Chapter 46 Uterine Leiomyomas
PREVALENCE
Uterine leiomyomas (also known as fibroids or myomas) are the most common benign pelvic tumors found in women. As many as 50% of reproductive-age women will have clinically apparent uterine leiomyomas, and 25% of women have symptomatic leiomyomas. On pathologic examination, 77% of hysterectomy specimens contain one or more uterine leiomyomas.1
Although benign, uterine leiomyomas are associated with significant morbidity, including abnormal uterine bleeding, chronic pelvic pain, impaired fertility, and recurrent pregnancy loss. In the United States, leiomyomas are cited as the primary indication for hysterectomy in more than 250,000 cases per year and account for over $5 billion in healthcare costs annually.2
CLASSIFICATION
Uterine leiomyomas are typically classified into subgroups by their position relative to the layers of the uterus. Subserosal myomas occur near the serosal surface of the uterus. They may have either a broad or pedunculated base and can extend between the folds of the broad ligament. Intramural myomas originate within the myometrium and may enlarge enough to distort the uterine cavity or the serosal surface. Submucosal myomas develop just below the endometrium and with progression protrude into the uterine cavity. These can also be either pedunculated or broad-based. Cervical myomas, on the other hand, are derived from cells in the cervix as opposed to the corpus of the uterus (Fig. 46-1).
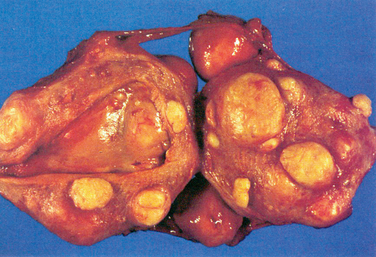
Figure 46-1 Hysterectomy specimen, demonstrating the presence of intramural, submucosal, and subserosal leiomyomas.
CLINICAL IMPACT
Abnormal Uterine Bleeding
Abnormal uterine bleeding is the most common symptom reported by women with leiomyomas. The most common types of leiomyomas associated with heavy bleeding are intramural or submucosal myomas. The typical bleeding patterns are menorrhagia and hypermenorrhea. Intermenstrual bleeding can represent an intracavitary myoma or specific endometrial pathology. Therefore, these patients must always undergo a more detailed evaluation of the uterine cavity. Very heavy vaginal bleeding can lead to problems such as iron deficiency anemia, which can be severe enough to require blood transfusions, and the necessity for frequent changes of sanitary protection can cause significant distress in work or social situations.3
Chronic Pelvic Pain
Pelvic pain or pressure, the second most common complaint in women with leiomyomas, is frequently described as analogous to the discomfort associated with uterine growth during pregnancy. The pain can occur both during and between bleeding episodes. Posterior leiomyomas may give rise to low back pain, whereas anterior leiomyomas may compress the bladder. Leiomyomas that become large enough to fill the pelvis may potentially interfere with voiding or defecation or may cause dyspareunia. Very large leiomyomas can on occasion outgrow their blood supply, leading to tissue ischemia and necrosis clinically manifested as acute, severe pelvic pain. Pedunculated leiomyomas can suffer torsion, also causing ischemia and acute pain. During pregnancy, leiomyomas have been known to undergo red degeneration, where hemorrhage occurs within the myoma, also leading to acute pain.3
Reproductive Function
Yet the indirect evidence is substantial. In one review, pregnancy rates among women with leiomyomas distorting and not distorting the uterine cavity were 9% and 35%, respectively, as compared to 40% among controls with no leiomyomas.4 Furthermore, the multiple reports of successful pregnancies among infertile women after myomectomy strongly suggest a connection.5–7
Although exact physiologic mechanisms for reproductive dysfunction are unclear, many plausible theories exist. There is a potential for reduced fecundity if a myoma occurs in the cornual region of the uterus, causing mechanical occlusion of a fallopian tube.3 It is possible that large leiomyomas may impair the rhythmic uterine contractions that facilitate sperm motility.8 It has further been documented that endometrial histology varies in relation to the location of the leiomyoma. Atrophy, as well as alterations in the vascular blood flow produced by sumbucosal leiomyomas, may impede implantation of an embryo, prevent delivery of hormones or growth factors involved in implantation, or interfere with the normal immune response to pregnancy.9–11 Submucosal leiomyomas that distort the uterine cavity are associated with first-trimester pregnancy loss, preterm delivery, abnormal presentation in labor, and postpartum hemorrhage.12
In regard to the effectiveness of assisted reproductive technology (ART), leiomyomas are generally thought to reduce the effectiveness of ART procedures. Early evidence demonstrated that both pregnancy and implantation rates were significantly lower in patients with intramural or submucosal leiomyomas.13,14 In one study, the presence of an intramural leiomyoma decreased the chances of an ongoing pregnancy after in vitro fertilization by 50%.15 The latest evidence suggests that patients with subserosal leiomyomas have ART outcomes consistent with patients lacking leiomyomas.4,16,17
EPIDEMIOLOGY OF UTERINE LEIOMYOMAS
The diagnosis of uterine leiomyomas increases with age throughout the reproductive years, with the highest prevalence occurring in the fifth decade of a woman’s life. The most common types of leiomyomas associated with heavy bleeding are intramural or submucosal myomas; these tend to be diagnosed at an earlier age and to result in more severe disease in African American women. (larger leiomyomas and greater incidence of anemia) as compared to white women.18,19
Nulliparous women have higher rates of leiomyomas than multiparous women, and the risk of developing leiomyomas decreases consistently with each subsequent term birth.20 Early age at menarche is associated with a twofold to threefold increased risk of developing leiomyomas.21
Leiomyomas clearly demonstrate their hormonal responsiveness in the fact that they form after puberty, have the potential to enlarge during pregnancy, and regress after menopause. However, studies of exogenous hormone treatments, including oral contraceptives and hormone replacement therapy, reveal conflicting data; no clear association can be inferred.22
Twin and family studies suggest a familial predisposition to developing leiomyomas, although further research in the genetics of leiomyomas has yet to be done.22 These studies are hampered by the extremely high incidence of leiomyoma formation in the general population.
According to some studies, an increase in body mass index (BMI) has been found to increase the risk for uterine leiomyomas by a factor of 2 to 3, and the evidence suggests that adult-onset obesity rather than excessive weight in childhood confers this risk. However, other studies have not observed similar associations with increased BMI.21
The majority of epidemiologic studies find that cigarette smokers are at a 20% to 50% reduced risk for the development of uterine leiomyomas through an unclear mechanism, and that the inverse association was independent of BMI. It is unclear whether this relationship varies as a function of pack-years. No clear relationship has been shown between leiomyomas and specific dietary factors or physical activity.21
PATHOLOGY AND PATHOPHYSIOLOGY
Genetics
Leiomyomas are defined as monoclonal proliferations of benign smooth muscle.23 Each monoclonal myoma may be associated with various chromosomal translocations, duplications, and deletions.24 Many but not all myomas contain nonrandom cytogenetic abnormalities while the myometrium has a normal karyotype—typically these involve chromosomes 7, 12, and 14. Most of the mutations occur in genes involved in cellular growth or responsible for architectural transcription.
Two hereditary disorders have been reported in which uterine leiomyomas are part of a syndrome complex that demonstrates the potential genetic contribution to myoma formation. The first is hereditary leiomyomatosis and renal cell cancer complex. This is an autosomal dominant syndrome with smooth muscle tumors of the uterus, skin, and kidney. The second is a syndrome of pulmonary leiomyomatosis and lymphangiomyomatosis (LAM) that is the result of mutations in one of the two genes responsible for tuberous sclerosis, a syndrome that results in multiple hamartomas.
Pathology
Grossly, myomas usually appear as discrete, round masses that are lighter in color than the surrounding myometrium, with a glistening, pearly white appearance. Histologic features include smooth muscle fibers that form interlacing bundles, with fibrous tissue in between the bundles. A more detailed description is found in Chapter 8.
Endocrinology
Tumor initiators and yet-to-be-determined genetic factors are involved in key somatic mutations that facilitate the progression of a normal myocyte into a leiomyocyte responsive to estrogen and progesterone. Estrogen receptors, progesterone receptors, and epidermal growth factor receptors (EGFR) are integral in the development of myomas.25 Studies have shown that, in comparison with the normal myometrium, myomas have an increased concentration of estrogen receptors and progesterone receptors.26,27
Aromatase p450 is overexpressed by leiomyomas.28,29 Therefore, in addition to circulating estrogen acting on the ER, the local conversion of circulating androgens to estrogens may be important in potentiating the actions of estrogen in the leiomyocyte (Fig. 46-2).30
Figure 46-2 Sex steroid hormone action. Estrogen and progesterone exert action through binding of specific receptors, which then bind to DNA at specific response elements. Binding of estrogen and progesterone at a variety of genes has different effects in various cells. Figure provided to the public via the internet by Fisher Scientific, Inc. (www.fishersci.com).
Traditionally, estrogen was thought to be the primary hormonal mediator of myoma growth. Although progestins have been applied for the treatment of bleeding from symptomatic myomas, recent studies have shown that progesterone may play a much greater role as a mediator of myoma growth than previously thought.31 The antiprogestin RU486 (mifepristone) has been shown to decrease the size of myomas,32,33 and another study showed that myomas in the secretory phase have increased mitotic counts compared to those in the proliferative phase.34
Growth of neoplastic tumors is the result of accelerated cellular proliferation, which outpaces the inhibitory effect of apoptosis. Apoptosis has been shown to be inhibited in uterine leiomyomas. Progesterone has been shown to increase the antiapoptotic protein, bcl-2.35 Therefore, the stimulation of myoma expansion may be a function of the suppression of apoptosis by progesterone. It has been observed in vitro that the addition of progesterone to cultured leiomyoma cells increased the expression of bcl-2 when compared to controls.35 Normal myometrium did not express increased levels of bcl-2 in the presence of progesterone.
The complex process of apoptosis involves not only the bcl-2 family, but Fas/FasL and Rb-1.36 Martel and coworkers have described the various apoptotic pathways deficient in leiomyomas and potential corresponding targets for therapy of myomas. The role of apoptosis in the pathogenesis of myomas is a promising area for future research, with a great potential for clinical application.
The synergistic interplay between estrogen and progesterone signaling in the pathophysiology of myoma growth has been observed as well. The increase in progesterone receptors as a result of increased estrogen has been well established. An in vitro study showed that progesterone up-regulates the expression of EGF, and estrogen also increases the expression of EGFR.25
DIAGNOSTIC IMAGING AND LEIOMYOMAS
Ultrasound
Traditional ultrasound is a cost-effective technology for assessing uterine leiomyomas (see Chapter 30 for details). The transvaginal approach is more accurate than abdominal ultrasound. However, abdominal ultrasound may be a useful adjunct to transvaginal ultrasound, if a large uterine size warrants such an approach.22 The presence of myomas may be detected by ultrasound, as uterine enlargement or a nodular contour of the uterus. They may also appear as discrete, focal masses within the myometrium.37,38 Myomas can appear hypoechoic or heterogeneous when compared with the appearance of the myometrium on ultrasound, and they may be characterized by calcification and posterior shadowing.37,39 Sagittal and axial views aid in providing information on the location and size of myomas.
Additional information regarding intracavitary masses, such as submucous myomas, may be obtained by means of saline infusion sonohysterography. This imaging technique consists of real-time transvaginal ultrasound during which sterile saline solution is injected into the uterine cavity. The saline is injected transcervically via a small-caliber catheter. As the uterine cavity is distended by the saline solution, intracavitary masses may be visualized as echogenic structures against the echolucent background of the distension media.40 Intramural myomas within close proximity of the endometrial cavity may also be assessed by sonohysterography. In addition, entities such as endometrial polyps and uterine anomalies such as adhesions may also be detected. Sonohysterography can be used not only to diagnose submucous myomas, but also to assess the potential access to surgical intervention.41
Three-dimensional ultrasound42 and color Doppler ultrasound43 are increasingly being applied to the evaluation of myomas for imaging. Color Doppler ultrasound highlights vascular flow, which is usually increased at the periphery of myomas and decreased centrally.42,43
Hysterosalpingography
HSG is a screening test for intracavitary anatomic defects and entails injection of iodine contrast dye transcervically, via a catheter, into the uterine cavity with radiologic assessment under fluoroscopy (see Chapter 29). HSG is performed in the follicular phase of the menstrual cycle to avoid interfering with ovulation or a potential pregnancy. Because the HSG instillation medium contains iodine, an iodine-allergic patient requires premedication with glucocorticoids and antihistamines before the procedure.44
Hysterosalpingography allows visualization of submucous myomas as the uterine cavity is distended by the contrast medium. The size and contour of the uterus may be altered by submucous myomas. Intramural myomas may enlarge the uterine cavity in a globular manner, and fundal myomas may enlarge the space between the cornuae. Subserosal myomas are not typically noted on HSG; however, if large enough, they may be detected as a mass effect on the uterine cavity.23 In cases where a submucous myoma must be differentiated from an endometrial polyp on HSG, hysteroscopy or sonohysterography play roles as complementary, potentially confirmatory adjuncts.
Magnetic Resonance Imaging
Disadvantages of MRI include cost, limited availability, and an inability to perform the procedure in patients with morbid obesity or claustrophobia. Traditionally, cost had been more of a disadvantage in the past; however, as the expense of MRI decreases, it is more commonly employed in clinical and presurgical evaluation. MRI is contraindicated in patients with pacemakers, defibrillators, metallic foreign bodies, and in rare cases of allergy to gadolinium.45
In T2-weighted images, the endometrial stripe is visualized as a central, high signal; the junctional zone is a low signal; and the myometrial areas are an intermediate signal.40 Leiomyomas are represented by variable signal density. Most of the time, they appear as hypodense, well-demarcated masses; however, increased cellularity47 and degeneration may be seen as high signal intensity.46
There is less distinction of the endometrial lining, junctional zone, and myometrium in T1-weighted images. These components are usually homogeneous and, consequently, obscured in appearance. Fatty or hemorrhagic degeneration may be represented by a high signal intensity.46 A detailed description can be found in Chapter 31.
MEDICAL TREATMENT OF LEIOMYOMAS
Gonadotropin-Releasing Hormone Agonists
Gonadotropin-releasing hormone (GnRH) agonists are an effective means of medically treating patients with symptomatic leiomyomas. After producing an initial flare of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), GnRH agonists down-regulate the hypothalamic-pituitary-ovarian axis via action on pituitary receptors. The flare effect is due to an initial stimulation of FSH and LH due to the binding of pituitary receptors, after which these receptors are desensitized, with a subsequent decrease in FSH and LH secretion.49 This results in decreased estrogen production.
Gonadotropin-releasing hormone agonists have been shown to directly inhibit local aromatase p450 expression in leiomyoma cells,50 thereby presumably resulting in decreased local conversion of circulating androgens to estrogens within the leiomyocyte. Several studies have concluded that GnRH agonists can directly induce apoptosis and also suppress the cellular proliferation of myomas, presumably via action on peripheral GnRH receptors.
Maximum reduction of the mean uterine volume occurs within 3 months of GnRH agonist administration. The decrease in volume is usually in the range of 40% to 80%. However, after the discontinuation of GnRH agonists, myomas will rapidly grow back to their pretreatment size, usually in the span of several months.51
Advantages of GnRH agonists include their use in the perimenopausal transition with add-back therapy for the goal of avoiding hysterectomy. Additionally, laparoscopic myomectomy may be made more feasible with GnRH agonist pretreatment, and GnRH agonists can also be beneficial in a patient who is to undergo hysterectomy to facilitate a vaginal approach rather than an abdominal incision. In a randomized clinical trial comparing the study group (patients receiving GnRH agonist and iron) to a control group (iron alone), preoperative hematologic parameters were improved.52
Although decreased tumor bulk and a decrease in associated symptoms are attained, the potential for unwanted long-term side effects exists; therefore, treatment with GnRH agonist is recommended for no more than 6 months. Common side effects include hot flashes, vaginal dryness, headache, and mood swings. Most importantly, in terms of bone health status, there is a recognized decrease in bone mineral density during therapy.53
Although add-back doses of steroidal hormones can be used with the aim of decreasing this bone loss, the long-term use of GnRH agonists with add-back is impractical and not recommended, especially in younger patients. Add-back therapy with progestins alone will impede the effectiveness of the GnRH agonist in reducing uterine size. In a randomized crossover study, patients were assigned to GnRH agonist alone versus GnRH agonist and a progestin. Uterine volume decreased to 73% of baseline if the agonist was used alone. When the progestin was added the uterine volume increased toward baseline. If the progestin was introduced when the GnRH agonist was started, the uterine volume did not decrease. This effect is attributed to the direct effect of progestins on the leiomyoma.54
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








