Vascular Diseases: Hemorrhage, Mesenteric Occlusive and Nonocclusive Disease, Ischemia, Radiation Enteritis, and Volvulus
Jonathan E. Efron
The only weapon with which the unconscious patient can immediately retaliate on the incompetent surgeon is hemorrhage.
—WILLIAM STEWART HALSTED
▶ HEMORRHAGE
Gastrointestinal (GI) bleeding can be due to numerous causes. It is self-evident that conditions such as colorectal cancer, inflammatory bowel disease, hemorrhoids, infectious colitides, ischemia, radiation, Meckel’s diverticulum, and virtually every disease that affects the mucosa of the intestinal tract can be associated, at some time, with bleeding.215,279 Lower GI tract hemorrhage as a consequence of renal transplantation, presumably due to immunosuppression, has also been reported.347 Uncommon conditions that can produce massive bleeding are coagulopathy, Osler-Weber-Rendu telangiectasia, Dieulafoy’s disease, blue rubber bleb nevus syndrome, Behçet’s disease, aortoduodenal fistula, rupture of a splenic artery aneurysm, microaneurysm of the superior hemorrhoidal artery, rupture of a pancreatic pseudocyst into the colon, and angiosarcoma.30,56,77,197,202,219,279,308 A rare cause of lower GI hemorrhage is variceal bleeding. This may be due to a congenital vascular abnormality, portal hypertension, obstruction of mesenteric venous circulation, splenic vein thrombosis, or a cardiac anomaly.175,382 Those with AIDS can present with GI tract hemorrhage from a number of causes: colitis from cytomegalovirus, herpes simplex, or bacteria; lymphoma; idiopathic proctocolitis; and Kaposi’s sarcoma.77 For our purposes, five specific conditions will be discussed in this section: diverticulosis, angiodysplasia, colorectal varices, Dieulafoy’s lesion, and Meckel’s diverticulum. Regardless of the cause
of bleeding, a systematic approach to diagnosis and management is required to care for the patient adequately. This includes initial workup and stabilization of the patient, followed by diagnostic studies to identify the source of bleeding, and finally intervention to stop the bleeding. Colonoscopy and angiography are used for both localization and intervention.
of bleeding, a systematic approach to diagnosis and management is required to care for the patient adequately. This includes initial workup and stabilization of the patient, followed by diagnostic studies to identify the source of bleeding, and finally intervention to stop the bleeding. Colonoscopy and angiography are used for both localization and intervention.
Initial Presentation
Patients who present with lower GI hemorrhage must be evaluated in a timely fashion and are approached much as one would approach a trauma patient at risk for hemorrhage. Initial assessment includes establishing that the patient is hemodynamically stable. However, whether someone is stable or not, the team first establishing contact should ensure there is adequate intravenous (IV) access with two large bore IV catheters as well as obtaining blood for routine analysis and for a type and crossmatch. Blood products must be available in cases of significant bleeding and/or hemodynamic instability. If the patient is unstable, rapid interventions are required, including massive resuscitation and transfusions. Usually, however, the individual’s condition is relatively stable, thus permitting time for evaluation.
Strate and colleagues performed a risk analysis of multiple factors that were thought to be predictive of severe bleeding.344 Severe bleeding was defined as persistent bleeding during the first 24 hours of admission requiring transfusion of two or more units of packed red blood cells or a decrease in the hematocrit of 20% or more. Also included were patients who rebled after 24 hours of admission and who required transfusion at that time, had a further drop of hematocrit of 20% or more, or those readmitted one week after discharge with a lower GI bleed. The following factors were found to correlate with severe lower GI bleeding: a pulse rate greater than 100 beats per minute on admission, a systolic blood pressure less than 155 mm Hg, the presence of syncope, nontender abdominal examination, bleeding per rectum during the first 4 hours of evaluation, the use of aspirin, and two or more active comorbid conditions.344 The authors then created a validated scale, assigning each of these risk factors a unit of one. Low-risk patients were those with no risk factors and were assigned a score of 0, moderate risk patients had a score between 1 and 3, and high-risk patients were those with greater than three factors (i.e., having a score of 4 or more). Those with higher scores have been shown to have a greater risk of requiring blood transfusions, needing surgery, recurrent hemorrhage, or mortality.345
Other clinical outcomes have been used in order to examine the results following lower GI bleeding episodes. Das and coworkers created an artificial neural network and multiple logistic regression models to predict poor outcomes in patients experiencing a lower GI hemorrhage.96 They defined poor outcome as those requiring therapeutic intervention, experiencing rebleeding, or death. Hemodynamic instability, persistent bleeding, and the existence of comorbidities were found to be persistent predictors of poor outcomes.96
Strate and Naumann propose the following risk factors for poor outcome in lower intestinal bleeding343:
Hemodynamic instability (hypotension, tachycardia, orthostasis, syncope)
Ongoing bleeding (blood at presentation or within 4 hours of presentation)
Older age
Comorbid illness
Bleeding while hospitalized for another process
Anticoagulation or antiplatelet medication
History of diverticular disease or angiogenesis
Nursing home resident
Nontender abdominal exam
Hematocrit less than 35%
Abnormal creatinine
Abnormal white blood cell count
Evaluation of Hemorrhage
The importance of obtaining an accurate history cannot be overemphasized. For example, knowledge of prior abdominal aorta surgery may be critical (Figure 28-1). However, patients usually present with no antecedent history and they frequently have no abdominal pain. Blood from the rectum
may be bright red or maroon and may contain clots. In addition to a detailed medical and surgical history, identifying risk factors for bleeding is essential in the history, such as the patient’s use of anticoagulants or antiplatelet drugs. Inflammatory conditions or other vasculitides are important to determine. One must also be wary and cognizant of an individual’s consumption of herbal agents because many of these products have anticoagulant properties.
may be bright red or maroon and may contain clots. In addition to a detailed medical and surgical history, identifying risk factors for bleeding is essential in the history, such as the patient’s use of anticoagulants or antiplatelet drugs. Inflammatory conditions or other vasculitides are important to determine. One must also be wary and cognizant of an individual’s consumption of herbal agents because many of these products have anticoagulant properties.
Physical examination of the bleeding patient is usually unrewarding. Even before one can begin investigations, the opportunity for identifying the source of bleeding may be lost because spontaneous cessation is not uncommon. The therapeutic effect of the administration of an enema before endoscopic examination or of a barium enema study is well recognized,4 but this may be simply coincidental. McGuire undertook a study to ascertain the course of bleeding in 78 individuals who were admitted for a total of 106 times for this complaint, with no specific cause other than colonic diverticula. Bleeding stopped spontaneously in 75% of episodes and in 99% of patients requiring fewer than four units per day of transfusion.
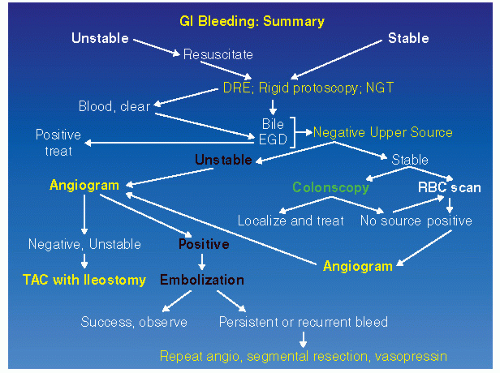
One should, obviously, perform a digital rectal examination and a limited rigid sigmoidoscopy with anoscopy as the initial examination. However, the yield from these diagnostic procedures is less than 10%.12,86,292 Still, if the source is found, appropriate therapy can be implemented. The treating physician must remember that bleeding suggestive of a lower GI source may actually be originating from the upper GI tract. The simple expediency of the placement of a nasogastric tube can eliminate the stomach as a potential source, especially if clear bile is returned. If the aspirate is positive, one should then proceed with upper GI endoscopy. A recent study by Laine and Shah demonstrated that 15% of patients who present with significant hematochezia had an upper GI source identified on endoscopy.194 An organized approach to the evaluation of the patient with hemorrhage of presumed lower GI origin is suggested. An algorithm is presented in Figure 28-2, which provides an overview for carrying out the proper sequence of investigations in the bleeding patient. The approach to the bleeding patient—after initial assessment is complete and the patient is believed to have a lower GI source for bleeding—is to proceed with colonoscopic or radiologic investigation.
Colonoscopy
Colonoscopy has been identified by some to be the firstline approach to diagnosing and intervening in lower GI bleeding.111,405 It identifies a definitive source of bleeding 40% to 90% of the time,19,144,181,336,346 and a generalized diagnosis for the bleeding 90% to 100% of the time. Zuckerman and Prakash performed an extensive meta-analysis of 13 studies examining colonoscopy in lower GI bleeding.406 They found a 1.3% complication rate in the 1,561 patients analyzed, thus indicating the procedure can be safely completed in the bleeding patient. Complications included congestive heart failure and worsening of the bleeding, but the rate of perforation was very low (0.3%). These low complication rates, along with high reported yields, make colonoscopy an enticing diagnostic and treatment modality for lower GI bleeding.
There are, however, certain prerequisites for colonoscopy performed during an active lower GI bleed, which include hemodynamic stability and the ability to perform a rapid bowel preparation on the patient. The oral preparation is performed over 4 to 5 hours and is often facilitated by placing a nasogastric tube in the patient. Polyethylene glycol (PEG) solution is delivered via the nasogastric tube over the next 4 hours. Rapid, high-volume preparations may lead to electrolyte abnormalities as well as significant fluid shifts that result in possible congestive heart failure. Aspiration pneumonia is another concern with a rapid prep through a nasogastric tube. Although these risks are rare, those patients undergoing rapid preparation for colonoscopy for suspected lower GI bleeding should receive the prep in a monitored setting in order to prevent or to recognize these potentially fatal complications. Adequate bowel preparation is essential for completing the colonoscopy. Ohyama and associates compared their early results for urgent colonoscopy when oral preparation was not performed with those after they initiated preparation with PEG solution and found significant improvement in their ability to complete the colonoscopy.258 Others have confirmed these results, with completion rates ranging from 55% to 70% in those with unprepped colons.81,352 Currently, if colonoscopy is to be attempted for diagnosis and managing lower GI bleeding, oral preparation is required.
The colonoscopy should be performed 1 to 2 hours after the preparation is complete, although controversy exists as
to the precise timing of colonoscopy for a suspected lower GI bleed. Jensen and colleagues found that colonoscopy performed 6 to 12 hours after admission resulted in significantly lower rebleed or surgery rates.346 Successful endoscopic intervention was seen in 29% of patients if instrumented within 12 hours of admission. This falls to 13% if one waits between 12 and 24 hours and drops to 4% if one waits longer. Predictors of rebleeding included active bleeding, a visible vessel, and adherent clot.181 The rebleeding rate in this study after intervention was 0%, and as one would expect this rate has been difficult to reproduce. Overall early rebleeding ranges from 0% to 24%, depending on the therapy used to terminate the bleeding, and late rebleeding rates that range from 0% to 17% have been documented.343
to the precise timing of colonoscopy for a suspected lower GI bleed. Jensen and colleagues found that colonoscopy performed 6 to 12 hours after admission resulted in significantly lower rebleed or surgery rates.346 Successful endoscopic intervention was seen in 29% of patients if instrumented within 12 hours of admission. This falls to 13% if one waits between 12 and 24 hours and drops to 4% if one waits longer. Predictors of rebleeding included active bleeding, a visible vessel, and adherent clot.181 The rebleeding rate in this study after intervention was 0%, and as one would expect this rate has been difficult to reproduce. Overall early rebleeding ranges from 0% to 24%, depending on the therapy used to terminate the bleeding, and late rebleeding rates that range from 0% to 17% have been documented.343
One of the key benefits of colonoscopy is that, in addition to being a diagnostic test, it is a therapeutic technique, if there is a skilled endoscopist available to stop active bleeding. Methods of controlling hemorrhage include injection, catheterization, banding, and clipping. The method used for hemostasis is often dependent on the comfort level of the endoscopist and the nature of the specific lesion that is bleeding. As mentioned previously, the overall complication rate, including perforation, is very low with colonoscopy for lower GI bleeding, so when a bleeding source is identified on endoscopy, every attempt should be made to control hemorrhage. If rebleeding occurs after an initially successful intervention, repeat endoscopy with intervention should be initiated. When attempting an intervention, having the patient in a well-controlled setting with adequate equipment is vital. If the patient is actively bleeding, having an anesthesia team to monitor and resuscitate the patient is recommended. Multichannel colonoscopies are required to facilitate active irrigation, suction, and simultaneous intervention to treat the bleeding site. The use of carbon dioxide insufflation may help decrease postprocedure distention.
Thermal ablation of lesions may be attempted by using a heater probe and bipolar or monopolar electrocautery. Argon plasma beam coagulation may also be used to ablate arteriovenous malformations or vessels. When using cautery, the endoscopist should generally use lower settings (10 to 20 W), with short bursts and minimal pressure.144,181 Electrocautery should be avoided in the right colon, especially within the cecum. The few reported cases of perforation are related to intervention in the right colon.315
When one encounters active bleeding from a vessel, an arteriovenous malformation (AVM), or a diverticulum, the endoscopist should attempt injection with epinephrine solution in four quadrants around the bleeding site in order to induce spasm and clotting of the bleeding vessel. A 1 to 10,000 or a 1 to 20,000 dilution of the epinephrine is employed, with 1 to 2 mL injected in each quadrant. Following induction of spasm of the vessel, definitive treatment of the bleeding site can be attempted. Injection of epinephrine alone has a rebleeding rate of approximately 15%.264,286 Some authors have advocated placement of endoclips as a safer option than electrocautery, although minimal data exists to support that statement.168,331 When hemodynamically stable, endoscopy with intervention is a viable option for management of lower GI bleeding.
Etiology Diverticulosis
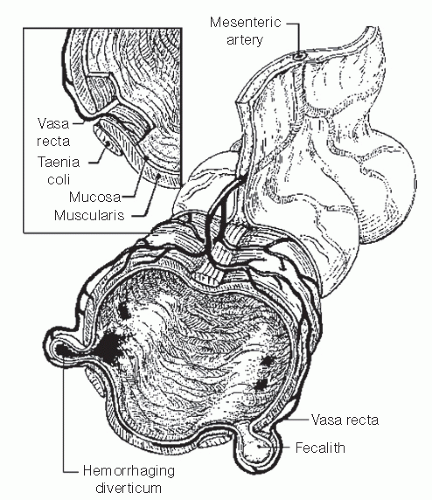
Classically, massive lower GI bleeding has been generally attributed to diverticular disease, usually without any evidence of diverticulitis.133,253,391 As discussed in Chapter 27, diverticular disease occurs where tunnels formed by the blood vessels weaken the muscle. Theoretically, the vasa rectum, through its proximity with the diverticulum, can rupture either at the apex or at the neck as the vessel proceeds into the submucosa of the colon (Figure 28-3). Baer demonstrated that 20 of 22 patients had a pathologically proved ruptured vasa rectum within a diverticulum as the source of lower GI hemorrhage (Figure 28-4).28
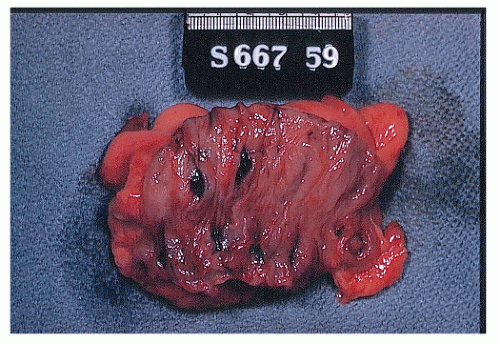 FIGURE 28-3. Portion of sigmoid colon removed for massive lower intestinal hemorrhage that was presumed due to diverticulosis reveals clot in several diverticula. |
The problem, however, is that most lower GI hemorrhage comes from the right side of the colon, where there are few or
no diverticula. Evidence suggests that unexplained vigorous lower intestinal bleeding, even in the presence of known diverticulosis, is most likely due to an arteriovenous malformation (vascular ectasia, angiodysplasia).8,38,39,339 With the availability of angiography and scintigraphy and the ability to identify preoperatively the site of bleeding, arteriovenous malformations have not uncommonly been observed in areas where diverticulosis is present.
no diverticula. Evidence suggests that unexplained vigorous lower intestinal bleeding, even in the presence of known diverticulosis, is most likely due to an arteriovenous malformation (vascular ectasia, angiodysplasia).8,38,39,339 With the availability of angiography and scintigraphy and the ability to identify preoperatively the site of bleeding, arteriovenous malformations have not uncommonly been observed in areas where diverticulosis is present.
 FIGURE 28-4. Diverticulum lined by hemorrhagic granulation tissue in a patient who had massive lower gastrointestinal hemorrhage. (Original magnification × 260; courtesy of Rudolf Garret, MD.) |
Angiodysplasia or Vascular Ectasia
The contemporary attitude is that lower GI bleeding originates from a vascular malformation. Because vascular ectasia is more commonly seen in the right colon and that is the most common site for lower GI hemorrhage, one can inferentially presume that this is the case.29,50,147,156,238,335,342,355 However, Höchter and colleagues challenged this theory when they reported 59 patients with angiodysplasia to have a more uniform bowel distribution.167 The sites of the lesions were as follows: cecum, 37%; ascending colon, 17%; transverse colon, 7%; descending colon, 7%; sigmoid, 18%; and rectum, 14%.167
SCOTT J. BOLEY (1927-PRESENT)
 |
Scott Boley was born in Brooklyn, New York, June 1, 1927. He attended the Wesleyan University in Connecticut and graduated from Jefferson Medical College in 1949. Following 11 years in private practice, he made a commitment to an academic career at the Albert Einstein College of Medicine and the Montefiore Medical Center and is currently professor of surgery and pediatrics. Boley has made numerous contributions to the field of colorectal surgery in particular. For example, he provided the initial description of the entity of noniatrogenic, noncatastrophic colonic ischemia (ischemic colitis) in 1963; he elucidated the cause of small bowel ulcers resulting from enteric-coated potassium diuretic tablets (1965); and he described the endorectal pull-through operation with primary anastomosis for Hirschsprung’s disease (1964). He was the first to identify the nature and etiology of vascular ectasia (angiodysplasia) of the colon (1977) and advocated an aggressive approach to the management of acute mesenteric ischemia through the use of early angiography and intra-arterial vasodilators. He also described the right colon patch endorectal pull-through operation for total aganglionosis of the colon. Boley has published more than 250 articles and book chapters and has edited several books and monographs. He continues in active teaching and consulting as of this writing.
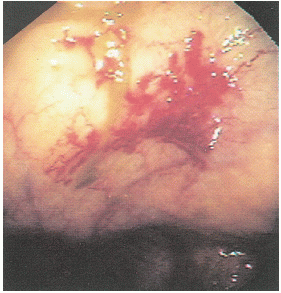
 FIGURE 28-6. An angiodysplastic lesion seen on colonoscopy is characteristically a focal, submucosal, vascular ectasia. |
Bleeding associated with ectasia is usually less severe than that from diverticular hemorrhage. It tends to be intermittent and is probably due to venous encroachment of the mucosa as compared with the ruptured vasa rectum of a bleeding diverticulum (Figure 28-5).
The etiology of the condition remains somewhat problematic. Boley and associates suggest that the vascular lesions are degenerative, from an acquired and progressive dilatation of previously normal blood vessels, the result of the aging process.50,53 They propose that muscular contraction or increased intraluminal pressure produces obstruction of the perforating veins.271 These submucosal structures become dilated and tortuous, with an associated arteriovenous communication (Figure 28-6). Others suggest a congenital etiology; some proposing an association with Meckel’s diverticulum,166 but this only serves to cause confusion. It is perhaps wiser to accept the concept that angiodysplasia is an acquired condition that should be distinguished from the blood vessel tumor, hemangioma (see Chapter 26).
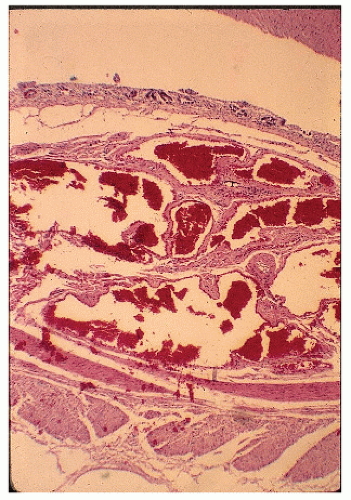 FIGURE 28-7. Angiodysplasia of the cecum. Note the irregular veins and arteries. (Original magnification × 120; courtesy of Rudolf Garret, MD.) |
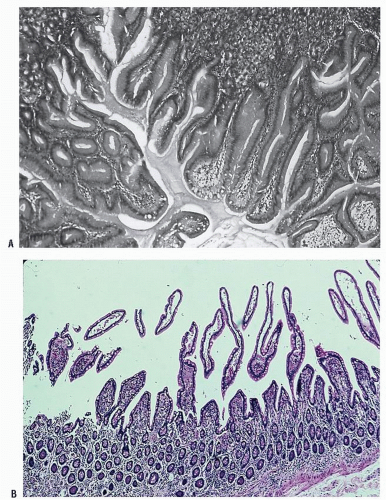
Pathologically, angiodysplastic lesions appear to be ectasias or dilatations of vascular structures. They represent collections of thin-walled, dilated vessels (either capillaries or veins) usually lying in the submucosa (Figures 28-7 and 28-8). Rarely, the condition may be associated with vascular malformations elsewhere in the GI tract (e.g., Osler-Weber-Rendu disease) (Figure 28-9).
There does not appear to be any gender predilection for vascular ectasias. However, two-thirds of the patients of Boley and Brandt were older than 70 years old.50 Richardson and associates reported 39 patients with bleeding due to vascular malformations of the intestine.291 There seemed to be a bimodal age distribution, with younger patients having no associated disease, whereas older people often had a cardiac lesion (especially aortic stenosis [see later] and severe atherosclerotic disease). The most common site of bleeding was the cecum, with resection controlling the hemorrhage in the vast majority of patients. However, bleeding can occur from angioplastic lesions in more than one area of the colon.335 Foutch and colleagues reviewed their experience with 964 patients diagnosed with angiodysplasia.122 They concluded with the following:
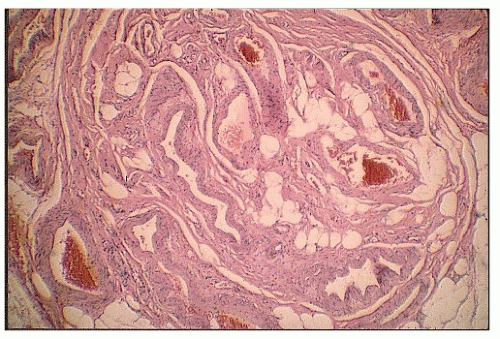 FIGURE 28-8. Vascular malformation showing thick-walled veins and arteries in an irregular distribution. (Original magnification × 250; courtesy of Rudolf Garret, MD.) |
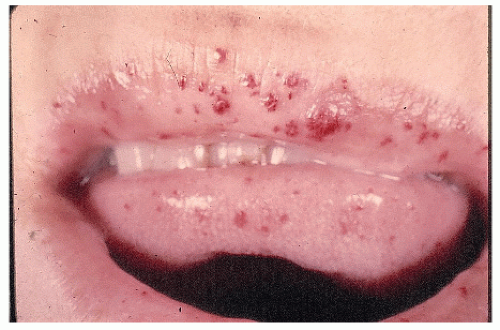 FIGURE 28-9. Telangiectasis of the lips in a patient with hereditary malformations and gastrointestinal hemorrhage. (Courtesy of Rudolf Garret, MD.) |
Colonic angiodysplasia is uncommon among healthy asymptomatic individuals (0.83%).
Lesions are usually small (< 10 mm) and are located proximal to the hepatic flexure.
The natural history is benign, with the risk of bleeding over a 3-year period nonexistent.
Most opine that endoscopic treatment for nonbleeding lesions is unnecessary.
Relationship to Calcific Aortic Stenosis
Love identified the syndrome of calcific aortic stenosis and GI hemorrhage, suggesting treatment of the bleeding by aortic valve replacement.212 Shbeeb and colleagues reviewed Love’s experience and confirmed the association of calcific aortic stenosis and obscure GI bleeding in the elderly.325 These authors and others believe that this operation not only corrects the cardiac hemodynamic instability but also stops the GI hemorrhage.73,145
A mechanism for the association and the ameliorative response to cardiac surgery is not clear. It may be a consumption phenomenon or a qualitative alteration of platelet function produced by the roughened stenotic valve in the area of greatest pressure and velocity of the bloodstream.212 This subtle coagulation defect combined with a thin-walled vascular lesion may tend to promote the hemorrhage. Another contributing factor may be the abnormal arterial inflow pulse wave.145 A coagulation panel, including platelet function, should be part of the preoperative assessment of any patient suspected of bleeding from angiodysplasia.335 One should be aware of this relationship so that earlier diagnosis may spare these patients from multiple hospitalizations and transfusions.
Dieulafoy’s Lesion
Dieulafoy’s lesion, also known as Dieulafoy’s malformation, was originally described by Paul Georges Dieulafoy in 1898 as a gastric, submucosal aneurysm.107 Significant, and often recurrent, hemorrhage occurs from a pinpoint nonulcerated arterial lesion, usually high in the gastric fundus. However, the condition has also been described as an unusual cause of small bowel hemorrhage and even rarely the source of bleeding in the colon, rectum, and anal canal.233
The lesion has characteristically been described as a solitary, protuberant, serpiginous, and abnormally wide artery
located in the submucosa that has the appearance of a submucosal tumor in an otherwise normal mucosa.233 Endoscopic criteria for diagnosis include a less than 3-mm mucosal defect in combination with any of the following25:
located in the submucosa that has the appearance of a submucosal tumor in an otherwise normal mucosa.233 Endoscopic criteria for diagnosis include a less than 3-mm mucosal defect in combination with any of the following25:
PAUL GEORGES DIEULAFOY (1839-1911)
 |
Paul Dieulafoy was born in Toulouse, France, November 18, 1839. He studied in Paris and received his doctorate there in 1869. He ultimately became professor of medicine and chief of medical services at the Hôtel-Dieu in Paris. Dieulafoy has been recognized as an innovative investigator and a keen observer who did seminal work on typhoid, Bright’s disease, and appendicitis. Among his eponymously associated contributions were his apparatus—a suction pump to evacuate fluid from the chest cavity; his erosion— an erosion or ulcer-complicating pneumonia and causing upper gastrointestinal hemorrhage; his pancreatic crisis—symptoms of acute abdomen at the onset of hemorrhagic pancreatitis; his triad— a hypersensitivity of the skin, tenderness, and muscular contraction at McBurney’s point in acute appendicitis; and, of course, his lesion or vascular malformation of the stomach. He wrote a manual on pathology and was elected president of the French Académie de Médecin in 1910. Dieulafoy died August 16, 1911, in Paris.
A protruding 1- to 2-mm blood vessel
Active arterial bleeding
Fresh adherent clot with a narrow point of attachment
Inactive lesion with associated intraluminal blood suggestive of recent hemorrhage
Microscopically, there is usually noted to be a thickwalled vessel without an associated inflammatory reaction.
Treatment may involve intra-arterial vasopressin (see Evaluation of Hemorrhage), sclerotherapy, oversewing (if within reach of rectal instrumentation), or resection.
Meckel’s Diverticulum
Meckel’s diverticulum is generally acknowledged to be the most prevalent congenital anomaly of the GI tract.221 Johann Meckel was not the first to recognize this entity, however. Fabricus Hildanus had reported this in 1598 as an unusual diverticulum of the small intestine, but it was Meckel who in 1809 published a meticulous description of its anatomy and embryonic origin.230 The condition is present in 1% to 2% of autopsies. It represents a diverticulum of the ileum derived from the unobliterated yolk stalk—that is, the remnant of the vitelline duct. Generally, it is found more commonly in males in the ratio of 2:1. In almost 90% of cases, the diverticulum arises on the antimesenteric border. When the remnant persists, it may result in a variety of intra-abdominal complications.
JOHANN FRIEDRICH MECKEL (1781-1833)
 |
Johann Meckel was born October 17, 1781, in Halle, Prussia. He is known as the Younger, having been born into a family of prominent physicians. His father, Philipp Friedrich Theodore Meckel, was professor of anatomy and surgical obstetrics at the University of Halle, and his grandfather, Johann Friedrich Meckel (the Elder), had occupied the same prestigious chair. Meckel’s younger brother, August Albrecht Meckel, also inherited the family’s academic attributes and became professor of anatomy and forensic medicine at the University of Bonn in 1821. The younger Meckel, however, as a child had an outspoken aversion to medicine in general and anatomy in particular, perhaps as a consequence of his having to help his father perform dissections. Despite this he ultimately became one of the greatest anatomists of his time. He began his medical studies at Halle and in 1801 moved to the University of Göttingen to expand his interest in comparative anatomy. He received his medical degree the following year in Halle. After several years of travel and study throughout Europe, he ultimately collaborated with the brilliant French anatomist, Cuvier, and translated Cuvier’s five-volume work into German, a task that he completed in 1810. Returning to his native Halle in 1806, he found that Napoleon himself had been using his home as temporary headquarters, an intrusion that may have aided in preserving the valuable anatomic collection of the Meckel family. In 1808, he was appointed professor of normal and pathological anatomy, surgery, and obstetrics at Halle. Meckel attracted large numbers to his lectures at Halle, which was then the center of comparative anatomy in Germany. Among his lasting contributions was the study of the abnormalities occurring during embryologic development. Meckel’s teratology was the first comprehensive description of birth defects. Johann Meckel died October 31, 1833, in Halle. (Photograph courtesy of the Anatomical Institute of the University of Halle).
The rule of 2’s is the classical description of this anomaly. It is located about 2 ft from the end of the small intestine, is often about 2 in. in length, occurs in about 2% of the population, is twice as common in males, and can contain two types of ectopic tissue—stomach or pancreas.
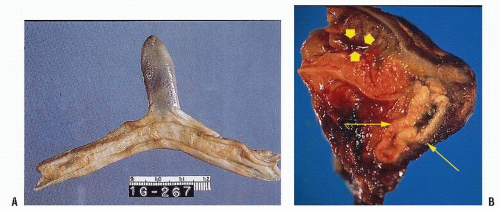
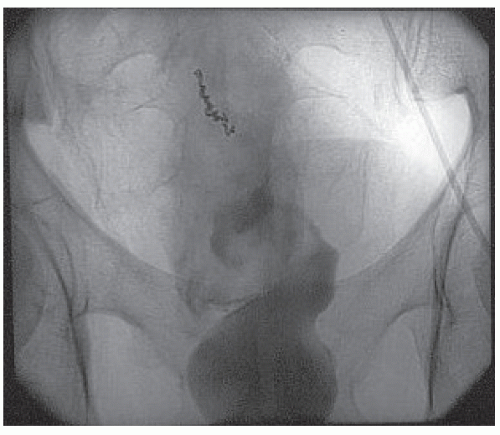
In the adult, the diverticulum is usually 1.5 to 2 in. long and occurs approximately 2 ft from the ileocecal valve (Figure 28-10). However, the distance is quite variable. The diverticulum contains all layers of the bowel wall, but in some cases it may harbor heterotopic, gastric, pancreatic, biliary, or even colonic tissues (Figure 28-11).61 The two most frequently observed complications are intestinal
obstruction and hemorrhage (Figure 28-12). So-called Meckel’s diverticulitis is a third presentation. Intussusception in young children may lead to intestinal obstruction and/or rectal bleeding. Serious hemorrhage from the rectum from a Meckel’s diverticulum is usually due to peptic ulceration. This occurs most frequently in children between the ages of 10 and 15, but it is not unusual to observe this presentation in adults. The blood is usually dark red as opposed to the tarry stool of an upper GI source for the hemorrhage or bright red rectal bleeding from a more distal location.
obstruction and hemorrhage (Figure 28-12). So-called Meckel’s diverticulitis is a third presentation. Intussusception in young children may lead to intestinal obstruction and/or rectal bleeding. Serious hemorrhage from the rectum from a Meckel’s diverticulum is usually due to peptic ulceration. This occurs most frequently in children between the ages of 10 and 15, but it is not unusual to observe this presentation in adults. The blood is usually dark red as opposed to the tarry stool of an upper GI source for the hemorrhage or bright red rectal bleeding from a more distal location.
Vane and colleagues reported 217 children with vitelline duct anomalies.371 Forty-eight presented with rectal bleeding, and at the time of surgery all were found to have ectopic gastric mucosa. Yamaguchi and coworkers identified ectopic gastric mucosa in only 9.1% of their 596 cases.398 In the experience of Mackey and Dineen, 25% of the individuals who were symptomatic presented with lower GI bleeding.221 Those who were younger than 40 years were most likely to have symptoms develop.
Diagnostic Studies
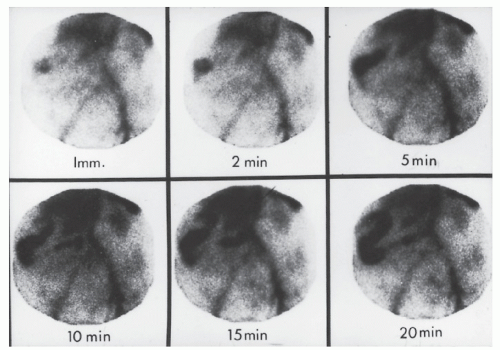
Kusumoto and colleagues compared the various modalities of evaluation of bleeding from Meckel’s diverticulum in 138 individuals.192 All underwent any one or more of three examinations: 99mTc scintigraphy, angiography, and barium enema study. Thirty-eight percent of patients had positive angiography. Forty-seven percent were diagnosed as having a Meckel’s diverticulum on barium study, but scintigraphy had a diagnostic accuracy rate of 83%. The authors concluded that99mTc-pertechnetate scintigraphy is the preferred test for evaluating bleeding when Meckel’s diverticulum is suspected.192 Schwartz and Lewis opined, however, that there is a relatively high false-positive and false-negative rate with scintigraphic imaging.320 Based on their findings, it was suggested that the scanning be supplemented with small bowel infusion or arteriography or both to improve preoperative evaluation in adult patients when this diagnosis is entertained.
Treatment
Excision of Meckel’s diverticulum can usually be accomplished by simple diverticulectomy, although a small bowel resection may be necessary, particularly if the base of the diverticulum is quite broad. Closure can be effected by conventional suturing or by any number of techniques using the stapling devices. A laparoscopically assisted approach to Meckel’s diverticulectomy has also been described.24
Incidental Removal
What is the natural history of Meckel’s diverticulum? Should it be removed incidentally when identified?
Soltero and Bill studied 202 cases over a 15-year period in an attempt to answer this question.338 Using the population averages and the number of cases in each age group (assuming a 2% incidence of Meckel’s diverticulum in the general population), they calculated the rates per year of a complication developing from a Meckel’s diverticulum using life- table techniques. They concluded that a Meckel’s diverticulum has a 4.2% likelihood of causing symptoms during a lifetime, decreasing to zero with old age. They also concluded that it would be necessary to remove approximately 800 asymptomatic Meckel’s diverticula in order to save one patient’s life from the complications of their presence. The obvious recommendation was that the removal of an asymptomatic Meckel’s diverticulum is rarely, if ever, justified.
Colorectal Varices
Since originally described in 1954, fewer than 100 cases of colonic varices have been reported in the literature.378 This rare cause of lower intestinal hemorrhage is almost always associated with cirrhosis, with resultant portal hypertension, or portal venous obstruction.174 The condition has been reported in approximately 2.5% of those undergoing sclerotherapy for esophageal varices.123 As few as 3.6% and as many as 56% of cirrhotic patients have been demonstrated to have concomitant rectal varices. Parenthetically, it must be remembered that hemorrhoids are not rectal varices, and this misnomer should never be applied to that condition.
Another presentation of variceal bleeding that is of interest to the colon and rectal surgeon is also a consequence of portal hypertension—that of stomal and parastomal varices, especially in an individual with sclerosing cholangitis and biliary cirrhosis as an extraintestinal manifestation of inflammatory bowel disease (see Chapters 29 and 31).158
A still rarer cause of colonic varices is the so-called familial or idiopathic variety.174 The condition may present at any age, including the first decade of life. To conclude that this disease truly represents idiopathic colonic varices, liver disease and portal venous obstruction must be excluded.
Contrast-enhanced, three-dimensional magnetic resonance angiography has been recommended as uniquely helpful for visualizing ectopic varices, that is, colorectal and stomal varices.158
Management
Generally, if the bleeding occurs in the group of patients whose colonic varices are secondary to liver disease, treatment parallels that of the management of bleeding esophageal varices from portal hypertension.326 Conversely, in those individuals whose colonic varices are attributed to a congenital etiology, favorable prognosis has been associated with colonic resection.378 Transanal application of the circular stapling instrument for the treatment of bleeding rectal varices has also been described.49
Diagnosis of Lower Gastrointestinal Bleeding
Colonoscopy
Therapeutic Colonoscopy
Specifically for the treatment of angiodysplastic lesions; sclerotherapy; electrocoagulation; and, more recently, endoscopic GI laser therapy have been successfully employed
(see Chapter 5).50,65,151,167,171,275,318,366 The recommended technique is to treat the periphery initially and the center last in order to reduce the vascular supply to the lesion and to diminish the potential for later bleeding.50
(see Chapter 5).50,65,151,167,171,275,318,366 The recommended technique is to treat the periphery initially and the center last in order to reduce the vascular supply to the lesion and to diminish the potential for later bleeding.50
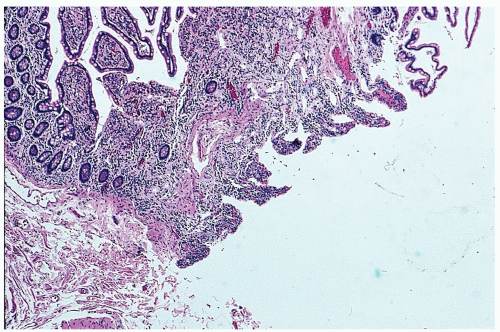 FIGURE 28-12. Meckel’s diverticulum. Loss of surface mucosa in an ulcerating, bleeding lesion. (Courtesy of Matthew Curran, MD.) |
Trudel and colleagues reported 71 patients with lower GI bleeding secondary to arteriovenous malformations, of whom 80% were diagnosed by colonoscopy.366 The mean number of prior hemorrhages was 6.1. Of the 28 individuals treated by endoscopic coagulation, bleeding ceased in approximately twothirds. A surgical procedure controlled the recurrent bleeding in six of seven cases where electrocoagulation had failed. Therapeutic colonoscopy has been reviewed earlier in this chapter.
Computed Tomographic Colonography
Computed tomographic (CT) colonography is an alternative imaging modality that could, in theory, be effectively used before colonoscopy in selected patients.358 The use of computed tomography as a diagnostic test for patients with GI hemorrhage has become a viable option since the development of multidetector row CT scans. This technique significantly decreases the time required for scanning, allowing for effective arterial phase imaging, and thereby permitting its use in cases of significant lower GI bleeding. Drawbacks to the technique include a high IV contrast load, with the potential risk of nephrotoxicity as well as the amount of radiation delivered during the scan. Like nuclear medicine scanning, the test is purely diagnostic; it does not have the option for intervention.
Yoon and colleagues performed a randomized, prospective trial to examine the feasibility of multidetector CT scanning for GI hemorrhage.399 Positive scans were reported in 84% of their patients. They also documented two episodes of renal insufficiency (11%) afterward, both patients having had preexisting diabetic nephropathy. Others have warned of the high contrast load required if progressing to mesenteric angiogram after CT angiography.178 When comparing multidirectional CT scans to nuclear scintigraphy, nuclear scintigraphy had a higher rate of detection (25% vs. 46%).403 At this point, the real role for CT localization may be in patients with active recurrent bleeding that has not been identified. One certain advantage of the procedure is the ability of CT scan to visualize the entire GI tract, perhaps with greater accuracy in localization than nuclear scanning or angiogram, although this has not been documented in the literature.
Angiography
If the source of bleeding has been identified by means of rectal examination, sigmoidoscopy, upper GI endoscopy, or colonoscopy, therapy can be instituted. Unfortunately, in patients who bleed massively from the colon and are unstable, these studies are usually not helpful except to eliminate another cause. The next investigative procedure that should be performed is either selective angiography or radionuclide scan. Unless angiography is not available at the hospital, the patient should not be taken to the operating room without this radiologic investigation. Abbas and coworkers identified instability as the patient having a blood pressure of less than 90 mm Hg and having received five units of packed red blood cells in 24 hours.2 This combination is predictive of identifying the source of bleeding by means of angiography. With both hypotension and the requirement for transfusion, they identified a bleeding site in 85% of their patients. However, when the patients were stable, the authors’ yield was only 15%. Clearly, the rate of bleeding determines the success of angiography in identifying the source. Steer and Silen demonstrated a required bleeding rate of 0.5 cc to 1 cc/minute in order to demonstrate a positive angiogram.341 The standard sensitivity that most publications suggest in order to identify the source of blood loss by means of angiography is 0.5 mL/minute. This is probably an unrealistically low estimate because it is based on the results of studies performed on the animal model. Bowel gas, the presence of fluid within the lumen, body habitus, and other variables may make it impossible for one to identify extravasation unless the rate of bleeding is as much as 5 mL/minute (300 mL/hour). The
literature varies widely in the reported success rates of localization with angiography—that is, from 25% to 70%.
literature varies widely in the reported success rates of localization with angiography—that is, from 25% to 70%.
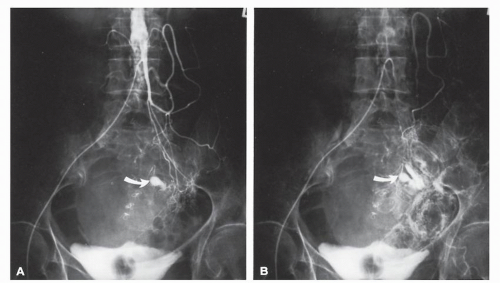 FIGURE 28-13. Bleeding from diverticulum. Early (A) and late (B) arterial phases of an inferior mesenteric arteriogram demonstrate an active bleeding site in the distal transverse colon (arrow). |
Direct selective catheterization of the celiac, superior mesenteric, and inferior mesenteric arteries is accomplished by way of the groin using a modified Seldinger technique.323 In suspected lower GI bleeding, the superior mesenteric artery is injected first because of the higher incidence of colonic bleeding from the right side.138 If the site of the bleeding is not found, the inferior mesenteric artery is studied. Finally, if no source is identified, a celiac injection should be made. As mentioned, on rare occasions upper GI bleeding may seem to be of colonic origin. Radiographic abnormalities include extravasation (Figures 28-13 and 28-14), arteriovenous malformation (Figure 28-15), a delayed emptying vein, and an
early-filling vein (Figure 28-16). The cecal branch of the ileocolic artery usually is the most likely site for a vascular malformation. The characteristic angiographic signs of angiodysplasia were described by Boley and colleagues and include a dense, slowly emptying vein (92%), a vascular tuft (68%), and an early-filling vein (56%).54 Extravasation of the contrast material is the least frequently observed finding (8%). Many reports testify to the success of identifying a bleeding site by angiography. For example, Allison and colleagues ascertained the source in 87% of patients in whom the study was undertaken as an emergency, and 74%, if performed electively.10
early-filling vein (Figure 28-16). The cecal branch of the ileocolic artery usually is the most likely site for a vascular malformation. The characteristic angiographic signs of angiodysplasia were described by Boley and colleagues and include a dense, slowly emptying vein (92%), a vascular tuft (68%), and an early-filling vein (56%).54 Extravasation of the contrast material is the least frequently observed finding (8%). Many reports testify to the success of identifying a bleeding site by angiography. For example, Allison and colleagues ascertained the source in 87% of patients in whom the study was undertaken as an emergency, and 74%, if performed electively.10
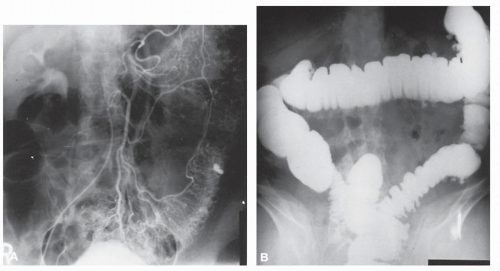 FIGURE 28-14. Bleeding from diverticulum. A: Angiogram demonstrates extravasation in upper sigmoid. B: Site of bleeding corresponds to larger diverticulum on barium enema. |
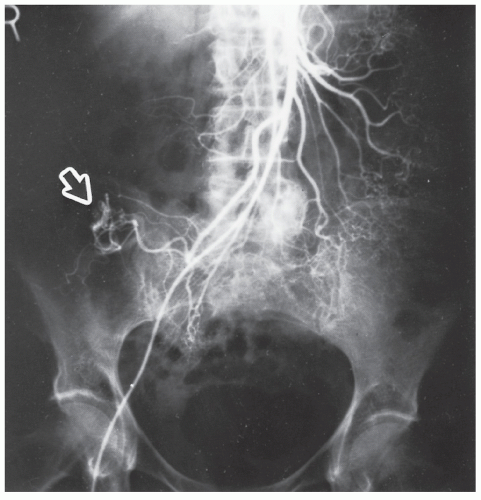 FIGURE 28-15. Arteriovenous malformation “vascular tuft” (arrow) demonstrated by SMA injection. (Courtesy of Brian R. Schnier, MD.) |
Injection of the major blood vessel in the resected specimen with silicon rubber compound and clearing with methyl salicylate is one means for possibly identifying a vascular tuft or dilated blood vessels (Figure 28-17). The potential value of this technique is to permit the pathologist to identify the lesion macroscopically.
Therapeutic Angiography
If the bleeding point is identified, it may be possible to control the hemorrhage by means of either an embolization technique (Figures 28-18 and 28-19) or by the infusion of Pitressin (vasopressin). Vasopressin causes contraction of smooth muscle, especially the capillaries, small arterioles, and venules, with less effect on the smooth musculature of the larger veins. Many papers have been published that deal with the efficacy of vasopressin in the treatment of GI hemorrhage.40,56,112,285,367 But the use of vasopressin has several disadvantages. The drug itself has a number of side effects, including decreased cardiac output, hypertension, and arrhythmias.138 Because many of these patients are elderly or perhaps have an unstable cardiovascular condition, it is important to carefully monitor the intake and output. Vasopressin has a profound antidiuretic effect. Additionally, there are potential concerns related to prolonged catheter use: embolism, hemorrhage around the puncture site, hematoma, and limitation of activity. An arterial pump is needed to administer the drug, and the position of the catheter has to be checked daily by means of a portable x-ray unit.
Embolization has been reported to be an acceptable alternative to infusing a vasoconstrictive substance.97,101,139,214,226,235,296,297,337 In the experience of DeBarros and coworkers, all 27 patients who had an angiographically visualized source for colonic hemorrhage underwent successful embolization.97 Six patients rebled (22%), 5 of whom required surgery. Two demonstrated ischemia (7.4%), 1 of whom required an operation.
Overall advancement in angiography with superselective embolization has allowed greater success and fewer complications in treating lower GI bleeding. Superselective embolization refers to using coaxial microcatheters to selectively cannulate and embolize distal arterial branches. Strate and Naumann compiled 20 studies that used superselective embolization techniques and found a total of 338 patients
out of 539 that could be embolized. Ninety-six percent achieved immediate hemostasis, with a rebleeding rate of 22%. Seventeen percent of these patients experienced major complications that resulted in surgery or death.343 Some studies have suggested that diverticular hemorrhage managed with embolization has a lower rebleeding rate and greater initial success rate than embolization performed for other causes of bleeding.2,185,273
out of 539 that could be embolized. Ninety-six percent achieved immediate hemostasis, with a rebleeding rate of 22%. Seventeen percent of these patients experienced major complications that resulted in surgery or death.343 Some studies have suggested that diverticular hemorrhage managed with embolization has a lower rebleeding rate and greater initial success rate than embolization performed for other causes of bleeding.2,185,273
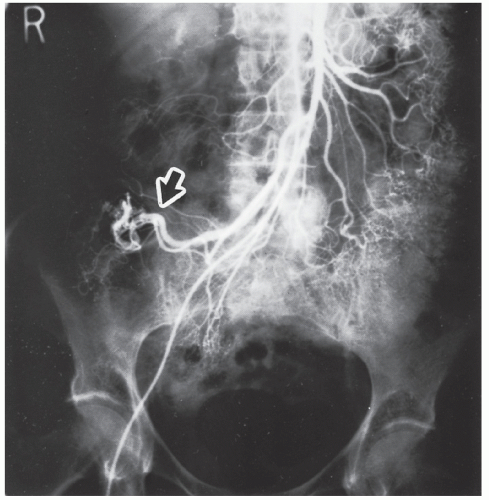 FIGURE 28-16. Early filling vein (arrow) draining from the arteriovenous malformation shown in Figure 28-14. (Courtesy of Brian R. Schnier, MD.) |
Currently, three agents are used for embolization: microcoils, polyvinyl alcohol particles, and Gelfoam. None has been shown to have a greater efficacy than another, but currently Gelfoam is rarely used. A potential benefit of alcohol particles is the ability to inject these proximal to a bleeding site when manipulating the microcatheters into the correct position is not possible.
Transcatheter embolization will inevitably lead, in some patients, to postembolic colonic ischemia and possibly even to infarction.297 Infarction of the embolized segment is the most common complication seen. However, contrast-related injuries such as allergic reaction or nephrotoxicity are also seen as procedure-related issues, as are vessel thrombosis or dissections and hematoma formation. Theoretically, the same segment of ischemic bowel would require removal, a procedure that might be performed under less urgent circumstances than hemorrhage. The incidence of ischemic complications may be reduced by using the least number of emboli required to control the hemorrhage.297 Not every patient is suitable, nor is every lesion amenable to such therapy. However, for those deemed appropriate and who achieve a satisfactory response, operative intervention may be avoided or at least delayed so that it can be undertaken at an elective time.
Nuclear Medicine Techniques
Technetium Sulfur Colloid Scintigraphy
Localizing the site of acute GI hemorrhage has been performed using technetium sulfur colloid scintigraphy (99mTc).218,260,275,319,330,381 The imaging agent used for conventional liver scans is injected into the venous circulation, and with the abdomen of the patient under the gamma camera, a radionuclide angiogram is obtained. The principle of the study is that the labeled colloid is rapidly cleared from the bloodstream by the reticuloendothelial system, but an active site of bleeding appears as a “hot spot” because the extravasated isotope is no longer recirculating and cannot be cleared by the system.330,381 The use of technetium sulfur colloid as a scanning method has been supplanted by tagged red blood cells scan.
Tagged Red Blood Cells
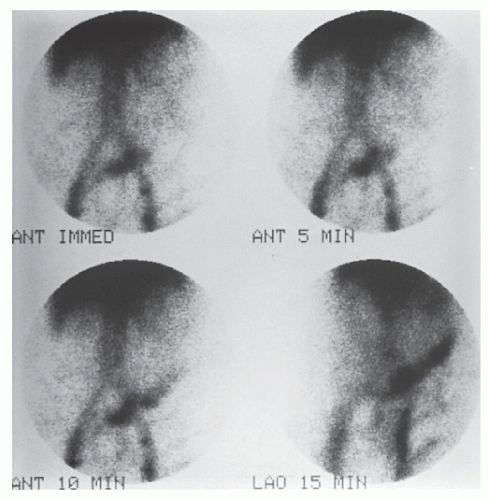
Another alternative is the use of technetium (99mTc) tagged red blood cells.392,393 This technique permits identification of a bleeding point due to hemorrhage of a lesser magnitude. In contrast to technetium sulfur colloid scintigraphy, the labeled red cells are not cleared rapidly and are available to produce a positive scan through repeated periods of imaging, even if
the extravasation occurs over a number of days. Retention of this blood-pool radiotracer in the vascular compartment permits this possibility (Figures 28-20 and 28-21). The disadvantage, of course, is that the radioactivity persists for a relatively long time. But the technique can be successful in detecting the presence of continuing hemorrhage, with transfusion requirements as little as 500 mL within 24 hours or 0.05 to 0.1 mL/minute.393
the extravasation occurs over a number of days. Retention of this blood-pool radiotracer in the vascular compartment permits this possibility (Figures 28-20 and 28-21). The disadvantage, of course, is that the radioactivity persists for a relatively long time. But the technique can be successful in detecting the presence of continuing hemorrhage, with transfusion requirements as little as 500 mL within 24 hours or 0.05 to 0.1 mL/minute.393
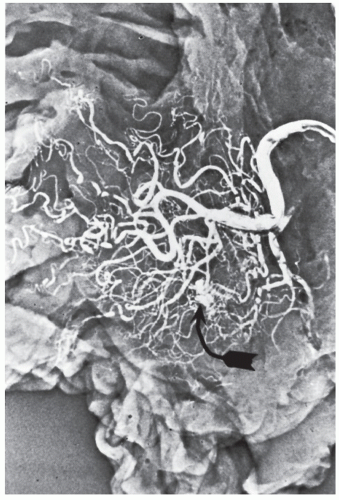 FIGURE 28-17. Angiodysplastic lesion (arrow) in an injected specimen following resection for cecal arteriovenous malformation. |
STANLEY BAUM (1929-PRESENT)
 |
Stanley Baum was born in New York City, December 26, 1929. He received his medical degree in 1957 from the Faculty of Medicine at the University of Utrecht in Holland and completed an internship at Kings County Hospital Medical Center in New York City and a radiology residency at the University of Pennsylvania in Philadelphia. He became a National Cancer Institute trainee before completing a fellowship in cardiovascular radiology at Stanford University Medical Center. Following a brief time on the faculty at Stanford, he returned to the University of Pennsylvania and advanced to the rank of professor of radiology. In 1971, he moved to Harvard as professor of radiology and chief of cardiovascular radiology at Massachusetts General Hospital. In 1975, he returned to the University of Pennsylvania as professor and chairman of the Department of Radiology. Baum held that post for more than 20 years, during which time he became the Eugene P. Pendergrass Professor of Radiology. He contributed to early MRI development and made a significant impact on angiography by describing the roles of vasoconstrictors in controlling gastrointestinal hemorrhage and of angiography in assessing vascular bleeding. He was one of the first interventional radiologists in the United States and was the founder and first president of the Society of Cardiovascular and Interventional Radiology. He was also one of the first diagnostic radiologists elected to the Institute of Medicine. Despite these monumental achievements, some of Baum’s most important work came after he stepped down as chairman. He was a founding member of the Academy of Radiology Research (ARR) and was ARR president when the bill to establish the National Institute of Biomedical Imaging and Bioengineering was introduced in the U.S. Senate. Among his numerous awards and honors are the Cannon Medal from the Society of Gastrointestinal Radiologists, and gold medals from the Association of University Radiologists, American Roentgen Ray Society, and Society of Cardiovascular and Interventional Radiology. In 2002, the University of Pennsylvania established the Stanley Baum Professorship in the Department of Radiology. (With appreciation to Arie Pelta, MD.)
Bunker and colleagues, in their initial study, confirmed the site of bleeding in 10 of 11 patients by this method.69 A later report of 100 individuals demonstrated clear superiority of 99mTc red blood cells over that of 99mTc sulfur colloid, with a sensitivity of 93%, a specificity of 95%, and an overall accuracy of 94% in detecting and localizing GI hemorrhage.70 In 32 patients with documented hemorrhage reported by Winzelberg and colleagues, 29 had positive scintiscans (sensitivity, 91%; 5% false negatives).392 The data of others support the concept of increasing application.252 Baum has suggested that as radionuclide scans are more widely employed, angiography will eventually be performed only in those patients with positive scans.37 Conversely, Bentley and Richardson noted that tagged scans accurately localized the site of bleeding in only 52% of cases and offered the opinion that it is a poor technique for identifying the source.43 They further questioned its use as a screening tool before angiography. Others also have expressed concern about the scan’s ability to accurately localize the site of bleeding. Hunter and Pezim found that performing a surgical procedure that relies exclusively on this technique for localization will “produce an undesirable result in at least 42% of patients.”172 More recent studies have demonstrated accuracy rates between 35% and 100%, with an average of 66%.64,94,153,200,206,259,403 When a positive result was found, the location of bleeding was confirmed by angiogram,
colonoscopy, or surgery. Improved sensitivity in localization is seen if scans are positive within 2 hours of injection.110
colonoscopy, or surgery. Improved sensitivity in localization is seen if scans are positive within 2 hours of injection.110
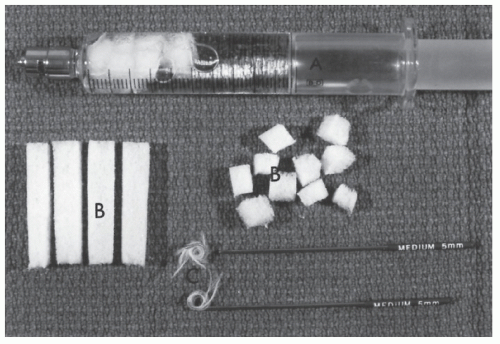 FIGURE 28-18. Thromboembolic devices. A: Syringe with Gelfoam. B: Gelfoam. C: Gianturco coils for occluding medium-sized vessels. (Courtesy of John G. Mardiat, MD.) |
The role of nuclear scintigraphy in the workup of the bleeding patient is still controversial. Tagged red blood cell scans are very sensitive at identifying the presence of bleeding but are not reliable for accurate localization. Although the scans are safe, they provide no ability to intervene and play a purely diagnostic role. There are authors who have advocated its use as a screening tool before angiography in order to confirm active bleeding prior to subjecting a patient to the risks of angiography. Gunderman and associates demonstrated an increased yield for angiography (from 22% to 53%) when patients were previously screened with a tagged red blood cell scan.150 However, this observation has not been born out in other studies.43,379 Some interventional radiologists claim the tagged blood scan helps one to localize the angiogram and decreases contrast load. Indeed, in some centers it may be difficult to obtain an angiogram without a prior tagged red blood cell scan. Moreover, there is some debate about using nuclear scintigraphy as an isolated test in order to allow a segmental colectomy in an actively bleeding patient. Suzman and coworkers examined 224 patients who underwent tagged red blood cell bleeding scans over a 5-year period.350 Fifty-one percent had positive scans, with 42.9% localizing to a specific segment. Fifty patients required surgery, and of those, 38 had undergone scintigraphy, 37 of whom were accurately localized. No patients who underwent segmental resection rebled, but many underwent preoperative localization with another technique, most commonly colonoscopy. Only 18% of the patients in this study underwent angiography, but angiography did not improve localization.
Opinion
There is no clear consensus at this time, but the overall accuracy rate of nuclear scintigraphy in the literature (66%) makes one wary of performing a limited resection without undertaking other preoperative tests. At this time, radionucleotide scintigraphy should be used as a screening test prior to angiography or applied to those patients where localization has not been possible in an individual with persistent or recurrent bleeding.
I concur that 99mTc-labeled red blood cell scintigraphy is of primary benefit in directing the patient’s diagnostic rather than therapeutic management.250
Treatment
As implied from the foregoing, if the bleeding point is identified by means of angiography, tagged red blood cell scan, endoscopy, or barium enema examination, appropriate therapy can be instituted: medical management, a local procedure, or resection, depending on the nature of the lesion and the patient’s clinical course.
Estrogen-Progesterone Therapy
A number of papers have appeared that indicate estrogen-progesterone therapy may be effective in controlling severe, recurrent bleeding from GI vascular malformations.143,244,290,370 The mechanism of action of hormonal therapy to control bleeding with this condition is not clearly understood. Theories include an effect on coagulation, induction of stasis in the mesenteric microcirculation, and improvement in the integrity of the vascular endothelial lining.244 Side effects are of some concern and include thromboembolic disease, an increased risk for the development of malignancies, nausea, vomiting, loss of libido, and gynecomastia. Still, hormonal therapy should be considered in situations when prolonged, obscure GI bleeding thought to be due to angiodysplasia cannot adequately be managed by other means.244
Richardson and Lordon present the typical patient who may be a candidate for this approach.290 They describe three individuals with chronic renal failure who had GI bleeding caused by angiodysplasia that responded to this treatment.
Obscure Bleeding
Thomas suggests the use of the expression “obscure bleeding” to describe the following situations:
The cause exists but has been difficult to diagnose or has been overlooked.
Bleeding results from multisystem disease.
Investigations have failed to localize the site of hemorrhage.361
The reality is, however, that it is only through an organized, algorithmic approach that one may hope to identify and treat the source of GI bleeding. The small bowel, in particular, has been the most difficult area to assess, an area of special concern in an individual with obscure bleeding or bleeding from an unknown source. In the elective situation, a small bowel series, enteroclysis, or capsule endoscopy imaging (see Chapter 5) must be considered.
Obscure bleeding is approached through a variety of new techniques. Most of these patients have already undergone multiple esophagogastroduodenoscopies and colonoscopies. Both tagged red blood cell scans and angiography should be performed, but these are the patients where localization is not possible. Push enteroscopy, video capsule endoscopy, and double-balloon endoscopy are all viable options to help isolate the bleeding source prior to surgery.
Push Enteroscopy. Push enteroscopy refers to the passage of an endoscope into the jejunum via an oral approach without the use of balloons to help advance the scope. Reported success in identifying a source of bleeding in obscure instances ranges from 24% to 56%.99,227,329 The yield is higher (41% vs. 26%) if there is an active bleeding at the time of the procedure.196
Capsule Endoscopy (see also Chapter 5). Capsule endoscopy uses a video capsule to identify lesions within the small intestine. It is diagnostic, not therapeutic. Triester and associates performed a meta-analysis examining the accuracy of capsule endoscopy as compared with other diagnostic techniques.365 They found capsule endoscopy identified potential bleeding sources at a higher rate than other modalities (23% to 67% yields). If positive findings are identified on video capsule endoscopy, referral for intervention with either surgical resection or double-balloon endoscopy is warranted.
Double-Balloon Endoscopy. Double-balloon endoscopy uses either a single- or double-balloon system in order to pass an enteroscope through the small intestine. It can be performed via either antegrade or retrograde approach. It allows not only for diagnosis but also for therapeutic intervention when lesions are identified. This may include injection, electrocauterization, or clipping. Tattooing marks the areas in the small intestine for possible future surgical resection. The reported positive yield rates for the procedure vary from 43% to 81%, with a similar percentage range of patients having a successful intervention for the bleeding site.76,232,247,400,401 The procedure appears to be safe, with a minimal complication rate. Zhong and colleagues reported 378 patients who underwent double-balloon endoscopy over 2 years. The most common side effect was mild-to-moderate discomfort, and no perforations occurred. The authors preferentially performed the procedure with conscious sedation, but general anesthesia was used for 42 individuals.
Operative Management
Resection
What is the proper treatment if the patient continues to bleed and the source has not been identified? In the past, individuals were submitted to exploratory laparotomy in the hope that a lesion could be found at the operating table. Multiple enterotomies were undertaken to identify the proximal limit of the bleeding and to perform operative colonoscopy. In someone at high risk, such a prolonged operative procedure exposes the patient to increased mortality as well as to the possibility of additional morbidity from infection. Alternatively, blind left colectomy has historically been advocated, and later, right colectomy. Most surgeons today, however, believe that subtotal colectomy is the preferred procedure when the source of bleeding has not been identified preoperatively or intraoperatively.
Operative Colonoscopy and Enteroscopy
Another option for operatively identifying the source of bleeding involves a combination of laparotomy and colonoscopy through the application of a two-team approach.44 A rapid, intraoperative cleansing of the bowel may be effected through a small cecostomy if this is felt advisable.321 This is analogous to the technique of on-table lavage (see Chapter 23). The abdominal surgeon can assist the colonoscopist in the passage of the instrument to make one final attempt to identify a discrete bleeding point.376 If one is seen, it may be dealt with through the instrument or it may be effectively treated by a less than total abdominal colectomy.
Intraoperative enteroscopy can be accomplished with the colonoscope passed per orum, guiding the instrument through the duodenum and into the small bowel. Segmental visualization can be accomplished, occluding the bowel at intervals to avoid overdistention.102 The endoscopic appearance can be supplemented by simultaneous viewing from the serosal aspect of the transilluminated bowel.
Desa and colleagues identified the source in 10 of 12 cases of obscure bleeding by the method of operative enteroscopy.102 These individuals had previously undergone multiple surgical procedures as well as the usual specialized testing. Flickinger and associates found that this technique influenced the operation in 93% of cases.120 When an angiodysplastic lesion is identified, one can treat it by electrocoagulation, laser photoablation, or suturing.1
Operative Arteriography
Intraoperative localization of vascular ectasias may be accomplished by placing the bowel to be examined on a sterile cassette cover.294 The segmental arterial branch feeding the area is isolated and injected with methylglucamine diatrizoate (Renografin 76). With the bowel exhibiting pallor and contracting, the film is exposed to identify the lesion. I have had no experience with this technique but would consider its applicability only for small bowel lesions. This method, though, has the potential advantage of a therapeutic option. McDonald and associates described the use of highly selective angiographic catheter placement combined with intraoperative methylene blue dye injection to precisely identify the source of hemorrhage in three patients who had a small bowel source of bleeding confirmed.229 At operation, 0.5 mL of methylene blue dye (50 mg/mL) was injected into the catheter, which resulted in immediate demarcation of the small intestine over a length of approximately 20 cm with a brilliant blue color. This enabled the surgeons to resect a limited amount of small intestine.
Results of Surgery
Smith and colleagues identified 24 patients with lower GI hemorrhage due to angiodysplasia, 17 of whom required surgery.335 With the bleeding point found preoperatively, no patient rebled following either a limited resection or a subtotal colectomy. But coagulopathy, specifically platelet disorders, contributed to a high mortality rate. Boley and Brandt accept a 20% rebleeding rate after right hemicolectomy for angiographically demonstrated ectasias and believe that the risk of subtotal colectomy is greater than the risk of rebleeding.50 Bender and
colleagues reported a mortality of 27% following total colectomy and opined that the procedure under these circumstances is associated with excessive morbidity and mortality.42 Whether this is a valid criticism of the surgery or a reflection of multiple blood transfusions, age, and other factors was not analyzed. Irrespective of the type of surgery, Leitman and associates found that those who failed transcatheter treatment had a mortality of 36%.202 Parkes and coworkers reviewed the records of 31 patients who underwent colon resection to determine the most effective surgical treatment for massive lower GI bleeding.268 The rebleeding rate for subtotal colectomy with a mean followup of 1 year was nil. With a segmental resection, even with a positive angiogram, the rebleeding rate was 14%, and if the angiogram was negative, a segmental resection was associated with a rebleeding rate of 42%. This last group of patients had the highest complication rate in the series (83%). This same group of patients also had an extremely high mortality rate (57%).
colleagues reported a mortality of 27% following total colectomy and opined that the procedure under these circumstances is associated with excessive morbidity and mortality.42 Whether this is a valid criticism of the surgery or a reflection of multiple blood transfusions, age, and other factors was not analyzed. Irrespective of the type of surgery, Leitman and associates found that those who failed transcatheter treatment had a mortality of 36%.202 Parkes and coworkers reviewed the records of 31 patients who underwent colon resection to determine the most effective surgical treatment for massive lower GI bleeding.268 The rebleeding rate for subtotal colectomy with a mean followup of 1 year was nil. With a segmental resection, even with a positive angiogram, the rebleeding rate was 14%, and if the angiogram was negative, a segmental resection was associated with a rebleeding rate of 42%. This last group of patients had the highest complication rate in the series (83%). This same group of patients also had an extremely high mortality rate (57%).
Comment
Fortunately, with the diagnostic studies available, the surgeon should rarely have to resort to blind resection. It is axiomatic that if a lesion is clearly demonstrated on angiography and cannot be controlled by minimally invasive means, a limited resection is appropriate. However, if there is not complete certainty about the source of the bleeding, the few minutes necessary to remove the remainder of the bowel should add very little risk. Furthermore, in the absence of blood in the small bowel, the maneuvers associated with operative colonoscopy and operative angiography truly prolong the surgical time with its attendant risks and have the potential hazards of contamination with the former technique and toxicity with the latter. Finally, ensuring proper examination of the anus and rectum prior to subtotal colectomy is essential in order to definitively determine that the bleeding site is not within this area.
▶ MESENTERIC OCCLUSIVE AND NONOCCLUSIVE DISEASE
The gut receives 20% of resting and 35% of postprandial cardiac output, of which 70% supplies the mucosa.55 In the fasting state, only one in five mesenteric capillaries is open. The bowel, as one can see, has therefore a remarkably resistant ability to withstand ischemia.55 However, if the blood pressure falls below 70 mm Hg, intestinal perfusion may be compromised. Below 40 mm Hg this mechanism fails, and the bowel becomes progressively more ischemic, with anaerobic metabolism replacing aerobic.55 The nature and rapidity of the ischemic process are affected by the collateral circulation and by disorders of splanchnic autoregulation.
Major occlusive disease is usually caused by mesenteric vascular obstruction as a consequence of atheroma, thrombus, or embolus. Other etiologies include dissecting aneurysm, arteritis, sepsis, intestinal obstruction, and trauma. Acute mesenteric ischemia usually occurs in patients older than 50 years of age, particularly in those with arteriosclerotic heart disease or valvular involvement.51 The characteristic person is an elderly man with prior heart disease and symptoms related to peripheral atherosclerosis.333 Hypercoagulable conditions and the use of oral contraceptives may also precipitate intestinal vascular thromboses, often involving the venous circulation. Other factors that predispose to ischemia include long-standing congestive heart failure, the prolonged use of diuretics, cardiac arrhythmias, recent myocardial infarction, hypovolemia, hypotension, burns, pancreatitis, and GI hemorrhage.51,52 Most patients, in fact, who develop ischemic changes of the small and large bowel do not have a demonstrably significant vascular lesion but have so-called nonocclusive vascular disease. Debus and colleagues classify acute mesenteric events into four categories98: T acute mesenteric embolus (50% of cases), acute mesenteric thrombus (25%), nonocclusive disease (20%), and mesenteric vein thrombosis (less than 10%).36
Signs and Symptoms
The most frequent symptoms of mesenteric occlusion are abdominal pain and rectal bleeding (up to 98%). An early characteristic feature is the disparity between the severity of the pain and the paucity of significant abdominal findings.51 In other words, the pain is generally out of proportion to the physical findings. Signs and symptoms of peritonitis rapidly ensue in the presence of intestinal infarction. Hypothermia is not uncommonly observed.333 Other complaints include back pain, nausea, vomiting, and diarrhea. In patients with so-called abdominal angina, abdominal pain is also evident, usually developing 15 to 20 minutes following the ingestion of food.124 Pain is characteristically epigastric or periumbilical, and weight loss and malnutrition may ensue.
Laboratory and Radiologic Studies
Laboratory investigations usually reveal an elevated white blood cell count and evidence of hemoconcentration— nonspecific abnormalities to be sure, but consistent with the diagnosis of mesenteric ischemia. Metabolic acidosis frequently is noted in patients with intestinal infarction. This is due to tissue hypoxia, persistent hypotension, and the release of vasoactive materials. Determination of arterial blood gases confirms the base deficit and should serve to alert the physician as to the severity of the illness. Plain films of the abdomen may demonstrate thickened bowel loops; a ground-glass appearance from ascites; the classic “thumbprinting”; and gas in the bowel wall, portal vein, or peritoneal cavity (Figure 28-22). However, one-fourth of
patients with mesenteric infarction have a normal plain film of the abdomen.
patients with mesenteric infarction have a normal plain film of the abdomen.
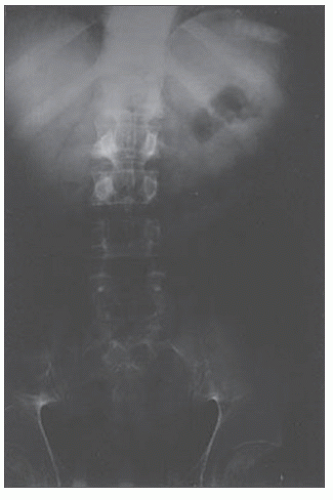 FIGURE 28-22. Plain film of the abdomen. Note thickened and ulcerated transverse colon, ground glass appearance from ascites, and “thumbprinting.” |
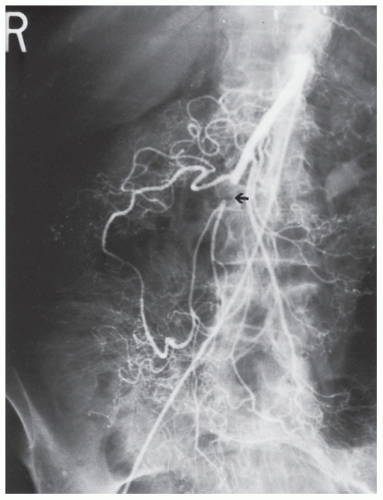 FIGURE 28-23. Superior mesenteric artery embolism (arrow). Note the collateral circulation through the meandering vessel. |
Although a wide range of investigations is available for the assessment of mesenteric ischemia, with the exception of angiography and computed tomography (CT), none is particularly helpful.55 With the widespread utilization and advances in multidetector computed tomography (MDCT) in conjunction with CT angiography, MDCT with angiography has replaced standard angiography as the procedure of choice for diagnosing mesenteric ischemia.27,126,169 The sensitivity of detecting bowel ischemia with MDCT has reached 82%.190
Findings on CT scan consistent with mesenteric ischemia include bowel wall thickening, intestinal attenuation and enhancement, dilatation, pneumatosis, portal vein gas, and fat stranding and ascites.380 All of these findings are relatively nonspecific with the exception of portal vein gas. The vascular findings on CT include abrupt termination of a vessel from embolic obstruction of an artery (Figure 28-23). Proximal obstruction is often required for adequate visualization. Venous thrombosis manifests visually as rounded or tubular low-attenuation focus of thrombus, with increased vein caliber in the affected vein.
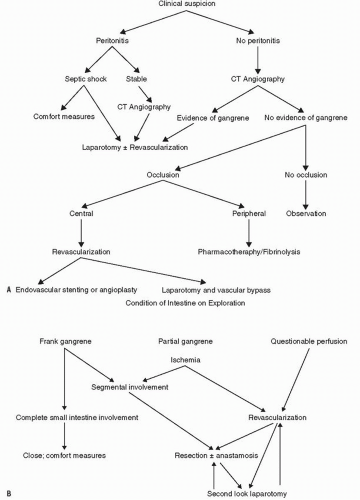
Evaluation and Treatment Protocol
The clinical pathway for patients with suspected mesenteric ischemia is dependent on the presence of peritonitis on physical exam. Peritonitis with signs of severe sepsis requires immediate laparotomy. If the patient is stable, but he or she has peritonitis, consideration for CT angiography should be given because the results may help guide the planned operation. Irrespective of the findings of the CT scan, however, one should proceed to the operating room. If ischemia is suspected but no signs of peritonitis are present, then CT angiography followed by either laparotomy or interventional angiography (depending on findings) should be performed.
If dead bowel is encountered in the operating room, the amount of intestine that should be removed is always a subject of concern. Visual appreciation of bowel injury based on capillary bleeding, color, and contractility is often misleading and commonly induces the surgeon to remove more than is necessary.68 A number of investigators have compared clinical judgment with other methods of determining intestinal viability. Clinical judgment was associated with a relatively high sensitivity, specificity, and overall accuracy, but had a low predictive value.316 Bowel was incorrectly assessed to be nonviable in 46% of patients in one series, leading to sacrificing more bowel than necessary. In an experimental study, Brolin and colleagues compared five methods for assessing intestinal viability: threshold stimulus level (TSL), the minimal electrical current necessary to produce a smooth muscle contractile response; intestinal color; peristalsis; Doppler ultrasound; and histologically evaluated resection margin.60 The bowel color, the presence of peristalsis, and the histologic findings failed to correlate with the intestinal survival rate. Conversely, blood flow measured by Doppler ultrasound and the myoelectric parameters established through an electronic contractility meter were directly related to viability. Bulkley and coworkers compared clinical judgment at the time of the operation with Doppler ultrasound and with the use of fluorescein to determine bowel viability in a prospective, controlled study of patients with intestinal ischemia.68 The fluorescein method was shown to be superior to the Doppler method.
Another option at the time of initial exploration is to resect frankly gangrenous intestine, leaving the questionable bowel in place with planned re-exploration, or second look procedure in 24 to 48 hours (Figure 28-24). Fluorescein may then be used to help determine viability, depending on the appearance of the intestine. An algorithm for the management of mesenteric ischemia is illustrated in Figure 28-25.
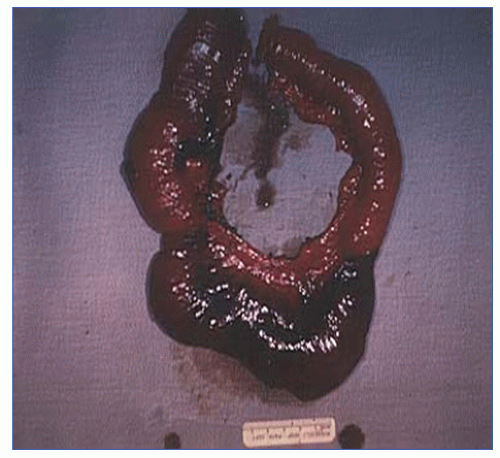 FIGURE 28-24. A resected segment of small bowel showing infarction from prolonged hypotension due to nonocclusive vascular disease. (Courtesy of Rudolf Garret, MD.) |
Results
Boley and colleagues reported their experience with 47 patients with intestinal ischemia due to superior mesenteric artery emboli.52 The overall mortality was 66%. Those with infarction of more than 50% of the small intestine did especially poorly (17 of 19 such patients died). A survival rate of 55% was obtained in patients managed according to the preceding protocol, whereas only 20% of those treated by traditional methods survived. The best results were obtained in patients who were diagnosed within 24 hours of the onset of pain.
Clavien and colleagues reported their experience of 81 individuals with mesenteric infarction documented by angiography.85 Almost one-half were felt to have an inoperable situation and were treated by supportive care only. Of those who underwent a laparotomy, 45% survived. Because of the high frequency of progressive infarction following resection (32%), the authors recommend preoperative perfusion of the superior mesenteric artery with vasodilators and postoperative anticoagulation. Sitges-Serra and coworkers identified 44 individuals who underwent massive small bowel resection (mean length of remaining bowel, 60 cm), noting a 46% mortality.333 Levy and associates reported an overall mortality in 62 surgically treated patients of 40%.205 They emphasize the importance of establishing double stomas whenever the viability of the remaining bowel is equivocal. Endean and coworkers assessed the University of Kentucky experience in 170 patients with acute intestinal ischemia.114 Nonthrombotic patients represented 60%; thrombotic, 34%; and indeterminate, 6%. The survival rate for arterial embolism was 41% and for thrombosis, 38%. These results are indicative of an improvement when compared with earlier reports.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree