CHAPTER 60 Tumors of the Pancreas
PANCREATIC CANCER
Pancreatic cancer is the second most common gastrointestinal malignancy in the United States, and approximately 37,680 new cases are expected to occur in 2008.1 Despite its relatively low incidence compared with other malignancies, it represents the fourth leading cause of cancer death in men and women (34,290 deaths expected in 2008). Overall, pancreatic cancer carries an unfavorable prognosis. For all stages combined, the one- and five-year relative survival rates are 24% and 5%, respectively.1 Early detection, accurate preoperative staging, and better treatment options remain a challenge.
EPIDEMIOLOGY
Incidence
Pancreatic cancer is very rare before the age of 45 years, but its occurrence rises sharply thereafter. It affects men more than women (ratio of 1.3 : 1), and is more common in blacks. The incidence in black men is 14.8 per 100,000, compared with 8.8 in the general population.2
Populations at Risk
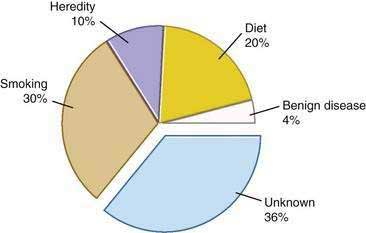
Genetic as well as environmental factors have been found to be associated with the development of pancreatic cancer (Fig. 60-1). Table 60-1 summarizes some of the genetic syndromes associated with an increased risk of pancreatic cancer. One of the most prominent of these syndromes is hereditary pancreatitis, even though it accounts for only a small fraction of pancreatic cancer cases (see Chapter 57). Affected patients have an abnormal trypsin gene that is transmitted as an autosomal dominant trait; their risk for development of pancreatic cancer by age 70 years is estimated at 40%.3 Patients with other, nonhereditary forms of chronic pancreatitis also have a higher likelihood of pancreatic cancer (see Chapter 59). A multinational study found this risk to be 2% per decade, independent of the type of pancreatitis.4
Table 60-1 Historical Features and Germline Genetic Alterations Associated with an Increased Risk of Pancreatic Cancer
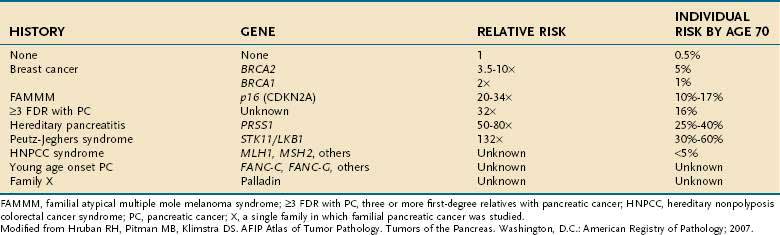
Individuals with familial Peutz-Jeghers syndrome, discussed in Chapter 122, also have an increased risk of pancreatic cancer, with an impressive 132-fold increased risk over the general population.5 Germline mutations in p16 are observed in kindreds with the familial atypical mole-malignant melanoma (FAMMM) syndrome, and these individuals are at risk for pancreatic cancer and melanoma.6 Lastly, patients with BRCA2 gene mutations (which predispose to hereditary breast cancer in women and men), also have a familial predisposition to pancreatic cancer.7
Other unknown genetic abnormalities are yet to be identified. In several population studies, 7% to 8% of patients with pancreatic cancer have a first-degree relative with the disease.8 Patients with three or more first-degree relatives with the disease, have a 32-fold increased risk over the general population; those with two first degree relatives have 6-fold increased risk; and those with one first-degree relative have a 2.3-fold increased risk.9
There are no specific recommendations for screening patients at risk for pancreatic cancer, because available techniques lack sensitivity for detection of very small lesions. The timing and frequency of such screening are also uncertain. The American Gastroenterological Association (AGA) suggests that screening should begin at age 35 in patients with hereditary pancreatitis. Patients with a history of familial pancreatic cancer should begin screening 10 years before the age at which pancreatic cancer has been first diagnosed in their relatives. The AGA also states that such screening is probably best done with spiral computed tomography (CT) and endoscopic ultrasonography (EUS).10,11
Environmental Factors
The most important environmental factor in pancreatic cancer, and possibly the only one that has been firmly established, is cigarette smoking.12 Multiple cohort and case-control studies have found that the relative risk for smokers of developing pancreatic cancer is at least 1.5.13–15 The risk may be particularly elevated in smokers who have homozygous deletions of the gene for glutathione S-transferase T1 (GSTT1), which is a carcinogen metabolizing enzyme.16 Furthermore, the risk rises with the amount of cigarette consumption, and the excess risk level returns to baseline by 15 years after cessation of the habit.15
The second most important environmental factor associated with pancreatic cancer appears to be dietary influences. A high intake of fat or meat has been linked to the development of this neoplasm,17 and a protective effect is ascribed to fresh fruits and vegetables.18 Reduced serum levels of lycopene, a carotenoid present in fruits, and selenium were found in subjects who subsequently had pancreatic cancer.19
Diabetes mellitus is very common in patients with pancreatic cancer. In most cases diabetes has been diagnosed within the preceding two years and may or may not be associated with obesity.20 Thus, recent onset of diabetes may help identify patients with pancreatic cancer, particularly in individuals older than 50 years. Destruction of the pancreas is unlikely to be sufficient to cause endocrine insufficiency in most patients with pancreatic cancer, and it has been proposed that higher production of islet amyloid polypeptide (amylin) by the tumor is responsible for the diabetogenic state. In fact, glucose tolerance frequently improves in patients who have undergone tumor resection.21
PATHOLOGY
As reviewed in Chapter 55, three different epithelial cell types are found in the normal pancreas: (1) acinar cells, which account for about 80% of the gland volume; (2) ductal cells, comprising 10% to 15%; and (3) endocrine (islet) cells, making up about 1% to 2%. More than 95% of the malignant neoplasms of the pancreas arise from the exocrine elements of the gland (ductal and acinar cells) and demonstrate features consistent with adenocarcinoma. Endocrine neoplasms account for only 1% to 2% of pancreatic tumors (see Chapter 32). Nonepithelial malignancies are exceedingly rare.22
The World Health Organization (WHO) has proposed a classification of pancreatic exocrine tumors that is widely used today (Table 60-2).23
Table 60-2 World Health Organization Classification of Primary Tumors of the Exocrine Pancreas
Data from Hamilton SR, Aaltonen LA. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System. Lyon, France: IARC Press; 2000.
Ductal adenocarcinoma accounts for 85% to 90% of pancreatic tumors.22 Autopsy series have shown that 60% to 70% of these tumors are localized in the head of the gland, 5% to 10% in the body, and 10% to 15% in the tail. On gross examination these tumors appear as firm masses with ill-defined margins blending into the surrounding pancreatic parenchyma. The average size of carcinomas in the head of the pancreas is 2.5 to 3.5 cm, compared with 5 to 7 cm for tumors in the body or tail. Differences in tumor size at presentation are related to the earlier development of symptoms and signs in proximal tumors than in distal neoplasms.
Tumors in the head of the gland have a propensity for obstruction of the distal common bile duct and pancreatic duct. Anatomic obstruction of these structures results in jaundice and chronic obstructive pancreatitis. Pancreatic pathologic changes observed include duct dilatation and fibrous atrophy of the pancreatic parenchyma. Some tumors can involve the duodenum or the ampulla of Vater. Extrapancreatic extension into the retroperitoneal tissues is almost always present at the time of diagnosis and can result in invasion of the portal vein or the superior mesenteric vessels and nerves. Neoplasms of the tail of the pancreas do not cause biliary or pancreatic duct obstruction. Extrapancreatic extension in distal tumors causes invasion of the spleen, stomach, splenic flexure of the colon, or left adrenal gland. In patients with advanced disease, metastases to the lymph nodes, liver, and peritoneum are common; the lung, pleura, and bone are less commonly involved.24
Microscopically, ductal adenocarcinomas are graded as well, moderately, or poorly differentiated.22 Well-differentiated tumors show irregular tubular neoplastic glands with mild cellular atypia, low mitotic activity, and significant mucin production. Loss of differentiation results from lack of cellular arrangement into glandular structures, increases in cellular atypia and mitotic figures, and cessation of mucin production. Some studies have demonstrated that histologic grading correlates with survival.25
Several immunohistochemical markers have shown diagnostic usefulness in mucin-producing tumors such as pancreatic adenocarcinoma. Among the better-known markers are MUC1, MUC3, MUC4, carcinoembryonic antigen (CEA), CA 19-9, DuPan 2, and CA 125.22 These markers are unable to differentiate between tumors of pancreatic and extrapancreatic origins, limiting their usefulness in the evaluation of liver metastases of unknown primary. However, they are particularly useful in separating neoplastic from non-neoplastic ductal changes and in distinguishing ductal from acinar or neuroendocrine tumors. Cytokeratins are other useful markers in differentiating among acinar, ductal, and islet cell tumors. Although all ductal adenocarcinomas stain for cytokeratins 7, 8, 18, and 19, most acinar and neuroendocrine tumors do not stain for cytokeratin 7.22
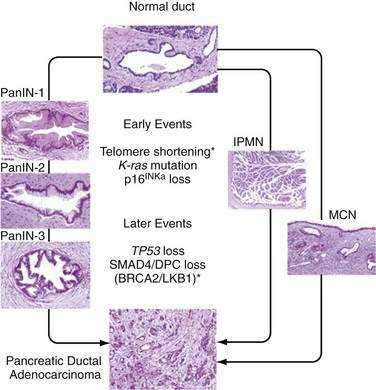
Progression of small pancreatic intraductal lesions to ductal adenocarcinoma has been described, similar to the adenoma-carcinoma model in colorectal cancer.26 These precursor lesions are referred to as pancreatic intraepithelial neoplasia (PanIN). PanINs are microscopic findings that are graded from 1 to 3, the last being equivalent to carcinoma in situ.
Molecular Pathology
Multiple combinations of genetic mutations are commonly found in pancreatic adenocarcinomas. Table 60-3 lists the most common. The accumulation of genetic alterations over time is thought to result in the development of pancreatic cancer. For example, progression of PanIN lesions from grade 1 to 3 has been linked to an increasing number of genetic mutations (Fig. 60-2).27
Table 60-3 Genetic Mutations in Pancreatic Cancer
| GENE (CHROMOSOMAL REGION) | PERCENT OF TUMORS WITH GENETIC MUTATION |
|---|---|
| K-ras (12p)* | >90% |
| p16 CDKN2A (9p)† | >95% |
| PK53 (17p)2 | 50%-70% |
| SMAD4/DPC4 (18q)† | 55% |
| AKT2 (19q)* | 10%-20% |
| MYB (6q)* | 10% |
| AIB1 (20q)* | 10% |
| BRCA2 (13q)† | 7%-10% |
| LKB1/STK11 (19p)† | <5% |
| MKK4 (17p)† | <5% |
| TGF-β-R1 (9q) or TGF-β-R2 (3p) | <5% |
| RB1 (13q) | <5% |
TGF-β, transforming growth factor-β.
Modified from Hruban RH, Wilentz RE. The pancreas. In: Kumar V, Abbas AK, Fausto N, editors. Robbins and Cotran pathologic basis of disease. 7th ed. Philadelphia: Elsevier; 2005.
A long list of oncogenes and their products has been implicated in the pathogenesis of pancreatic cancer.28 Mutations in the K-ras gene are a hallmark of pancreatic adenocarcinoma and appear to be present in more than 90% of tumors. Studies in intraductal papillary mucinous neoplasms (IPMNs) and mucinous cystic neoplasms (MCNs) of the pancreas have shown that the frequency of K-ras mutations correlates with the extent of microscopic dysplasia within tumors.29,30 Evidence suggests that K-ras mutation may be an early genetic event in pancreatic carcinogenesis, but K-ras mutations may be detected even in the setting of chronic pancreatitis without frank neoplasia.31
Loss of function in several tumor suppressor genes has also been found in pancreatic tumors, notably p16 (also called INK4A), TP53, and DPC4 (also called SMAD4). The combination of p16 and K-ras mutations is uncommon in other human tumors and may be a molecular “signature” for pancreatic adenocarcinoma.32 Loss of p16 function is observed in 80% to 95% of sporadic pancreatic cancers; p16 has a critical role in regulation of the cell cycle, and disruption of its function can lead to accelerated cell division and growth.33 Germline mutations in p16 are associated with the FAMMM syndrome, which is characterized by a high incidence of melanoma and pancreatic cancer.34
The most commonly mutated tumor suppressor gene in human cancer, TP53, is also mutated in a high percentage of pancreatic tumors.28,35 Disruption of TP53 function has been linked to alterations in the cell cycle, regulation of transcription, deoxyribonucleic acid (DNA) repair, and apoptosis, leading to the rampant genetic instability that characterizes pancreatic cancer (see Chapter 3). Another tumor suppressor gene involved in pancreatic carcinogenesis is DPC4.36 The current evidence suggests that DPC4 is a key transcription factor involved in the regulation of transforming growth factor-β expression and subsequent growth inhibition.37 Therefore, disruption of DPC4 could have critical effects in cell cycle regulation and cell differentiation.
Besides oncogenes and tumor suppressor genes, other genetic pathways have been implicated in pancreatic carcinogenesis. Up-regulation of epidermal growth factor (EGF) receptors and their ligands has been well established.38 Telomere shortening and dysfunction also has been found.39 More recently, abnormal activation of genes involved in pancreatic organogenesis has been described (see Chapter 55). One study suggests that the sonic hedgehog gene product is abnormally present early as well as late in pancreatic carcinogenesis.40
CLINICAL FEATURES
Most patients with pancreatic cancer experience symptoms late in the course of disease. The lack of early symptomatology leads to delays in diagnosis, and less than 20% of patients present with resectable masses.41 Tumors of the head of the pancreas produce symptoms earlier in the course of disease. In contrast, tumors of the distal gland are characterized by their “silent” presentation, with physical findings appearing only after extensive local growth or widely metastatic disease has developed. Clinical signs and symptoms can offer clues to the resectability of pancreatic tumors (Table 60-4).42
Table 60-4 Demographics and Presenting Symptoms and Signs in Patients with Unresectable (Palliated) and Resected Pancreatic Cancer*
| PALLIATED(N = 256) | RESECTED (N = 512) | |
|---|---|---|
| Demographics | ||
| Age, average (yr) | 64.0 | 65.8 |
| Men/women | 57%/43% | 55%/45% |
| Race | 91% white | 91% white |
| Symptoms and Signs (%) | ||
| Abdominal pain | 64 | 36* |
| Jaundice | 57 | 72* |
| Weight loss | 48 | 43 |
| Nausea/vomiting | 30 | 18* |
| Back pain | 26 | 2* |
* P = 0.001 vs. palliated group.
Modified from Sohn TA, Lillemoe KD, Cameron JL, et al. Surgical palliation of unresectable periampullary adenocarcinoma in the 1990s. J Am Coll Surg 1999; 188:658.
Pain can be a major symptom in many patients with pancreatic cancer. Pain is primarily due to invasion of the celiac or superior mesenteric arterial plexus.43 The pain is of low intensity, dull, and vaguely localized to the upper abdomen. In advanced disease, pain may be localized to the middle and upper back. The pain also may be postprandial and lead patients to reduce their caloric intake, a situation that ultimately results in weight loss or cachexia.
Less common symptoms include those of diabetes and pancreatitis. New-onset diabetes mellitus may herald pancreatic cancer and can be observed in 6% to 68% of patients.44 Acute pancreatitis is occasionally the first manifestation of pancreatic cancer,45 and the clinician must keep this fact in mind especially when dealing with an older adult patient who presents with acute pancreatitis of unclear etiology.
DIAGNOSIS
Computed Tomography
Although transabdominal ultrasonography is frequently the first modality used in many patients with pancreatic cancer (because 50% of them present with jaundice), the method of choice for diagnosis and staging of pancreatic cancer is CT.46–48 The pancreas is ideally imaged by means of the thin-section, pancreatic protocol, helical CT.49,50 In large series, a correct diagnosis of pancreatic cancer using CT can be made in up to 97% of patients.47 Refinements in the CT resolution of peripancreatic blood vessels has rendered routine angiography obsolete for the evaluation of suspected pancreatic masses.46,47
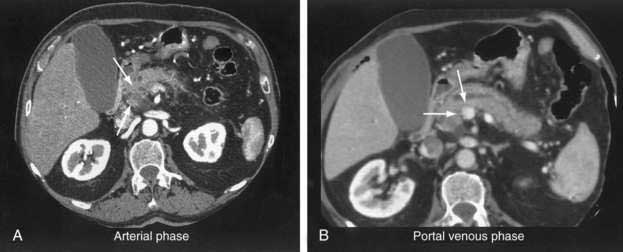
The pancreatic protocol CT consists of dual-phase scanning using intravenous and oral contrast agents. The first, arterial (pancreatic) phase is obtained 40 seconds after administration of intravenous contrast agent. At this time maximum enhancement of the normal pancreas is obtained, allowing identification of nonenhancing neoplastic lesions (Fig. 60-3A). The second, portal venous phase is obtained 70 seconds after injection of intravenous contrast agent and allows accurate detection of liver metastases and assessment of tumor involvement of the portal and mesenteric veins (see Fig. 60-3B).
Current CT criteria for unresectability of a pancreatic tumor are as follows: (1) distant metastasis (e.g., liver, peritoneum, other), (2) arterial encasement of the celiac axis or superior mesenteric artery, and/or (3) occlusion of the portal vein or superior mesenteric vein.46–48 With these criteria, CT has been shown to be almost 100% accurate in predicting unresectable disease.46 However, approximately 25% to 50% of patients predicted to have resectable disease according to these CT criteria are found to have unresectable lesions at laparotomy.46–48 These patients clearly do not benefit from surgical exploration, and their identification by preoperative imaging remains a challenge.
The most common causes of unresectability of a pancreatic tumor are small peritoneal or liver tumor implants and vascular involvement by tumor. The advent of helical pancreatic protocol CT has helped improve the preoperative determination of surgical resectability, particularly in relation to vessel invasion. Later studies have shown that assessment of the degree of circumferential vessel involvement by tumor can help predict unresectability.51–53 Other efforts aimed at detecting small peritoneal and liver metastases beyond the resolution of CT have focused on the development of staging laparoscopy, as discussed later.
Endoscopic Retrograde Cholangiopancreatography
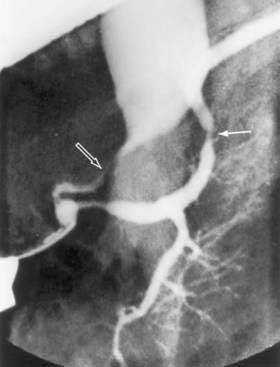
Since its introduction in 1968, endoscopic retrograde cholangiopancreatography (ERCP) has become a mainstay in the differential diagnosis of various tumors of the periampullary region.54 The majority of these tumors originate from the pancreas (85%), and less commonly from the distal bile duct (6%), ampulla (4%), or duodenum (4%). ERCP allows visualization of the pancreatico-biliary tree to distinguish benign (stones) from malignant causes of obstruction. A “double-duct sign,” representing strictures in biliary and pancreatic ducts, is classically found in many patients with pancreatic cancer (Fig. 60-4).
Tissue sampling can also be obtained during ERCP. For tumors of the ampulla or duodenum, biopsy of mucosal lesions is readily obtained with endoscopic forceps. Tumors of the distal bile duct may also be sampled via brush biopsy for routine cytology or genetic analysis.55,56
As discussed earlier, CT allows for identification of pancreatic tumors in the majority of patients with pancreatic cancer, rendering ERCP unnecessary in most cases. In practice, however, many patients with pancreatic cancer undergo ERCP, not for the purpose of diagnosis but rather for stenting of the biliary duct (see Chapters 61 and 70). Routine preoperative biliary duct stenting to relieve jaundice has not been shown to decrease postoperative morbidity and mortality, and the procedure can increase the likelihood of surgical infectious complications.57–59 Therefore, its practice in patients with resectable tumors (as assessed by CT) cannot be recommended, unless it is anticipated that surgery will delayed for more than two weeks. For patients with jaundice and unresectable or metastatic disease, endoscopic biliary stenting, preferably with expandable metal stents, offers excellent palliation.60
Endoscopic Ultrasonography
EUS may be the most accurate test for the diagnosis of pancreatic cancer.61 Several studies have shown that EUS has a higher sensitivity and specificity than CT for detecting pancreatic masses (Fig. 60-5).62,63 EUS also has been found to be more accurate than CT in assessing vascular invasion and predicting tumor resectability. Other advantages of EUS include accurate assessment of peripancreatic nodal disease, and allowance of tumor biopsy by fine-needle aspiration (FNA).64
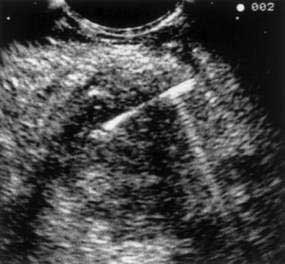
Figure 60-5. Endoscopic ultrasonographic image of pancreatic cancer. The figure shows the needle during biopsy of the tumor.
Limitations of EUS are manifold. EUS is highly operator-dependent, and it is estimated that experience with 100 such examinations is needed to be considered proficient.63 Imaging by EUS can be compromised by the presence of a biliary stent, which results in imaging artifacts and loss of tissue detail. Due to technical and anatomic constraints, imaging of the portal vein and splenic vein is generally superior to imaging of the superior mesenteric artery and vein.65 For this reason EUS may lack accuracy when assessing vascular invasion at the level of the superior mesenteric vessels. Lastly, EUS provides no information regarding metastatic disease, and a complementary CT or magnetic resonance imaging (MRI) scan is required for complete staging of disease.
Magnetic Resonance Imaging
MRI has been increasingly used in the evaluation of pancreatic tumors, and several groups have shown results that rival those of helical CT.66,67 In one study, pancreatic tumor detection was reported in 90% of patients for MRI versus 76% for helical CT.66 Optimal MRI resolution is obtained with T1-weighted images and the use of dynamic gadolinium enhancement. Tumors are viewed as low-signal masses against the high-signal background of normal pancreatic parenchyma. Pancreatic masses, ductal dilation, and liver metastasis can be demonstrated in exquisite detail. Additionally, MR angiography and MR venography techniques using gadolinium contrast enhancement can demonstrate vascular involvement by tumor, obviating conventional angiography. Unlike CT, MRI does not involve radiation and uses an iodine-free contrast agent with rare renal toxicity. Limitations of MRI are related to cost, availability, and clinicians’ familiarity with and predilection for CT imaging.
Magnetic resonance cholangiopancreatography (MRCP) can also be obtained at the time of MRI.68 In a prospective, controlled study, MRCP was found to be as sensitive as ERCP in detecting pancreatic carcinomas.69 MRCP uses heavy T2-weighted images that emphasize fluid-containing structures such as ducts, cysts, and peripancreatic fluid collections. Images obtained are highly comparable with those obtained with ERCP and readily demonstrate pancreatic ductal obstruction, ectasia, and calculi. In contrast to ERCP, MRCP is noninvasive and does not require injection of contrast into the pancreatico-biliary tree, avoiding possible complications such as allergy, pancreatitis, or infection. However, no therapeutic or diagnostic intervention can be performed with MRCP.
Positron Emission Tomography
Positron emission tomography (PET) is a noninvasive imaging tool that provides metabolic rather than morphologic information on tumors.70,71 This diagnostic method is based on greater use of glucose by tumor cells than by normal pancreatic parenchyma. The radioactive glucose analog fluorodeoxyglucose (FDG) F 18 is administered intravenously, followed by detection of FDG uptake by the PET scanner. The normal pancreas is not usually visualized by FDG-PET. In contrast, pancreatic carcinoma appears as a focal area of increased uptake in the pancreatic bed. Hepatic metastases appear as “hot spots” within the liver. Owing to the lack of anatomic detail, PET scanning is not a principal diagnostic modality for pancreatic cancer. However, FDG-PET can be helpful in differentiating benign from malignant pancreatic masses when morphologic data are equivocal.72 It can also be useful in assessing tumor recurrence after pancreatic resection, when scar tissue or postoperative changes may be difficult to differentiate from carcinoma. Finally, FDG-PET can be of benefit in assessing tumor response to neoadjuvant chemoradiation, which may lead to alteration in clinical management.
Percutaneous and Endoscopic Ultrasonography–Guided Aspiration Cytology
FNA cytology of the pancreas has been one of the major advances in the management of patients with pancreatic tumors. CT-guided biopsy has been used for more than 20 years and is regarded as a safe, reliable procedure, with a reported sensitivity of 57% to 96% and virtually no false-positive results.73,74 Experience with EUS-guided FNA shows similar results.64 Whenever a patient is deemed to have unresectable or metastatic pancreatic cancer, CT- or EUS-guided FNA biopsy is indicated for histologic confirmation of disease, unless a palliative surgical procedure is required. Even if the diagnosis of chronic pancreatitis is reasonably eliminated, proof of malignancy will exclude other rare benign diseases of the pancreas, such as tuberculosis and sarcoidosis. Furthermore, FNA cytology can usually distinguish between adenocarcinoma and other pancreatic tumors, such as neuroendocrine neoplasms and lymphomas, which carry a better prognosis (see Chapters 29 and 32).75
Serum Markers
A wide variety of tumor markers have been proposed for pancreatic cancer, but currently the only one with any practical usefulness is CA 19-9. Although not suitable for screening, this marker is a valuable adjunct in pancreatic cancer for diagnosis, prognosis, and monitoring of treatment.76
For diagnosing pancreatic cancer, the sensitivity and specificity of CA 19-9 vary with the threshold value set. One of the major caveats of this tumor marker is that in the presence of jaundice, and especially with cholangitis, very high values can be found in the absence of malignancy (false-positive results). In addition, patients with a Lewis blood group phenotype (-a-b) do not express the CA 19-9 antigen, resulting in false-negative results. Despite these shortcomings, one study reported a sensitivity and specificity of 86% and 87%, respectively, using a cut-off value of 37 U/mL.77
Initial reports of CA 19-9 response to chemotherapy indicated that a decrease in value greater than 20% during treatment correlated with improved survival in patients with locally advanced or metastatic disease.78 A more contemporary study using a larger patient population did not confirm these findings. Based on these latest results, CA 19-9 response to chemotherapy cannot be used as an independent prognostic factor for survival.79
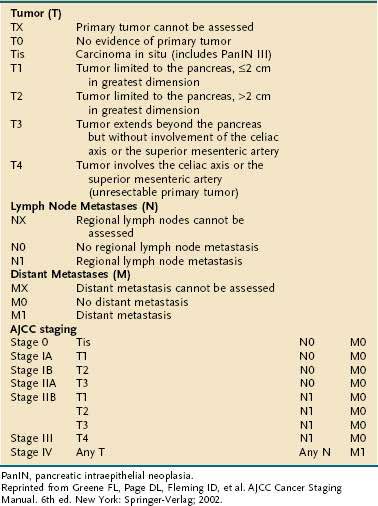
STAGING
The American Joint Committee on Cancer (AJCC) staging system for pancreatic cancer is shown in Table 60-5.80 The system was last revised in 2002, and modifications were made to better identify unresectable (T4, stages III and IV) from resectable disease (T1-3, stages I and II). Despite these changes, several limitations of the staging system still exist. First, adequate evaluation of lymph node status cannot be performed without surgical intervention; this drawback may lead to understaging of locally advanced disease in patients who are not candidates for laparotomy. Second, the margins of resection, which carry great prognostic significance, are not taken into consideration when assigning clinical stage. Because of these and other shortcomings, the AJCC staging system has found limited clinical applicability.
Staging of pancreatic cancer is predicated on the identification of three distinct patient groups. The first group involves those presenting with metastatic disease. Surgery is best avoided in these patients in view of their short survival, and chemotherapy is their principal treatment modality other than palliative care measures.81 The second group comprises patients who have locally advanced (unresectable) disease but no metastases. These patients can benefit from neoadjuvant chemoradiation and, according to their treatment response, may be candidates for surgical exploration or intraoperative radiation therapy.82–85 A third group consists of patients with resectable disease for whom surgical resection should be advocated.
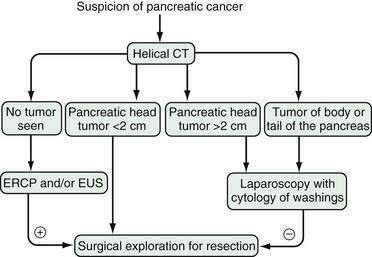
A minimal staging workup for a patient with pancreatic cancer should include a physical exam, and a CT or MRI of the abdomen and pelvis.80 As discussed, contemporary CT or MRI is extremely accurate in identifying unresectable disease.46,47,66 In selected cases, EUS may complement CT scanning by allowing further assessment of vascular invasion and tissue sampling. However, CT imaging fails to correctly predict resectability in 25% to 50% of patients. In most cases, lesions missed are beyond the resolution of current radiologic imaging; they include small implants on the peritoneal surfaces of the liver, abdominal wall, stomach, intestine, or omentum. Additionally, micrometastases only detectable by peritoneal washings are also missed. Successful detection of such tumor dissemination depends on access to the peritoneal cavity and visual inspection, which at present can be achieved only by laparoscopy or laparotomy.
Several large studies have documented the value of staging laparoscopy in the evaluation of patients with pancreatic cancer.86–90 The staging procedure consists of a simple diagnostic laparoscopy with biopsy of suspicious nodules and collection of peritoneal washings for cytologic analysis. Peritoneal cytology results are important for staging because patients with occult metastases detected by this method have been shown to carry a prognosis similar to that of patients with M1 disease (stage IV).91,92 More complicated staging procedures involving extended laparoscopic dissections of the peripancreatic bed and laparoscopic ultrasonography can also be added if necessary.89,90
Published data demonstrate that approximately 25% of patients in whom localized disease is demonstrated by CT also have unsuspected metastatic implants.87,88 A further 10% have positive peritoneal cytology results despite a lack of gross metastatic disease at laparoscopy. For most of these patients, laparoscopy has prevented unnecessary surgical explorations to assess tumor resectability.93 The combination of CT scan and staging laparoscopy has enhanced the identification of patients with metastatic, localized-unresectable, and resectable pancreatic cancer and has helped in stratifying patients to different treatment protocols.88 Our current algorithm for the diagnosis and staging of pancreatic cancer is shown in Figure 60-6. We recommend laparoscopy for all patients with tumors in the body and tail of the pancreas (in which the frequency of unsuspected metastases approaches 50%) and for patients with tumors in the head of the pancreas larger than 2 cm, because the yield of laparoscopy in lesions smaller than 2 cm is less than 10%.