiency | ||
| Pancreatic insufficiency | ||
| Reduced mucosal digestion | ||
| Intraluminal consumption of nutrients | Vitamin B12 (cobalamin) | |
| Malabsorption | ||
| Reduced mucosal absorption | ||
| Decreased transport from the intestine | ||
| Other Mechanisms | ||
| Decreased gastric acid and/or intrinsic factor secretion | Vitamin B12 | |
| Decreased gastric mixing and/or rapid gastric emptying | ||
| Rapid intestinal transit | Fat | |
CCK, cholecystokinin.
FATS
DEFECTIVE MIXING
For sufficient digestion and absorption of lipids, dietary fat must adequately mix with digestive secretions. Gastric resections or gastrointestinal motility disorders that result in rapid gastric emptying or rapid intestinal transit, such as autonomic neuropathy resulting from diabetes mellitus or amyloidosis, can cause fat malabsorption consequent to impaired gastrointestinal mixing of dietary fat.1
REDUCED SOLUBILIZATION OF FAT
Fat malabsorption due to decreased formation of micelles occurs if the luminal concentrations of conjugated bile acids are lower than the critical concentration required for forming micelles.2,3 Table 101-31,4 details the pathophysiologic mechanisms and representative diseases that cause luminal bile acid deficiency.
Table 101-3 Pathophysiologic Mechanisms That Result in Deficiency of Luminal Conjugated Bile Acids
| PATHOPHYSIOLOGIC MECHANISM | CAUSES |
|---|---|
| Decreased synthesis and/or secretion of conjugated bile acids | |
| Intestinal loss of conjugated bile acids | |
| Luminal deconjugation of bile acids | Small intestinal bacterial overgrowth |
| Binding of bile salts or insolubilization of bile salts due to low luminal pH |
CCK, cholecystokinin.
DECREASED LIPOLYSIS
If exocrine pancreatic function is severely reduced, impairment of pancreatic lipase and colipase secretion results in decreased luminal hydrolysis of dietary fat.5 Chronic pancreatitis, cystic fibrosis, pancreatic duct obstruction by pancreatic and ampullary tumors, and pancreatic resection are the most common causes of pancreatic insufficiency.1 Even when pancreatic enzyme concentrations are normal, reduced pancreatic lipase activity due to a low luminal pH,6 excessive calcium ingestion,7 or ingestion of the specific lipase inhibitor orlistat8 can cause pancreatic steatorrhea. Selective congenital lipase or colipase deficiency is a rare cause of pancreatic fat malabsorption.9
DECREASED MUCOSAL ABSORPTION AND CHYLOMICRON FORMATION
Generalized mucosal diseases, such as celiac disease or tropical sprue, often are associated with fat malabsorption. Defective uptake of free fatty acids and monoglycerides results from reduced mucosal surface area because of villus shortening, reduced enterocyte function, and mucosal inflammation.1 Intestinal fat absorption also is impaired in diseases that result in disturbance of intracellular formation of chylomicrons and accumulation of lipids within the enterocytes, including abetalipoproteinemia, hypobetalipoproteinemia, and chylomicron retention disease.10
DEFECTIVE LYMPHATIC TRANSPORT OF CHYLOMICRONS
Impairment of lymphatic transport of chylomicrons is a cause for postmucosal malabsorption of dietary fat. Decreased lymphatic transport can result from congenital diseases such as primary intestinal lymphangiectasia or from obstruction of lymphatic vessels due to metastatic solid tumors, lymphoma, Whipple’s disease, retroperitoneal fibrosis, or trauma6 (see Chapter 28). Usually, lymphatic vessels in the mucosa become dilated (lymphangiectasia), and chylomicrons are lost into the intestinal lumen postprandially and also in the fasting state11; steatorrhea in these situations usually is only mild to moderate.10
PROTEINS AND AMINO ACIDS
Defective digestion or absorption of dietary proteins has to be differentiated from excessive loss of serum proteins into the gastrointestinal tract, which is termed protein-losing enteropathy (see Chapter 28).
DEFECTIVE INTRALUMINAL PROTEOLYSIS
Protein digestion may be impaired in patients who have undergone partial or total gastric resection, presumably as a result of poor mixing with digestive secretions, although gastric pepsin deficiency could be contributory. Defective proteolysis also occurs with exocrine pancreatic insufficiency.1,12,13 In congenital diseases, pancreatic proteolysis can be impaired by inborn errors in the synthesis of proteolytic enzymes (trypsinogen deficiency)13 or by defective activation of pancreatic proenzymes resulting from congenital deficiency of intestinal enterokinase (see later).14
DEFECTIVE MUCOSAL HYDROLYSIS OF PEPTIDES AND DECREASED ABSORPTION OF OLIGOPEPTIDES AND AMINO ACIDS
Generalized mucosal diseases, such as celiac disease and tropical sprue, result in global malabsorption, which includes malabsorption of oligopeptides and amino acids due to lack of mucosal hydrolysis of oligopeptides and defective mucosal absorption.13 Reduction of intestinal absorptive surface, as in short bowel syndrome or jejunoileal bypass, also results in protein and amino acid malabsorption.13,15 Congenital defects of amino acid transporters on the enterocytes, such as Hartnup’s disease and lysinuric protein intolerance, can lead to selective malabsorption of a subgroup of amino acids (see later on).
CARBOHYDRATES
DEFECTIVE INTRALUMINAL HYDROLYSIS OF CARBOHYDRATES
Pancreatic α-amylase normally is secreted in excess into the intestinal lumen. In mild forms of pancreatic insufficiency, carbohydrate digestion usually is at least partially preserved,16 but severe pancreatic insufficiency results in clinically apparent carbohydrate malabsorption and diarrhea due to decreased luminal hydrolysis of ingested starch.17
MUCOSAL DEFECTS OF CARBOHYDRATE DIGESTION AND ABSORPTION
The most common cause of carbohydrate malabsorption is late-onset lactose malabsorption due to decreased levels of the intestinal brush border enzyme lactase (adult-type hypolactasia, acquired primary lactase deficiency). Depending on ethnic background, lactase is present in less than 5% to more than 90% of the adult population; its deficiency results in a selective malabsorption of lactose. Acquired malabsorption of carbohydrates occurs commonly after extensive intestinal resections, in diffuse mucosal diseases such as celiac disease or Crohn’s disease, or temporarily after self-limited gastrointestinal infections (postinfection carbohydrate malabsorption).16,17 The pathophysiologic mechanisms of carbohydrate malabsorption are reduction of the intestinal mucosal surface area and a reduced activity or expression of intestinal oligo- and disaccharidases or transport proteins for monosaccharides.16 Congenital disaccharidase deficiencies (lactase, sucrase-isomaltase, and trehalase)18 and congenital deficiency or malfunction of transport molecules as in congenital glucose-galactose malabsorption19 can cause early onset of malabsorption of mono- or disaccharides (see later on). Intolerance of fructose is discussed in a subsequent section.
VITAMINS
FAT-SOLUBLE VITAMINS
Diseases causing malabsorption of dietary fat commonly cause malabsorption of fat-soluble vitamins, because they require similar absorptive mechanisms. This is especially important in diseases that result in impaired micelle formation from bile salt deficiency.20 Fat-soluble vitamins also are malabsorbed in diffuse diseases of the mucosal surface area, in diseases affecting chylomicron formation and transport,21 and in exocrine pancreatic insufficiency.22 Some authors have suggested that absorption of fat-soluble vitamins is less affected by exocrine pancreatic insufficiency than by small intestinal diseases resulting in steatorrhea.23
WATER-SOLUBLE VITAMINS
Vitamin B12 (Cobalamin)
Decreased release of dietary vitamin B12 from food sources because of impaired pepsin and acid secretion, as in atrophic gastritis24 or use of acid inhibitory drugs such as proton pump inhibitors,25 usually results in only mild cobalamin malabsorption without clinical consequences. By contrast, deficiency of gastric intrinsic factor secretion, as occurs in pernicious anemia or after gastric resections, or secretion of an abnormal intrinsic factor, as in some congenital diseases, results in severe vitamin B12 malabsorption with clinical consequences.24
Autoimmune gastritis of pernicious anemia is the most common cause of vitamin B12 malabsorption.26 Cobalamin malabsorption in pernicious anemia is caused by decreased intrinsic factor secretion resulting from parietal cell destruction and by blocking autoantibodies that inhibit intrinsic factor binding to vitamin B12.26 Mild cobalamin malabsorption may be found in patients with pancreatic insufficiency and in patients with Zollinger-Ellison syndrome, owing to decreased proteolytic release of vitamin B12 from its complex with R-binding protein24,27 (see Chapters 32, 59 and 100).
In bacterial overgrowth syndrome (see Chapter 102) or helminthic infection with Diphyllobothrium latum (see Chapter 110), dietary cobalamin is made unavailable to the host or is consumed by the microorganisms or parasites in the intestinal lumen and, therefore, is not available for intestinal absorption.26
Diseases and conditions affecting the ileal mucosa, such as Crohn’s disease or ileal resection, lead to a reduction of specific absorptive sites for the intrinsic factor-vitamin B12 complex.24 Ileal resections of more than 60 cm usually result in clinically significant vitamin B12 malabsorption.28 Imerslund-Gräsbeck syndrome, a disease of autosomal recessive inheritance due to malfunction of the cubilin-amnionless (AMN) complex, is characterized by selective ileal malabsorption of the intrinsic factor–vitamin B12 complex despite normal ileal morphology.24,29 Congenital diseases affecting transcobalamin II also result in malabsorption of cobalamin.24,30
Folate
Folate malabsorption occurs with mucosal diseases affecting the proximal small intestine, such as celiac disease, Whipple’s disease, and tropical sprue.31 Folate deficiency is common in chronic alcoholism, in which it is postulated to be caused by decreased dietary intake as well as decreased intestinal absorption of folate.32 As discussed later, several drugs result in impaired intestinal uptake of folate, and an inherited form of selective folate malabsorption has been described. In contrast with cobalamin, body stores of folate are small relative to the daily requirements; therefore, folate deficiency develops faster than cobalamin deficiency in the setting of malabsorption. Increased serum folate levels resulting from bacterial formation of tetrahydrofolate have been reported in small intestinal bacterial overgrowth states.33
Other Water-Soluble Vitamins
Other water-soluble vitamins, such as ascorbic acid and the B-complex vitamins, are absorbed in the small intestine either by carrier-mediated transport or by passive diffusion. Generalized malabsorption syndromes from intestinal causes impair the absorption of these vitamins, thereby leading to deficiency states.34,35 Deficiency of these water-soluble vitamins also occurs in chronic alcoholism, probably owing to decreased oral intake and reduced intestinal absorption.32
MINERALS
CALCIUM
Severe calcium malabsorption can occur in diseases that affect the small intestinal mucosa, such as celiac disease. In these disease states, calcium absorption is impaired directly because of the reduction of the intestinal surface area and indirectly because of formation of insoluble calcium soaps with malabsorbed long-chain fatty acids. Therefore, diseases causing malabsorption of long-chain fatty acids by other mechanisms, such as bile acid deficiency, also can result in calcium malabsorption.21 In many of these diseases, malabsorption and deficiency of vitamin D further contribute to intestinal calcium malabsorption.21 Selective intestinal malabsorption of calcium—that is, without fat malabsorption—can occur in renal disease, hypoparathyroidism, and inborn defects in formation of 1α,25-dihydroxyvitamin D or in the intestinal vitamin D receptor.21 Calcium malabsorption also occurs commonly after gastric resection (see subsequent section, “Malabsorption after Gastric Resection”).
MAGNESIUM
In many generalized malabsorptive disorders, magnesium malabsorption can result in magnesium deficiency.36 Malabsorption is due to the reduction in mucosal absorptive surface area and to luminal binding of magnesium by malabsorbed fatty acids; a congenital form of selective intestinal magnesium malabsorption also has been reported.37
IRON
Iron deficiency is common in patients with gastric resection or with celiac disease. Reduction in the mucosal surface area of the small intestine as a result of diffuse mucosal disease, intestinal resection, or intestinal bypass also can result in impaired iron absorption, potentially leading to iron deficiency38; a congenital form of iron malabsorption also has been described (see Table 101-14).39 Intestinal loss of iron from chronic gastrointestinal bleeding is, however, the most common gastrointestinal cause of iron deficiency.40 Worldwide, hookworm infection is a common cause of iron deficiency.
ZINC
Zinc, like other minerals, is malabsorbed in generalized mucosal diseases of the small intestine.41 A congenital selective defect of zinc absorption, acrodermatitis enteropathica, is caused by a defect in the zinc transport protein hZIP4.42
OTHERS
Generalized malabsorption can cause deficiency of copper and selenium.43,44 In Menkes disease (kinky hair disease), an inherited disorder of cellular copper transport, selective intestinal copper malabsorption results (see later on). It is uncertain whether malabsorptive diseases result in deficiencies of chromium and manganese.41
MECHANISMS THAT COMPENSATE FOR MALABSORPTION
ROLE OF THE COLON
The colon has the capacity to absorb a limited number but a wide variety of substances and nutrients including sodium, chloride, water, oxalate, short chain fatty acids, calcium, and vitamin K. Although colonic nutrient absorption does not play a major role in health, the nutritive role of the colon in patients with severe malabsorption is clinically relevant.45 Colonic preservation of malabsorbed nutrients also can result in symptoms and complications of malabsorption,46 such as colonic hyperabsorption of oxalate, which contributes to formation of renal stones (see later on).
Colonic Salvage of Incompletely Absorbed Carbohydrates
In healthy people, between 2% and 20% of ingested starch escapes absorption in the small intestine47; pancreatic insufficiency or severe intestinal disorders further increase this amount.17 Carbohydrates that reach the colon cannot be absorbed by the colonic mucosa, but they can be metabolized by the colonic bacterial flora. Metabolism by anaerobic bacteria results in the breakdown of oligosaccharides and polysaccharides to mono- and disaccharides, which are metabolized further to lactic acid; short-chain (C2 to C4) fatty acids (SCFAs) such as acetate, propionate, and butyrate; and to odorless gases, including hydrogen, methane, and carbon dioxide.48
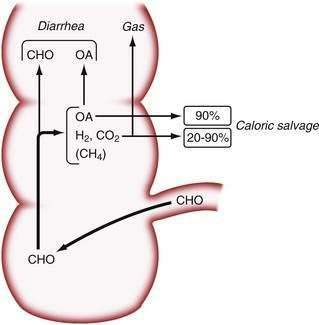
Studies in normal subjects have suggested that the bacterial metabolism of starch to small carbohydrate moieties is a rapid process in the normal colon. The rate-limiting step in the overall conversion of polysaccharides to SCFAs appears to be the conversion of monosaccharides to SCFAs.17 Colonic absorption of SCFAs results in a reduction of the osmotic load and, as a result, in mitigation of osmotic diarrhea.49 In normal subjects, more than 45 g of carbohydrates must reach the colon to cause diarrhea, and up to 80 g of carbohydrates per day can be metabolized by bacteria to SCFAs; approximately 90% of these SCFAs are absorbed by colonic mucosa50 (Fig. 101-1). Chronic carbohydrate malabsorption causes adaptive changes in bacterial metabolic activity that result in an even higher efficiency of the bacterial flora to digest carbohydrates,51 although at the expense of increased flatus production (see later).
Because SCFAs have caloric values between 3.4 and 5.95 kcal/g,52 their colonic absorption can contribute positively to overall calorie balance. In patients with short bowel syndrome, colonic salvage of malabsorbed carbohydrates can save up to 700 to 950 kcal/day, provided that a substantial part of the colon remains in continuity with the small intestine.53 Not all SCFAs are absorbed by the colon, and those that are not absorbed contribute to osmotic diarrhea.
The beneficial effects of colonic bacterial carbohydrate metabolism may be accompanied by side effects due to gas production (see Chapter 16). Up to 10-fold differences in the volume of gas produced in the colon have been observed in normal persons.54 The colon also can absorb gas. If intracolonic gas volumes are low, up to 90% of the volume of intracolonic gas can be absorbed; if gas volumes are high, however, this proportion can decrease to 20%54 (Fig. 101-2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree