Urinary Diversion & Bladder Substitutions: Introduction
Selected patients with lower urinary tract cancers or severe functional or anatomic abnormalities of the bladder may require urinary diversion. Although this can be accomplished by establishing direct contact between the urinary tract and the skin surface, it is most often performed by incorporating various intestinal segments into the urinary tract. Virtually every segment of the gastrointestinal tract has been used to create urinary reservoirs or conduits. No single technique is ideal for all patients and clinical situations. A decision is based on a patient’s underlying disease and its method of treatment as well as on renal function, individual anatomy, and personal preference. An ideal method of urinary diversion would most closely approximate the normal bladder: it would be nonrefluxing, low pressure, continent, and nonabsorptive.
Individual methods of urinary diversion can be categorized in various ways, such as (1) by the segment of intestine used and (2) by whether the method provides complete continence or simply acts as a conduit carrying urine from the renal pelvis or ureter to the skin, where the urine is collected in an appliance attached to the skin surface. Continent forms of urinary diversion can be categorized further according to whether they are attached to the urethra (ie, as a bladder substitute) or are placed in the abdomen and rely on another mechanism for continence (continent urinary reservoir).
Preoperative Counseling and Preparation
All candidates for urinary diversion or bladder substitution should undergo careful preoperative counseling and preparation, including a detailed discussion of the objectives and potential complications of each method. Any potential impact of a procedure on sexual function, body image, and lifestyle should be discussed. Overall satisfaction of most patients undergoing urinary diversion appears to be high (Allareddy et al, 2006; Dutta et al, 2002; Fujisawa et al, 2000a; Hara et al, 2002). However, because they allow freedom from an external appliance, continent forms of urinary diversion, especially bladder substitution, may be of great psychological and functional benefit to well-selected patients (Bjerre, 1995; Okada et al, 1997). More recent data suggest that the differences in quality of life between continent and noncontinent diversion may not be as significant as previously expected (Gilbert et al, 2007).
A careful history taken from the patient should note any previous abdominal or pelvic surgery, irradiation, or systemic disease. A history of intestinal resection or irradiation, renal failure, diverticulitis, regional enteritis, or ulcerative colitis would be especially important to consider when selecting a method of urinary diversion or bladder substitution. A complete blood count and measurement of serum electrolytes, urea nitrogen, and creatinine should be performed. The upper urinary tract should be imaged with intravenous urography, ultrasound, or computed tomography to determine whether hydronephrosis, renal parenchymal scarring, or calculous disease exists. Contrast imaging of the small or large bowel or colonoscopy should be considered preoperatively for patients with a history of significant intestinal irradiation, occult bleeding, or other gastrointestinal diseases. Patients with benign bladder diseases—such as a reduced bladder capacity due to neurologic disorders or irradiation, bladder fistulas, or interstitial cystitis—are occasionally considered candidates for urinary diversion or bladder substitution to manage urinary incontinence; however, with such patients, careful evaluation of bladder function and anatomy is required, as adequate urinary function can often be restored by urinary tract reconstruction, pharmacologic manipulation, or intermittent catheterization.
Patients undergo a standard mechanical and oral antibiotic bowel cleansing program beginning 1 or 2 days before surgery. Recent data suggest that mechanical bowel preparation can be avoided in colon and rectal surgery without increased risk of postoperative infectious complications. It may actually contribute to postoperative ileus. Hence use of clear liquid diet without mechanical bowel preparation prior to surgery may be adequate (Slim et al, 2009). Administration of combined oral and intravenous antibiotics prior to bowel surgery is advised as it appears to reduce the risk of wound infections by as much as 75% (Nelson et al, 2009). Much of patients’ dissatisfaction with urinary diversion can be attributed to poor stomal construction or siting (Fitzgerald et al, 1997). The patient should be preoperatively evaluated in the lying, sitting, and standing positions with their clothes on. The stoma should preferably be located above or below the belt line. The most common site for stoma placement is through the rectus abdominis muscle sheath to avoid the later development of a parastomal hernia. The ideal location is free of creases, folds, previous incisions, and in a location visible to the patient to ensure ability to care for stoma. In obese patients or patients with a short mesentery, the stoma should be placed closer to the level of the umbilicus or in the right upper quadrant.
