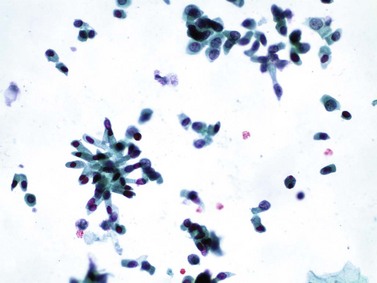
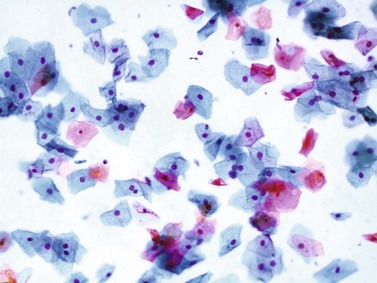
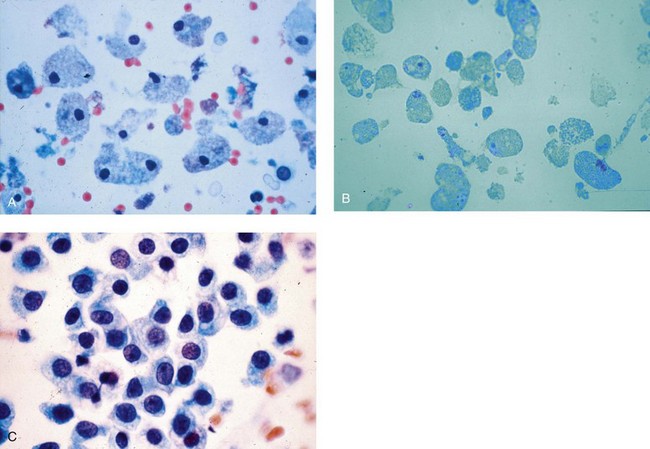
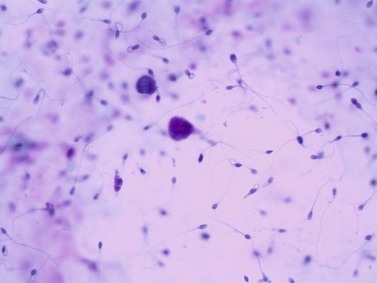
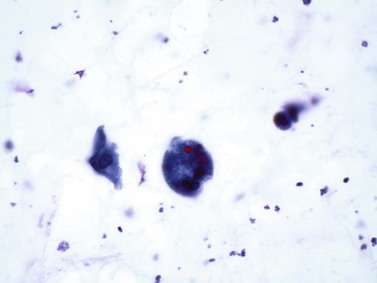
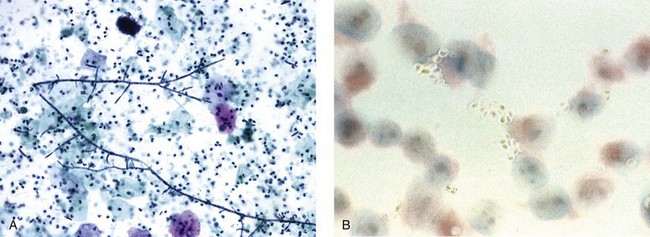
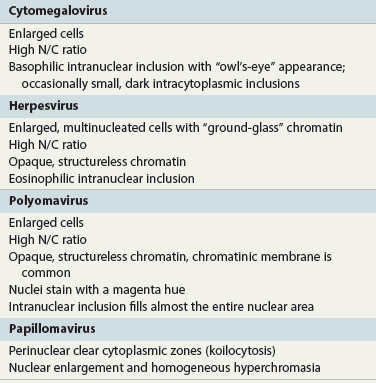
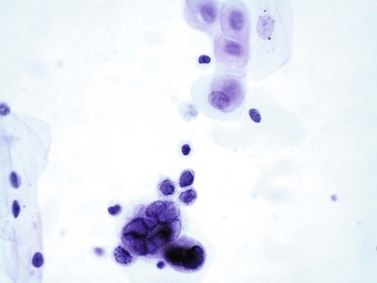
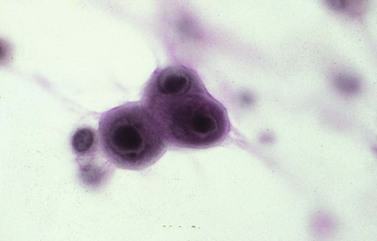
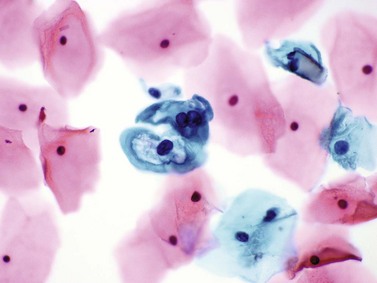
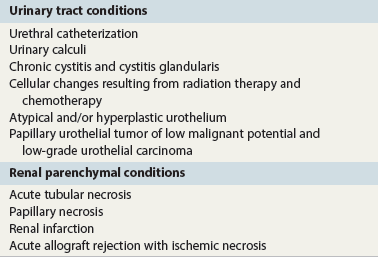
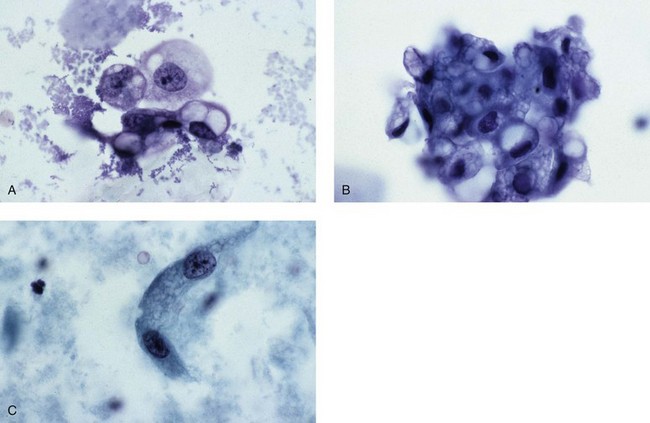
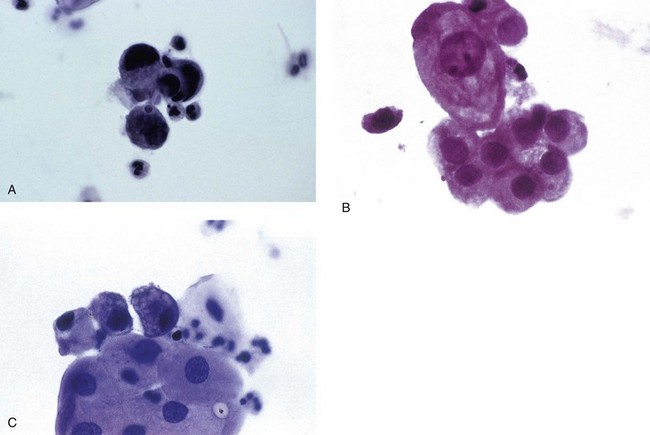
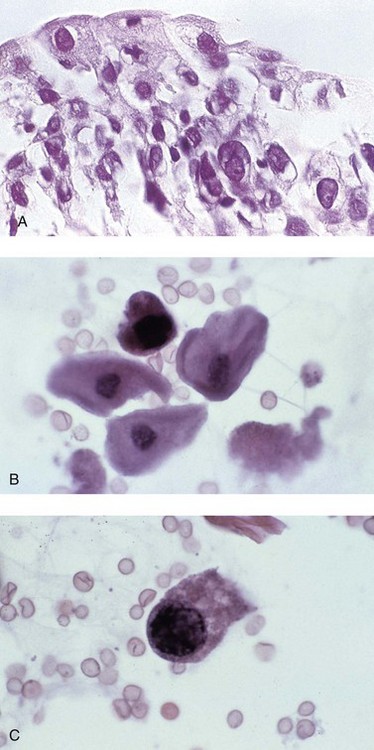
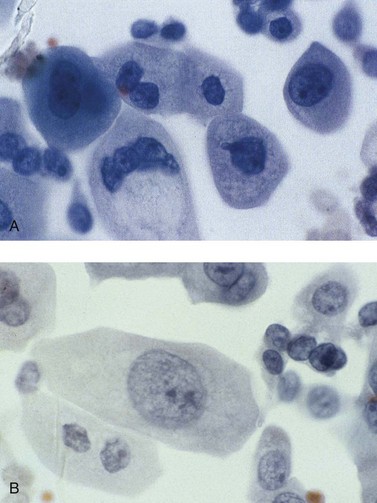
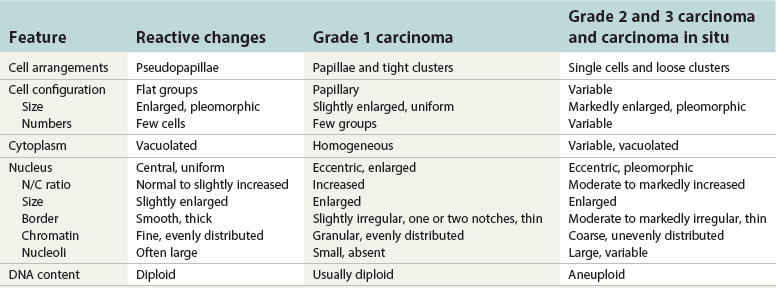
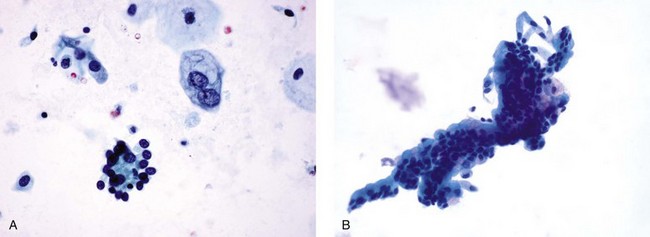
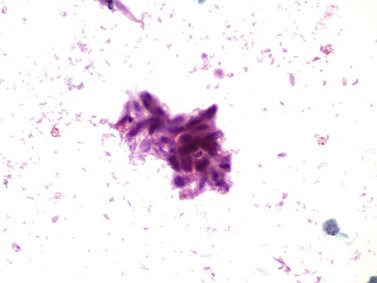
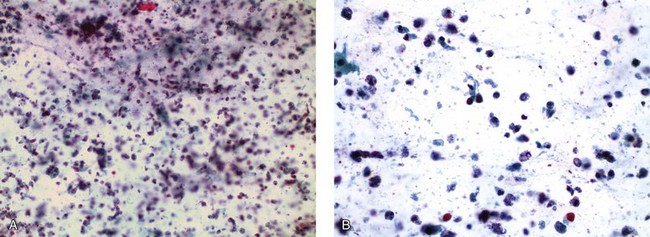
Chapter 7 Normal components of the urinary sediment Noncellular components of the urinary sediment Blood group antigens and other tumor markers detected by immunohistochemistry HER2 and high-molecular-weight cytokeratin Tyrosine-phosphorylated proteins Examination of urine is one of the oldest medical tests, used by Sumerians, Babylonians, Egyptians, Indians, and Greeks in their traditional medicine. It was not until Papanicolaou published his article in Science in 1945 that urine cytology was widely used to detect urothelial carcinoma.1 Subsequently, Koss and Melamed characterized urine cytology and histology in 1960.2–5 This chapter discusses the spectrum of cytologic abnormalities in voided urine samples and washings to allow comparison with the biopsy findings described in Chapters 5 and 6. The clinically significant and common problem of hematuria is also addressed from the perspective of the cytopathologist. Cytologic examination of the urine sediment is of value in the diagnosis of a wide variety of benign and malignant diseases of the bladder, urethra, ureter, and kidney.6–9 The principal indications for the use of cytology in disorders of the urinary tract include the following: 1. Diagnosis of carcinoma in situ, urothelial dysplasia, and high-grade carcinoma 2. Follow-up of patients with urothelial tumor, regardless of grade 3. Monitoring of patients with urothelial tumor undergoing or following treatment7,10,11 4. Evaluation of hematuria, including separation of kidney (upper tract disease) and nonkidney (lower tract) causes Over the decades, several reporting schemes for urine cytology have been published in the literature, each of which has relative strengths and weaknesses. Unlike cervical cytology, there has not been widespread acceptance and use of any particular reporting scheme for urine cytology studies. Thus terminology and criteria for urine cytology reporting are not uniform among pathologists.12 The major diagnostic categories that we use at our laboratory are presented in Table 7-1. Table 7-1 Cytologic diagnostic categories in urine sediment *These two atypical categories can be combined into a single “atypical” category; the predictive value for cancer is only slightly higher in our experience with the “favor neoplasm” than with the “neoplasm should be considered” category. †In addition, evaluation of urinary sediment in patients with hematuria allows separation of kidney (upper tract disease) and nonkidney (lower tract) causes. The sources of urologic cytology specimens include voided or randomly voided urine, catheterized urine, bladder washing (barbotage),13 brushing,14 ureteric and renal pelvic brushing and washing, and neobladder urine from an ileal conduit or colonic pouch. Collection methods are significantly associated with specimen adequacy and cell count.15 Regardless of the type of sample and collection technique used, superficial urothelial cells are a common component of the urine sediment. These cells have one or more nuclei that are large, measuring up to 3 µm in diameter, comparable to superficial squamous cells (Fig. 7-1, A).7 Binucleate and multinucleate cells are common. Such cells are often larger than the mononucleate superficial cells, and their nuclei are somewhat smaller. Large multinucleate superficial cells are by far the most striking component of the urinary sediment, particularly in washings or brushings of the bladder or ureter. Multinucleate superficial cells are particularly large and may be mistaken for giant cells. A potential error in diagnosis is misinterpretation of large superficial cells as macrophages or tumor cells. The DNA content of superficial cells may be polyploid.16,17 Fig. 7-1 Normal superficial (umbrella) cells. A, Superficial cells in voided urine. B, Deeper layer cells (parabasal cells). Epithelial cells smaller than the superficial cells that are derived from the deeper layers of the urothelium often exfoliate in clusters, particularly if the specimen was obtained with an instrument. Single small urothelial cells are observed in voided urine. Clusters of urothelial cells may be tightly packed and assume spherical “pseudopapillary” configurations with sharp borders. Such clusters are often misinterpreted as low-grade papillary carcinoma.18,19 When the deep cells are removed by an instrument, they often appear in loose clusters. These cells are polygonal or elongate, sometimes columnar, and almost always display cytoplasmic extensions in contact with other cells. The amount of basophilic cytoplasm in such cells depends on the layer of origin, and this cytoplasm is more abundant in cells derived from superficial layers. Single cells resemble parabasal squamous cells in size and configuration. These cells are often spherical or round, particularly in voided urine, but they may also show cytoplasmic extensions.7 The nuclei of the smaller urothelial cells are approximately the same size, measuring approximately 5 µm in diameter (Fig. 7-1, B). They are usually finely granular and benign appearing, with one or rarely two small chromocenters. In voided urine, the nuclei may be pale or opaque and occasionally somewhat darker. Columnar urothelial cells are common, particularly in specimens obtained through instrumentation.20 Columnar cells often derive from cystitis cystica or the urethra. They can be single or in small groups, often with a tail by which they are attached to the basement membrane (Fig. 7-2). Squamous cells of varying size and degrees of maturation are common in urine sediment, particularly in voided specimens (Fig. 7-3). Such cells are more abundant in female than male patients.7 In women, these cells originate in the urethral squamous epithelium and in the trigone of the urinary bladder, and they are usually glycogenated. Voided urine sediment may also contain squamous cells derived from the vulva, vagina, or uterine cervix. In men, the origin of the squamous cells is the terminal portion of the urethra or, in rare cases, a vaginal type of squamous metaplasia. Among the benign squamous cells, one may see superficial cells, intermediate cells, and smaller parabasal cells. Navicular cells are intermediate squamous cells with abundant cytoplasmic glycogen content and peripheral nuclei; these cells stain yellow with Papanicolaou stain. Such cells may be observed during pregnancy and in early menopause, and sometimes they are noted in women or men receiving hormonal therapy (androgen deprivation therapy for prostate cancer). Squamous cells may also be anucleate and fully keratinized. In such cases, these features should be reported in the diagnosis because the presence of such “ghost” cells may be of considerable significance, representing leukoplakia or squamous cell carcinoma of the bladder.6 Cells derived from renal tubules sometimes appear in the urine sediment. These cells are small and usually poorly preserved, with pyknotic, hyperchromatic, condensed, spherical nuclei, and granular eosinophilic cytoplasm. Occasionally, the tubular cells form small clusters or casts. The significance of tubular cells in urine sediment remains uncertain. In patients following kidney transplant, the presence of renal tubular cells may indicate rejection of the allograft.21 Proximal tubular cells in urine are easily identified by their large size (20 to 60 µm in diameter), their irregular, elongate, or cigar-like appearance, and their coarsely granular basophilic cytoplasm (Fig. 7-4, A). Cytoplasmic borders are indistinct and may be ragged or torn. The granular cytoplasm contains large numbers of mitochondria ultrastructurally. Nuclei are slightly larger than erythrocytes and may occasionally be multinucleate. Interestingly, proximal and distal tubular cells appear singly, not in fragments or clusters. These cells are often mistaken for granular casts in unstained bright-field microscopy. Proximal and distal renal tubular cells slough from their basement membranes and can be found in urine as intact preserved cells or as ghost or necrotic forms that retain their size and cytoplasmic characteristics (Fig. 7-4, B). Renal tubular cells lining the proximal and distal collecting ducts are small (12 to 18 µm diameter), and each cell contains a single slightly eccentric nucleus with coarse and evenly distributed chromatin. An occasional nucleolus may be present because these cells may be reactive, with prominent nucleoli, but they are never multinucleate. The cytoplasm is polygonal to columnar, finely granular, and uniformly basophilic, with distinct borders (Fig. 7-4, C). Vacuolization may occasionally be seen, especially in reactive states. The cells may phagocytize castlike material, crystals, and pigments. Occasionally, cells of prostatic and seminal vesicle (Fig. 7-5) origin may be present in the urinary sediment. Such cells accompany spermatozoa and are common after prostatic massage.22,23 Erythrocytes are a frequent component of the urinary sediment, particularly in patients with clinical evidence of hematuria (see later).6 Polygonal transparent crystalline precipitates of urates are common in voided urine. Their presence results from changes in the acidity of urine after collection, but it has no diagnostic significance. Crystals derived from true uric acid are exceedingly rare. Other crystals are very rarely of diagnostic value.24 Voided urine and occasional specimens obtained by instrumentation may contain contaminants and renal casts. A wide variety of bacteria may affect the epithelium of the urinary tract. Most are coliforms and other gram-negative rods. Cystitis may be acute or chronic. Acute cystitis is usually associated with symptoms that rarely require confirmatory tissue biopsy or cytologic examination. In those cases in which urine is studied, the sediment may contain numerous exfoliated urothelial cells, necrotic material, and inflammatory cells, with a predominance of neutrophils (Fig. 7-6, A). Marked necrosis and inflammation may also occur in the presence of necrotic tumors, particularly high-grade urothelial carcinoma and squamous cell carcinoma. Fig. 7-6 A, Acute cystitis, consisting of marked inflammation, degenerate urothelial cells, and scattered superficial cells. B, Necrosis and macrophages in tuberculosis of bladder. C, Acid-fast bacilli in urine (Ziehl-Neelsen stain). The urinary sediment in chronic cystitis usually contains a background of chronic inflammation with macrophages and erythrocytes.6 Urothelial cells may be abundant and poorly preserved and may occasionally form small clusters. The cytoplasm in these cells tends to be granular and vacuolated; when the cells are degenerate, the cytoplasm contains spherical eosinophilic inclusions (Melamed-Wolniska bodies) (Fig. 7-7).25 These may have slight nuclear enlargement and hyperchromasia, but the contours of the nuclei are usually regular, and the chromatin texture is finely granular without the coarse granularity of urothelial cancer cells. There may be necrosis of urothelial cells, with nuclear pyknosis and marked cytoplasmic vacuolization. In ulcerative cystitis, large sheets of urothelial cells may be observed. Interstitial cystitis, a form of chronic cystitis associated with chronic inflammation, displays nonspecific cytologic changes.7 Eosinophilic cystitis has a predominance of eosinophils, a pattern that may be seen in patients with allergic disorders or previous biopsies, as well as following mitomycin C treatment.26 Tuberculous cystitis may be observed in patients with AIDS and those receiving treatment for urothelial carcinoma with bacille Calmette-Guérin (BCG). In such patients, the urine or bladder wash shows inflammatory cells and necrosis (see Fig. 7-6, B); rarely, it contains fragments of tubercles consisting of clusters of elongate, carrot-shaped epithelioid cells, sometimes accompanied by multinucleated Langhans-type giant cells, and reactive atypia of urothelial cells.27–29 Ziehl-Neelsen staining may reveal acid-fast bacilli (see Fig. 7-6, C). The sediment or bladder biopsy occasionally contains “decoy” cells with glassy hyperchromatic nuclei.28 Similar findings may occur in patients with tuberculosis of the bladder. Fungi occasionally affect the lower urinary tract, particularly the urinary bladder. Candida albicans, the most common fungus, is usually seen in pregnant women, diabetic patients, and persons with impaired immunity such as patients with AIDS, those undergoing chemotherapy for cancer, and bone marrow transplant recipients. In urinary sediment, the fungi may appear as yeast forms, with small oval bodies, or pseudohyphae, and oblong branching nonencapsulated filaments (Fig. 7-8). Other fungi are uncommon, including Blastomyces dermatitidis, Aspergillus, and Mucor. A fungus of the species Alternaria is a common laboratory contaminant.7 Several important viruses cause significant morphologic changes in the urothelial cells, many of which may be confused with malignancy. The dominant feature of viral infection is the formation of nuclear and cytoplasmic inclusions (Table 7-2). Herpes simplex is an obligate intracellular virus, and florid infection with permissive replication of the virus causes abnormalities in urothelial cells that are readily recognized. In the early stages of viral replication, the nuclei of infected cells appear hazy with a ground-glass appearance. Multinucleation is commonly observed in such cells. Multiple nuclei are often densely packed, with nuclear molding and tightly fitting contoured nuclei (Fig. 7-9). In later stages of infection, the viral particles concentrate in the center of the nuclei and form bright eosinophilic inclusions with a narrow clear zone or halo at the periphery. Infected cells may contain single or multiple nuclei.7,24 Cytomegalovirus is usually seen in newborn infants with impaired immunity. The infection is common in adults with AIDS. The characteristic changes are readily recognized in the urinary sediment, including large cells with large basophilic nuclear inclusions surrounded by a large peripheral clear zone (Fig. 7-10). A distinct outer band of condensed nuclear chromatin is evident. Polyomavirus infection is widespread, according to serologic studies of adults. The occult virus can become activated and recognized in voided urine sediment. One form of polyomavirus, the BK virus, plays a major role in urine cytology because it produces cell abnormalities that may be readily confused with cancer; these cells are also known as “decoy cells” (Fig. 7-11, A).30 In permissive infections, the BK virus produces large, homogeneous, basophilic nuclear inclusions that occupy almost the entire volume of the nuclei.31,32 Occasionally, a narrow rim of clearing separates the inclusion from the chromatinic rim. The infected cells are often enlarged, and they usually contain a single nucleus, but binucleation and occasional large multinucleated cells may be seen.33 The cytologic picture in some cases may be quite dramatic and has led to misdiagnosis of carcinoma.34 We found that decoy cells do not exhibit aneuploidy by fluorescence in situ hybridization (FISH), and acid hematoxylin stain appears to be superior to Papanicolaou stain in identifying and confirming the presence of polyomavirus infection (Fig. 7-11, B).35 Urine cytology is an effective screening method for monitoring renal transplant recipients; it has high sensitivity and a high negative predictive value and can therefore be used routinely in the follow-up of these patients.36 Fig. 7-11 Decoy cells in polyomavirus infection. A, These may be mistaken for malignant cells. B, Nuclear details of polyomavirus infection (acid hematoxylin stain). More than 70 types of human papillomavirus have been identified, and types 6 and 11 are associated with condyloma acuminatum. Condyloma may also appear in the urethra and invariably induces koilocytosis. Urothelial carcinoma exhibits a low incidence of human papillomavirus types 16 and 18 infection (Fig. 7-12).37 The most important of these parasites is Schistosoma haematobium (Bilharzia). The two important cytologic manifestations of infection with S. haematobium are recognition of the ova and the malignant tumors that may be associated with it.26 The ova are elongate structures with a thick, transparent capsule and a sword-shaped protrusion known as the terminal spine located at the narrow end of the ovum. Fresh or calcified ova may be readily recognized in the urinary sediment. The embryonal form of the parasite, known as miracidium, is released in human stool and urine, and it retains the shape of the ovum with its terminal spine. Other common intestinal parasites that affect the bladder include Ascaris lumbricoides, Enterobius vermicularis, and agents of filariasis. Numerous reactive changes involving the urothelium may be mistaken for malignancy (Table 7-3). Approximately 40% of patients with calculi have abnormal cytologic findings in voided urine.18 These patients have numerous large, smooth-bordered clusters of benign urothelial cells with an abundance of superficial cells (Fig. 7-13, A). These changes may overlap with the spectrum of findings with low-grade urothelial carcinoma, but the cells tend to cluster, with fewer single cells.18 Calculi are abrasive to the mucosa when present in the renal pelvis, ureter, or urinary bladder, and the resultant cytologic specimens closely resemble the effects of instrumentation. Significant atypia of urothelial cells resulting from lithiasis is uncommon,7 and the clusters have smooth borders (Fig. 7-13, B and C). Nonetheless, lithiasis remains a major diagnostic pitfall in urine cytology interpretation. Intravesically administered agents and drugs, including BCG (see the earlier section on bacteria), Mitomycin C, and thiotepa, are commonly used for treatment of primary and recurrent bladder tumors (Figs. 7-14 and 7-15). They may induce cell enlargement, cytoplasmic vacuolization, and other reactive changes. Intravesical chemotherapy can contribute to false-positive results in urine cytology.38 Fig. 7-15 Thiotepa-induced changes, including urothelial detachment with nuclear atypia and cytoplasmic vacuolization. Systemically administered drugs such as the alkylating agents cyclophosphamide and busulfan have a marked effect on the urothelium, by inducing significant cytologic abnormalities (Fig. 7-16). These drugs may cause changes that include bizarre urothelial cells with marked nuclear and nucleolar enlargement, thus mimicking poorly differentiated carcinoma.7,39,40 Large doses of cyclophosphamide have been shown to induce urothelial carcinoma, leiomyosarcoma, and carcinosarcoma.41,42 Radiation therapy typically induces marked cell enlargement (most reliable criterion), with bizarre cell shapes and vacuolated nuclei, polychromatic cytoplasm, and sometimes multiple nucleoli (Fig. 7-17). These findings may persist for years after treatment.7 Clinical history is essential to diagnosis. Degenerating cells with pyknotic, crenated nuclei are often a source of concern in urine cytology caused by inflammation, stone, and trauma, for example. Although these changes mimic malignancy, the chromatin is usually smudged and degenerated (Fig. 7-18), in contrast to the cancerous cells, in which the chromatin is crisp and distinct. Large numbers of superficial cells and intermediate cells can be seen in catheterized urine, bladder washings, and brushings (Fig. 7-19, A). Small pseudopapillae, cellular enlargement, and pleomorphism with large nucleoli can be intimidating features (Fig. 7-19, B), but careful examination of the entire sample may be helpful for distinguishing reactive atypia from malignancy (Table 7-4). Marked cellular spindling is common after laser coagulation of the bladder. The spindled cells occur singly, in loose clusters, and in lamellar stacks, and they have elongate nuclei with dense chromatin and bipolar cytoplasm (Fig. 7-20). Cytologic interpretation should not be undertaken during the immediate posttreatment period.43 The samples are dominated by degenerated glandular cells. The nuclei are usually dense and hyperchromatic secondary to degeneration. Urothelial cells are usually sparse or absent. Eosinophilic cytoplasmic inclusions (Melamed-Wolniska bodies) are common (Fig. 7-21). Debris, cytoplasmic fragments, granular deposits, bacteria, few inflammatory cells, and red blood cells are seen in the background.44,45 Fresh specimens should be examined; urine from a collection bag is unsatisfactory for cytologic examination.
Urine cytology
Utility of urine cytology
Indications

Types of cytology specimens
Normal components of the urinary sediment
Superficial (umbrella) cells
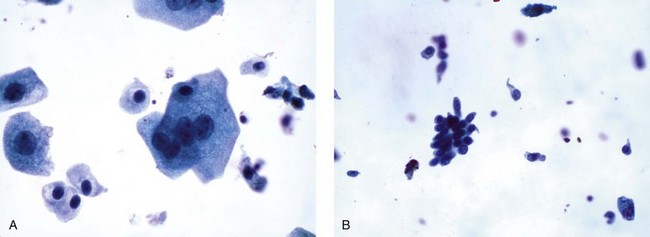
Cells originating from the deeper layers of the urothelium
Columnar cells
Squamous cells
Renal epithelial cells
Convoluted tubular cells
Collecting duct cells
Other benign cells
Noncellular components of the urinary sediment
Diagnostic criteria
Inflammatory processes
Bacteria
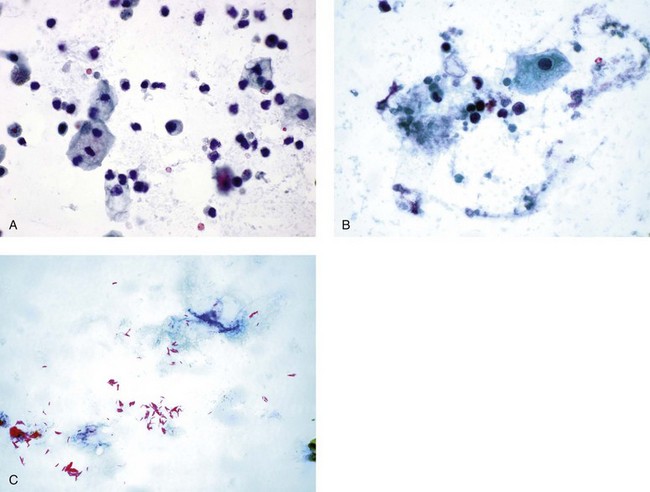
Fungi
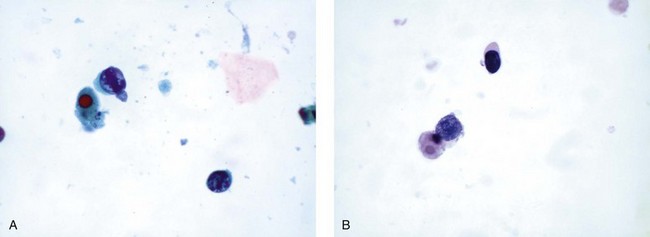
Viruses
Trematodes and other parasites
Reactive cytologic changes
Lithiasis
Drug effects
Effects of radiation therapy
Degenerative changes
Instrumentation atypia
Laser-induced changes
Neobladder and ileal conduit urine
Urine cytology