Mild
Moderate
Severe
Shields
Depend® Shields for Men
Prevail® Belted Shields
Guards
TENA® MEN™ Protective Guards—Level 1
Prevail® Male Guards
TENA® MEN™ Protective Guards—Level 2
Depend® Guards for Men
TENA® MEN™ Protective Guards—Level 3
Underwear
Prevail® Boxers for Men
Prevail® Per-fit® Underwear
TENA® MEN™ Protective Underwear (Super Plus, (Plus and Extra) Absorbency
Depend® for Men Underwear
With FIT-FLEX™ Protection
Briefs
TENA® Classic Briefs
Depend® Real Fit® Briefs for Men
Pad/pants systems
TENA® Day (Light, Regular, Plus) and Night Super Pads
Prevail® Pant Liners
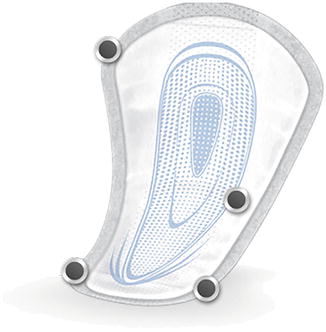
Fig. 3.1
Male shield
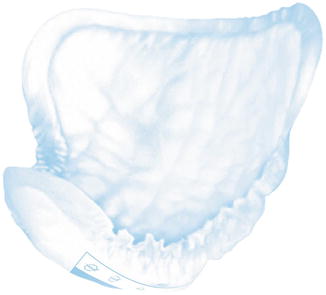
Fig. 3.2
Male guard

Fig. 3.3
Male brief type garment (diaper configuration)

Fig. 3.4
Male absorbency underwear garment
In the individual patient, absorbent products alone may constitute a long-term management strategy. However, it has been demonstrated that the use of even one pad per day is a source of bother and decreased patient satisfaction [5]. Additionally, the use of pads may be associated with skin irritation and dermatitis, especially in the intertriginous areas. In those who need to change their pad or garment more than several pads per day, financial considerations may influence the ability to change pads in a timely fashion. Therefore, it is important to ensure that the patient is utilizing the most effective product based on their degree of incontinence.
Occlusive Therapy (Penile Clamps)
Occlusive devices may function as a stand-alone therapy for incontinence or as an adjunct to absorbent products. Combination therapy between the two types of devices decreases the number of pads required during active periods with a resultant decrease in incontinence products expenditure. Penile clamps, while somewhat unsophisticated, are very effective when utilized properly. Satisfaction with this device is generally dependent on the ability to tolerate the penile compression in the mid-shaft. One of the most commonly used products of this type is the Cunningham clamp (Fig. 3.5). The clamp is available in three sizes (juvenile, regular, and large) corresponding to shaft length. Patients must be instructed to release the clamp every 2 h to allow for circulation regardless of whether it coincides with a void attempt. Additionally, the clamp should not be left on the phallus overnight due to the risks of constant pressure. Mechanical compression devices are not suitable for patients with memory deficits, poor manual dexterity, impaired sensation or a significant component of overactive bladder.


Fig. 3.5
Cunningham penile clamp
Clamp type devices are available in three general styles: (1) Padded Straight, (2) Circular, and (3) Pouch (Table 3.2). The pouch type (Acticuf®) is distinguished by the addition of an absorbent pouch attached to a spring action clamp (Fig. 3.6a, b). One disadvantage of this model is the inability to customize the degree of compression. Additionally, device usage is defined by the limits of the pouch absorbency. Regardless, it is a viable option for patients with intermittent, low volume leakage.
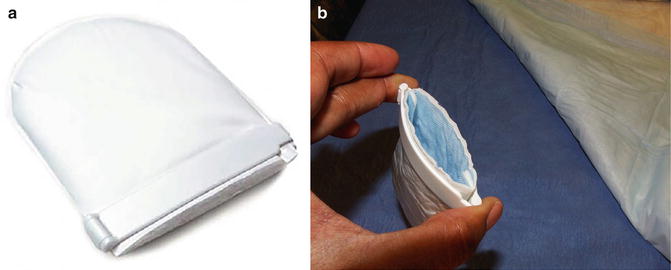
Table 3.2
Mechanical penile compression devices
Name | Manufacturer | |
|---|---|---|
Padded clamp | Cunningham Clamp | BARD |
Dribble Stop | DribbleStop | |
Squeezer Klip | Life Control | |
Circular | J Clamp | Jackson Medical Products |
C3 | Personal Medical Corporation | |
Uriclak | Uriclak | |
U-Tex | ||
Pouch | Acticuf | GT Urological |

Fig. 3.6
(a) Acticuf penile clamp with pouch. (b) Open acticuf
Though simple in concept, penile clamps are not complication free even in appropriately selected patients. Moore et al. compared the Cunningham clamp, U-Tex adjustable strap, and the C3 clamp. While the Cunningham clamp was most successful in decreasing urine loss, it was associated with decreased penile Doppler flow and more discomfort [6]. In general, there are no predictors for which patients will find the clamps to be an indispensable tool versus those who describe it as “weapon of torture.” Therefore, it should be suggested to all incontinent patients who do not have a contraindication.
Catheters (Condom, Urethral and Suprapubic)
Patients with severe or total incontinence may resort to a catheter and drainage system as the best method to obtain complete control of urinary incontinence. This form of management is advantageous when the number or frequency of absorbent product changes is disruptive and/or financially prohibitive.
Condom type catheters or urinary sheaths are an effective method of urinary containment for men with severe incontinence. In comparison to compressive devices, condom catheter systems are acceptable for patients with any degree of urge incontinence. Theoretically, this approach would also be superior to urethral catheterization due to the avoidance of mechanical bladder irritation. However, this management is unsuitable for patients with a retractile phallus, skin excoriation, concomitant urethral stricture, poor manual dexterity, or a large glans/narrow phallus configuration [7]. In the appropriate patient, external catheters have been demonstrated to be superior to absorbent products in patient satisfaction. However, the success of a condom catheter is wholly dependent on proper sizing. The condom or sheath varies based on the material (latex or silicone), length of adhesive surface, circumference and overall length [8]. Ideally, the assistance of a continence nurse is highly desirable to ensure that the patient uses the best type for his anatomy. Alternatively, catheter suppliers will generally forward patients an assortment of condom catheters to allow for selection of the style that maintains an adherent and comfortable fit.
Urethral Catheter Drainage is a decision of last resort in a patient who is unsuitable for alternative management. The need for urethral catheterization may also reflect the presence of an unstable bladder neck due to an intractable stricture. Suprapubic catheter drainage is not an answer for the patient with severe incontinence secondary to an open bladder neck due to continued urethral leakage.
Behavioral Therapy (Pelvic Floor Muscle Exercises, Biofeedback, Pelvic Floor Stimulation, and Lifestyle Adjustments)
Pelvic Floor Muscle Therapy (PFMT)
PFMT is a generic label for exercises meant to strengthen the pelvic floor musculature with resultant decreased leakage during periods of increased intra-abdominal pressure combined with a reflex inhibitory effect on the detrusor body. Levels of instruction may range from simple verbal description to supervised physical therapy sessions. Assessment of the quality and strength of the contractions can arise from direct physical examination or via the use of biofeedback equipment. Pelvic floor muscle therapy programs are highly variable depending on the involvement of a therapist, specific pelvic floor contraction instructions, utilization of biofeedback, time of initiation, and number of exercises (contractions) prescribed per session and daily. A direct comparative analysis of the literature in this area has been complicated by varying exercise protocols and heterogeneous endpoints [9–11].
Basic PFMT initiates with verbal and written instruction in the outpatient setting combined with specific details regarding the number of contractions to be performed daily. While formal PFMT session with a licensed physiotherapist is an asset, it may not be critical to long-term continence outcomes. Moore et al. demonstrated equivalent continence outcomes in patients randomized to a formal PFMT session versus written/verbal instructions with phone support [10]. In this particular study, the self-directed program consisted of 10–12 pelvic floor muscle (PFM) exercises three times per day. In our clinical setting, the patients are given written and verbal instructions to perform 4–5 sets of 15 PFM’s per day. Physical confirmation via digital examination while performing an exercise or consultation with a physiotherapist is reserved for patients with difficulty consistently recruiting the correct muscles and/or poorly sustained contractions.
Initiation of PFMT
There have been several randomized control trials (RCTs) evaluating the utility of starting PFMT prior to prostatectomy with the goal of improving time to continence and overall dry rates [12–15]. As previously stated, comparison of studies is difficult due to heterogeneity in the patient populations, therapeutic protocols and endpoints. Differences in radical prostatectomy technique (open, robotic assisted, and laparoscopic assisted) introduce another source of incongruity between trials. Meta-analysis of RCTs preoperative and postoperative PFMT to postoperative PFMT only has failed to demonstrate a difference in continence between the two groups at 1-month, 3-months, 6-months and 1 year [16]. Due to lack of standardization in PFMT protocols and endpoints, individual studies may demonstrate significant results in a particular endpoint with no basis for comparison. In a non-randomized, retrospective study of 284 open radical prostatectomy patients, Patel analyzed time to continence in men undergoing physiotherapist-guided (PG) PFMT 4 weeks prior surgery versus the control group who received preoperative verbal instruction from the surgeon. At the 6-week time point, significantly fewer subjects in the PG-PFMT were incontinent as measured by a 24 h pad test [17]. A RCT by Centemero in 118 open radical prostatectomy patients, self-reported continence at 1 and 3-months was significantly different in patients who received PF-PFMT 4 weeks prior to surgery versus postoperative only. Additionally, the Quality of Life (QoL) from the International Continence Society male short form (ICSmalesf) was superior in the preoperative intervention group [12]. The available data suggests possible benefit in time to continence and patient satisfaction that are not fully analyzed in meta-analysis due to statistical limitations. However, the long-term benefit of preoperative PFMT has not been proven [18].
Pelvic Floor Stimulation: Electrical Stimulation [Transcutaneous Electrical Nerve Stimulation (TENS), Percutaneous Electrical Nerve Stimulation and Anal Stimulation] and Electromagnetic Therapy
Electrical stimulation (ES) of the pelvic floor is focused on stimulating motor fibers of the pelvic floor. It is unclear whether this solely acts by strengthening the periurethral musculature or whether it is also function as a form of biofeedback [19–21]. Surface electrode placement may be transcutaneous or percutaneous [22–24]. Surface electrodes may be position in multiple locations: perianal, suprapubic, sacral dorsal penile and/or thigh. Percutaneous stimulation is primarily focused on posterior tibial nerve stimulation (PTENS) . Stimulation protocols vary tremendously based on the following parameters: current source, pulse width and duration, current intensity (range), stimulus frequency, pulse shape, time and total number of sessions and rest to work ratio, type of urinary incontinence and type of electrical stimulation [22]. Direct rectal or anal stimulation has been used with the goal of directly simulating the pudendal nerve with elicitation of pelvic floor contraction. This modality is believed to be more efficacious in patients who demonstrated good pelvic floor control [25, 26]. Extracorporeal magnetic innervation (ExMI) was developed to allow for stimulation to the pelvic floor without the placement of surface electrodes or cavitary probes. The energy is delivered while in the sitting position through a padded seat containing the magnetic coils – Neocontrol (Neotonus, Inc., Marietta, GA).
Studies comparing groups of patients treated ES plus PFMT versus PFMT only tend to demonstrate benefit at 3- and 6-months but equivalence between groups at 1 year. Yamanishi randomized between postoperative anal surface electrode stimulation and PFMT versus written/verbal instruction PFMT only in 56 men post open radical prostatectomy. Combination therapy groups had significant improvement in 24 h pad test ICImale SF continence scores and Qol scores. However, these differences were not present at the 12 month time point [25]. Conversely, other series have reported an increased level of adverse events in patient receiving either rectal probe or perianal surface electrode ES [27]. Similar results are mirrored in ExMI literature; Koo randomized patients to ExMI or PMT starting 1 week after catheter removal. The ExMi group demonstrated decreased 24-h pad weight at 1 and 3 months post-op; this difference was no longer present at 6 months. Although the advantage in the ExMI group was not maintained, it does demonstrate a possible shortened time to continence [28].
Based on the failure to identify a clear sustained benefit for electrical stimulation over PMFT with the potential for increased adverse events, we currently do not uniformly utilize these forms of electrical stimulation in our continence rehabilitation program.
Lifestyle Adjustments
Behavioral modifications including diet, fluid management and weight loss have been demonstrated to be effective in significantly reducing daily urine loss [29]. Therefore, patients should be instructed to avoid caffeine and monitor their responses to acidic and spicy foods with the goal of eliminating bladder irritants. In addition to influencing leakage volume, weight has been linked to inferior outcomes of male incontinence procedures (slings and artificial urinary sphincter) [30, 31]. Optimization of comorbid conditions affecting urinary symptoms and incontinence is included in lifestyle modification. Improvement of diabetic management includes more rigid diet control and exercise. However, if the patient is unable to satisfactorily control his sugars with conservative measures collaboration with his endocrinologist or internist is required [32]. Total body conditioning has also gained attention as an adjunct to recovery of incontinence. Adjustment of diuretic timing can be critical to decreasing episodes of nocturnal enuresis. While all of these measures are intuitive and linked to reductions in urinary incontinence, there are no randomized studies testing lifestyle interventions to objective urinary outcomes.
Expectant Management/Watchful Waiting
Although data suggest that incontinence seems to stabilize at the 6-month point after robotic assisted radical prostatectomy, observational studies in the past have shown that a 10–15 % of patients may experience improvement up to 24 months [1, 33, 34]. Therefore, we are careful to ask whether the patient has experienced improvement within the last 4–6 weeks. In those with milder incontinence, continuation of pelvic floor muscle exercises combined with maximal lifestyle modification is a viable option. At any point, the management can be changed to a surgical intervention if the patient’s progress stagnates or the level of bother exceeds his willingness to continue waiting.
Medical Management of Stress Incontinence
The primary management of male stress incontinence, especially severe incontinence, has not been pharmacological. However, there are categories of medications which have been studied for this purpose—alpha-adrenoceptor agonists, Beta 2-adrenoceptor agonists, and serotonin–noradrenaline reuptake inhibitors [35].
α-Adrenoceptor Agonists
Ephedrine, phenylpropanolamine and midodrine have been studied in distant past in mixed gender studies for use in stress urinary incontinence [36–38]. Though the studies were small, there were significant improvements in incontinence. Unfortunately, ephedrine is highly regulated and not approved for use in urinary incontinence. Phenylpropanolamine was pulled from the US market due to the risk of hemorrhagic stroke. Midodrine is available in the USA with approved indication of symptomatic orthostatic hypotension. However, there have been no recent studies evaluating its effectiveness for postprostatectomy incontinence. Anecdotally, the decongestant agents in Zyrtec D® and Seldane D®, pseudoephedrine, can be used on a prn basis for incontinence. However, this has not been evaluated in a clinical trial.
β2-Adrenoceptor Agonists
Clenbuterol is a sympathomimetic amine used by sufferers of breathing disorders as a decongestant and bronchodilator. Although there are two studies evaluating its effectiveness for male stress urinary incontinence, there no randomized clinical trials in male patients [39, 40]. Currently, this agent is not approved for any indication by the FDA.
Serotonin–Noradrenaline Reuptake Inhibitors
The use of imipramine for urinary incontinence stems from initial usage in the pediatric population for nocturnal enuresis and combination agent for neurogenic detrusor overactivity. Studies in post-prostatectomy patients have been limited [41]. Duloxetin e, a selective serotonin (5-HT) and norepinephrine (NE) reuptake inhibitor, decreases stress urinary incontinence by augmenting urethral sphincter contractility [42]. The drug inhibits the presynaptic reuptake of 5-HT and NE at Onuf’s nucleus in the sacral spinal cord resulting in increased in rhabdosphincter activity due to increased postsynaptic receptor stimulation. In 2013, Neff reported on the usage of duloxetine in 94 post prostatectomy patients. While pad usage, IIQ-7 scores and QOL scores were statistically improved, 65 % of patients discontinued the medication due to side effects and/or lack of efficacy. While the agent is approved by the FDA for major depressive disorder, generalized anxiety disorder, fibromyalgia and neuropathic pain; it is not indicated for stress urinary incontinence.
Urethral Bulking Agents
Urethral bulking agents remain the least invasive surgical technique in the treatment of male stress urinary incontinence (SUI). The utilization of materials to improve urethral coaptation evolved from initial application in females for intrinsic sphincter deficiency (ISD) [43]. Development of the perfect injectable agent—durable, efficacious, easily injectable, and safe—is ongoing. Polytetrafluoroethylene (Teflon) was one of the first agents utilized; however, due to its particle size, migration occurred to regional lymph nodes, lungs, and brain. Consequently, Teflon was withdrawn from the market [44]. This clinical experience was utilized to guide the design of future synthetic injectable agents. Secondary to concerns associated with the migration of synthetic compounds, there was a natural interest in harvest and reinjection of an autologous substance. This reasoning formed the basis for the utilization of subcutaneous fat. Although it proved to be technically feasible in female patients, the success rates for the treatment of continence were poor [45].
Irrespective of gender, the most studied urethral bulking agent has been bovine collagen (Contigen®). Although no longer commercially available for urological applications in the USA, it served as the gold standard for efficacy. Currently, the most commonly used synthetic agents for urethral bulking are polydimethylsiloxane (Macroplastique®,Uroplasty, Minnetonka, MN, USA), carbon-coated zirconium oxide beads (Durasphere®,Carbon Medical Technologies Inc., St. Paul Minnesota) and hydroxylapatite spheres in carboxymethylcellulose carrier [Coaptite (Boston, MA, Boston Scientific Corporation)]. Secondarily, materials with primary indications for pediatric vesicoureteral reflux [Deflux (Q-Med, Uppsala, Sweden), Zuidex (Q-Med AB, Uppsala, Sweden)] have been studied for use for in female urethral bulking without demonstrating equivalence to bovine collagen [46]. While the success/improved rates range between 20 and 70 % in women, they are modest at best in male patients. Irrespective of gender, injectable therapy is a consideration in patients who are unable to tolerate or refuse more invasive surgical therapy. In male patients, the best success rates have been described in patients with a high Valsalva leak point pressure, unscarred vesicourethral anastomosis and no radiation history (5, 6) [47, 48]. Each of these agents is discussed in further detail including methods of administration and available literature. No currently available agents have FDA approval for the treatment of male incontinence. However, Macroplastique® and Durasphere™ are approved outside of the USA for this indication.
Assessment
The minimum assessment prior to proceeding with injectable therapy consists of cystoscopic evaluation to confirm suitability of the anastomosis and proximal urethra. Scarred, noncompliant tissue will not accommodate the material resulting in a difficult and ineffective injection. Although urodynamic evaluation is routinely performed in the evaluation of post prostatectomy incontinence, its utility for predicting the success of anti-incontinence therapy has not been proven [49]. However, patients with complaints suggestive of detrusor overactivity and/or detrusor hypocontractility should be evaluated prior to proceeding.
Antimicrobial Prophylaxis
In the past, a 2–3 day course of oral antibiotics was recommended as appropriate course length for injectable therapy [50]. At a minimum, the AUA Best Practice Policy Statement for Antimicrobial Prophylaxis for Urological Procedures recommends a 24-h course of a fluoroquinolone or trimethoprim-sulfamethoxazole for cystoscopic procedures with manipulation [51].
General Technique
Antegrade and retrograde techniques have been utilized for the injection of bulking agents in the male patient. Periurethral and transurethral approaches are described as methods for delivery of injectable materials to the male bladder neck; however, the retrograde transurethral technique is accepted as the standard outpatient clinic approach. Therefore, we describe the use of bulking agents in this context.
Injection Basics
The posterior urethra is made up of four layers histologically; mucosa, lamina propria, muscularis, and adventitia. The lamina propria is the appropriate layer for urethral bulking agent injection as it contains the potential space where the product can be delivered [47]. In the post-prostatectomy male this layer may be obliterated by scar just distal to the anastomosis secondary to prolonged catheterization, urine leak, or retropubic hematoma. All materials require a needle for delivery of the material into the submucosal space. The size of the needle is dependent on the characteristics of the agent. Materials composed of a larger average particle size and higher viscosity will require a larger gauge needle. In general, however, dedicated injection needles range in size from 18 to 22 gauge. A standard rigid cystoscope or an injection scope with a working element can be utilized (Table 3.3). Injection systems are currently available from the following cystoscopic equipment manufacturers: Richard Wolf (Fig. 3.7) and Karl Storz (Fig. 3.8). These systems allow for more precise guidance of the needle using a resectoscope element rather than making manual adjustments via the working channel.
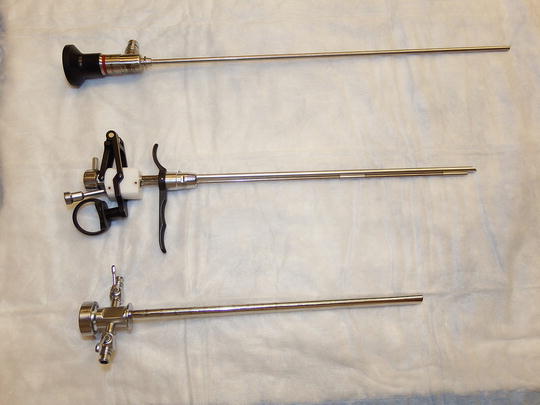

Table 3.3
Injectable agents with equipment specifications
Injectable material | Trade name | Syringe sizes (ml) | Needle (manufacturer, product #) |
|---|---|---|---|
Polydimethylsiloxane | Macroplastique | 2.5 | Rigid endoscopic needle- 3.8 Fr. shaft × 14.5″ (370 mm) long with 20 gauge tip × 0.54″ (14 mm) long (Uroplasty: MRN420) Uroplasty rigid endoscopic needle 5 Fr. shaft × 15″ (380 mm) long with 18 gauge tip × 0.54″ (14 mm) long (Uroplasty: MRN520) |
Carbon coated zirconium oxide | Durasphere | 1 and 3 | Bent spinal tip, 15 in., 18 gauge (Coloplast: 890205) Needle: pencil point tip, 15 in., 20 gauge (Coloplast: 890209) |
Calcium hydroxylapatite (CaHA) and sodium carboxymethylcellulose (NaCMC) | Coaptite | 1 | Sidekick™ needle 14.6 in., 21 gauge (Boston Scientific: M0068903040) |
Glutaraldehyde cross-linked bovine collagen | Contigen | 2.5 | Rigid endoscopic needle—22 gauge (Richard Wolfe) |

Fig. 3.7
Richard Wolf injection scope

Fig. 3.8
Storz injection scope
Preparation for Injection
The patient is positioned in the dorsolithotomy position. The perineum is prepped and draped in a standard fashion. The urethra is infused with 20 cc of 2 % lidocaine jelly and allowed to dwell for at least 10 min after placement of a penile occlusive device. To improve analgesia, 1 % lidocaine solution (0.25–0.5 ml) may injected in the planned injection locations. Attempted re-cannulation of these needle sites for the bulking agent delivery decreases the likelihood of material extravasation from additional punctures in the urethral mucosa. For all agents with the exception of Macroplastique®, it is possible to use the same needle for the local and injectable in series, thus reducing the possibility of creating multiple mucosal defects. Prior to placing the scope, the needle should be primed with local anesthetic and passed through the working channel.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