Intestinal Conduit Urinary Diversion
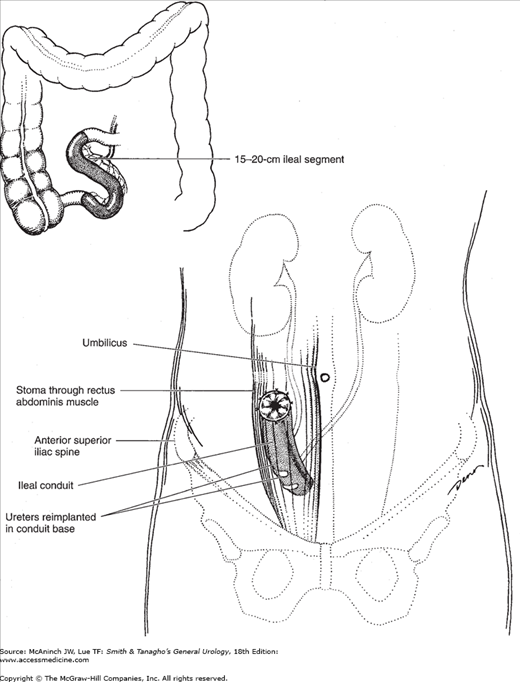
Ureteroileal urinary diversion is the most common method of urinary diversion in the United States. The conduit is constructed using a segment of ileum 18–20 cm long and located approximately 15–20 cm proximal to the ileocecal valve (Figure 25–1). Longer conduits may be required in obese patients, but a short segment minimizes the absorptive surface of the bowel in contact with urine. Once the appropriate length of bowel is selected and isolated, the mesentery is divided proximally and distally and individual mesenteric blood vessels are ligated. The bowel is divided, thus isolating the segment selected for conduit construction. The continuity of the small intestine is reestablished, with the anastomoses being above the plane of the conduit allowing for normal bowel function. The conduit is usually positioned in the right lower quadrant of the abdomen in an isoperistaltic direction. The posterior closed end of the conduit can be sewn with an absorbable suture to exclude the staple line and fixed to the posterior peritoneum or the sacral promontory to prevent volvulus of the conduit. The ureters are reimplanted individually in an end-to-side fashion or joined together (Wallace technique) and anastomosed in an end-to-end fashion, creating a refluxing ureteroileal anastomosis. Ureteral stents (7–8F, single-J, Silastic) are usually placed through the ureteral anastomosis and conduit and into the renal pelvis to facilitate urinary drainage while the anastomosis is healing.
The preselected stoma site is identified and a small circle of skin and underlying fat excised. The fascia is incised in a cruciate fashion. The end of the conduit is brought through the rectus abdominis muscle and anchored to the fascia, and the stoma is then formed. The stoma should protrude, without tension, approximately 1–1.5 in above the skin surface.
Jejunal conduit urinary diversion is used rarely and mainly in cases where other segments cannot be used due to significant ileal and colonic disease caused by previous irradiation or inflammatory bowel disease. Electrolyte disturbances are more common when the jejunum is used for conduit construction and hence this bowel segment is used only if none of the other intestinal segments can be used to fashion a conduit.
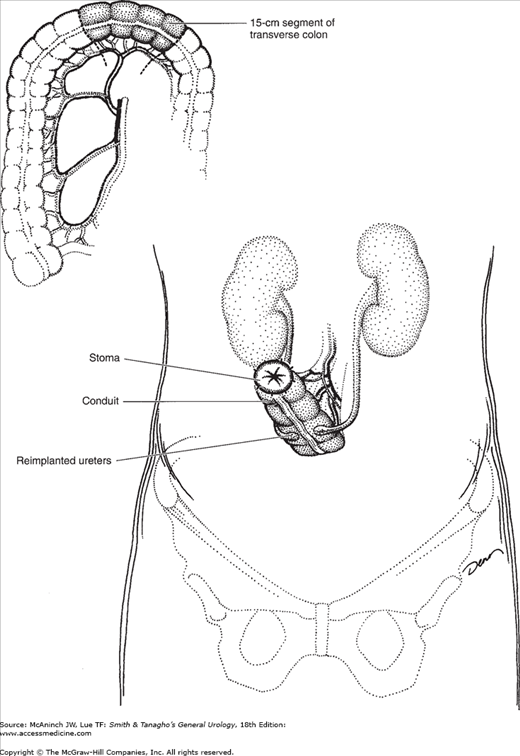
There are several advantages to using the large bowel in construction of urinary conduits: nonrefluxing ureterointestinal anastomoses are easily performed, possibly abrogating the deleterious effects of reflux on the upper urinary tracts (Richie and Skinner, 1975); stomal stenosis is uncommon because of the wide diameter of the large bowel; limited absorption of electrolytes occurs; and the blood supply to the transverse and sigmoid colon is abundant. Either the transverse or the sigmoid colon can be used, allowing for placement of the conduit high or low in the abdomen, depending on the integrity and condition of the ureters. Use of the transverse colon for conduit construction is especially well suited for patients who have received extensive pelvic irradiation or when the middle and distal ureters are absent.
The blood supply of the transverse colon is based on the middle colic artery. The greater omentum is separated from the superior surface of the transverse colon, and a segment of bowel, usually 15 cm in length, is selected for the conduit (Figure 25–2). Short mesenteric incisions are made, and the colon is divided proximally and distally. Once the conduit is isolated, bowel continuity is reestablished. The proximal end of the conduit is closed and fixed in the midline posteriorly. The ureters are brought through small incisions in the posterior peritoneum and reimplanted into the base of the conduit. The stoma may be positioned on either the patient’s right or left side.
A sigmoid conduit is constructed in a similar manner. Great care should be taken to preserve the blood supply by carefully selecting a segment with a good blood supply and by making short mesenteric incisions. The conduit is positioned lateral to the reapproximated sigmoid colon. Ureteral reimplantation and stoma construction are completed.
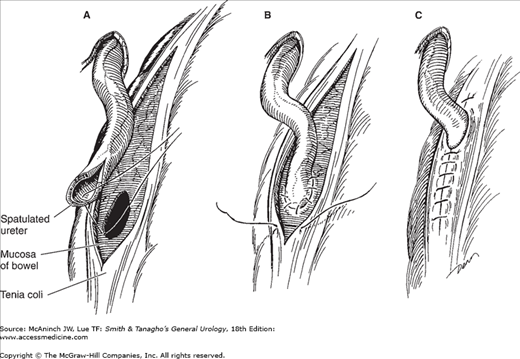
The ureters can be reimplanted into the large intestine either in a nonrefluxing submucosal tunnel or in a refluxing end-to-side manner. A submucosal tunnel can be created by incising the tenia up to the mucosa of the large bowel for a distance of 3–4 cm. A “button” of mucosa is removed, and the ureter is anastomosed to the mucosa of the bowel. The muscularis of the tenia is reapproximated over the ureter to complete the tunnel (Figure 25–3).
Continent Urinary Diversion and Bladder Substitution
Various procedures have been developed for construction of bladder substitutes or continent urinary reservoirs that preclude the need for an external urine collection appliance. Such reservoirs or bladder substitutes are composed of three segments: ureterointestinal (afferent limb) anastomosis, the reservoir itself, and the conduit carrying the urine from the reservoir to the surface (efferent continence mechanism). Bladder substitutes rely on the intact urethra and sphincter to provide outlet resistance and carry urine to the urethral meatus. In men and women whose urethrae are involved by cancer or are not functional owing to benign diseases, an efferent continence mechanism can be constructed with the appx or short segments of tapered, intussuscepted, or reimplanted intestine.
The decision to proceed with bladder replacement is dependent on the risk of urethral recurrence and the continence of the patient. Both men and women who have low risk of urethral recurrence and those who have intact external urinary sphincters should be considered for bladder replacement rather than construction of a continent urinary reservoir. The risk of urethral occurrence or recurrence in men who undergo radical cystectomy is 6.1–10.6%. Carcinoma in situ and tumor multifocality appear to be risk factors for prostatic urethral tumor involvement in men with bladder cancer (Nixon et al, 2002). Although prostatic urethral disease is a risk factor for urethral recurrence, recent evidence suggests that orthotopic diversion may be considered in those with only proximal prostatic urethral involvement and negative urethral margins at cystectomy (Iselin et al, 1997). Although orthotopic bladder replacement was once reserved for men, it is now commonly performed in women as well (Stein et al, 1997). Women with bladder cancer whose tumors are not located at the bladder neck and have a clear urethral margin at the time of cystectomy are candidates for this procedure. Approximately 66% of women undergoing radical cystectomy for the management of bladder cancer fall into this group (Stein et al, 1995, 1998a; Stenzl et al, 1995). Intraoperative inspection and frozen section assessment of the bladder neck limits the risk of urethral recurrence.
The bowel segments should be opened and refashioned (detubularized) to interrupt the normal high-pressure contractions of the intact intestine (Hinman, 1988). A large radius is preferred, as this results in a reservoir with a larger geometric capacity and lower pressure. Continent reservoirs and bladder substitutes may be made of small intestine, large intestine, or a combination of both. Even though bladder substitution is perceived by some as a more complex procedure, prior studies have demonstrated complication and reoperation rates similar to those of ileal conduit urinary diversion in the hands of experienced surgeons (Gburek et al, 1998; Parekh et al, 2000). Long-term results of bladder replacement demonstrate excellent functional outcomes (Abol-Enein and Ghoneim, 2001; Elmajian et al, 1996; Hautmann et al, 1999; Stein et al, 1997; Stenzl et al, 2001; Steven and Poulsen, 2000). Daytime continence can be expected in 87–100% of men and 82–100% of women. Nighttime continence is achieved in 86–94% of men and 72–82% of women. Nearly all men are able to void to completion, while approximately 25–50% of women require intermittent catheterization to completely empty the neobladder. It is beyond the scope of this chapter to review all techniques and minor modifications; instead, the most common techniques and the general principles of continent diversion are reviewed.
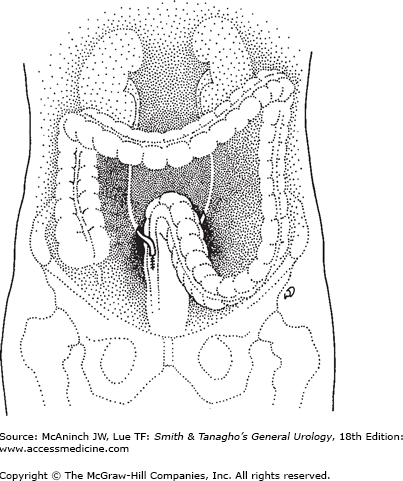
The first direct anastomosis of the ureters into the intact colon was performed by Smith in 1878 (Smith, 1879). Peritonitis (from fecal spillage) and pyelonephritis (resulting from ascending infection and stricturing of the ureteral anastomosis) led initially to very high surgical mortality rates. Recognizing that ascending infection from the rectum into the kidney was a major problem, surgeons developed techniques to reimplant the ureters into the colon in an antirefluxing fashion. Since patients will retain large amounts of urine and fecal material simultaneously in the rectum, assurance of adequate rectal sphincter function is important preoperatively. Since ammonia may be absorbed across the bowel surface, patients with liver disease who may be at an increased risk of hyperammonemic encephalopathy should not undergo this procedure. Patients who have primary diseases of the colon or have received extensive pelvic irradiation should undergo alternative forms of diversion.
The ureters are identified at or below the common iliac arteries. The overlying peritoneum is incised and the ureters are mobilized carefully to preserve their blood supply. A site low in the sigmoid is selected for ureteral reimplantation. The ureters are reimplanted separately into the respective ipsilateral tenia coli, using the antirefluxing techniques described earlier. The peritoneum is sutured over the completed ureteral anastomosis (Figure 25–4).
One particularly worrisome complication of ureterosigmoidostomy is development of adenocarcinoma at the site where the ureters have been reimplanted into the large intestine. The incidence of anastomosis site adenocarcinoma is uncertain, but there seems to be a several thousandfold increase in its incidence in patients who have undergone ureterosigmoidostomy over individuals who have not undergone this type of surgery. The induction period varies but may be approximately 20 years; thus, the risk is especially high in young patients. Experimental studies have shown that the development of adenocarcinoma seems dependent on the presence of urine, feces, urothelium, and colonic epithelium in close approximation. All those who undergo ureterosigmoidostomy should be counseled to undergo yearly sigmoidoscopy starting 5 years after the procedure and at any time occult or gross gastrointestinal bleeding or a major change in bowel habits is noted.
Nils Kock was responsible for the early and ongoing development of a continent urinary reservoir fashioned entirely of small intestine (Kock et al, 1982; Nieh, 1997). This reservoir includes the construction of nipple valves as the proximal and distal ends of the reservoir to prevent ureteric reflux (proximally) and incontinence (distally). The Koch reservoir is no longer commonly used due to a high incidence of complications related to the nipple valves.
In an attempt to decrease the complication rate associated with the afferent intussuscepted antireflux nipple of the Kock ileal neobladder, Stein and colleagues (1998b) have described an innovative antireflux technique (Figure 25–5). This new reservoir, the T pouch, has several important advantages over the Kock ileal neobladder: (1) It requires a smaller segment of ileum to create the antireflux technique, (2) the serosal-lined antireflux mechanism eliminates the need for intussusception, (3) blood supply is better preserved, and (4) urine is not in contact with the implanted portion of the ileum.
Figure 25–5.
Construction of a T pouch. A: Two segments of small intestine are isolated; one portion will form the reservoir, and the smaller, more proximal portion will form the antireflux segment. B: The longer reservoir portion of the segments is folded into a V. The smaller, antireflux segment is fixed to the serosa of the reservoir portion. C: The antireflux segment has been opened and tapered with a stapling device. The ileal segments selected for the reservoir portion of the pouch are joined anteriorly and then opened, exposing the mucosa. As the opening reaches the ostium of the antireflux segment, the incisions are carried laterally and create wide flaps that can then be closed over the ostium to cover the tapered antireflux segment. D: The reservoir portion is closed.
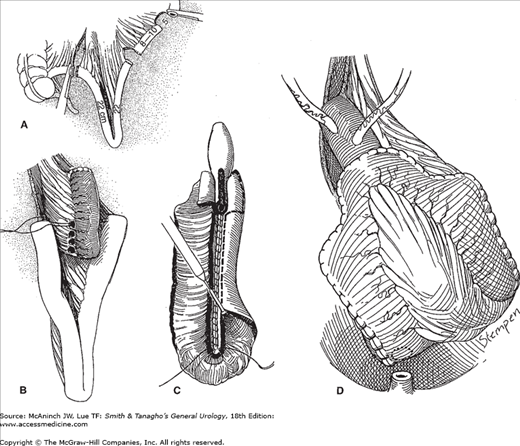
Camey described a technique of bladder substitution whereby an intact segment of ileum was anastomosed directly to the urethra (Lilien and Camey, 1984). A 40-cm segment of ileum was isolated from the gastrointestinal tract, and its midpoint was anastomosed, without tension, directly to the urethra. The ureters were reimplanted into either end of the ileal segment in an antirefluxing fashion. However, failure to detubularize the ileal segment led to a high incidence of urinary incontinence compared with substitutes fashioned from detubularized small-bowel segments (Hautmann et al, 1999; Studer and Zingg, 1997). Forty- to sixty-centimeter segments of ileum can be detubularized and folded into U-, S-, or W-shaped reservoirs, which can be connected directly to the urethra (Figure 25–6
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree