Chapter 47 Tumors of the biliary tree
Pathologic features
Overview
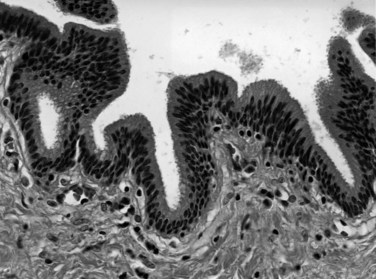
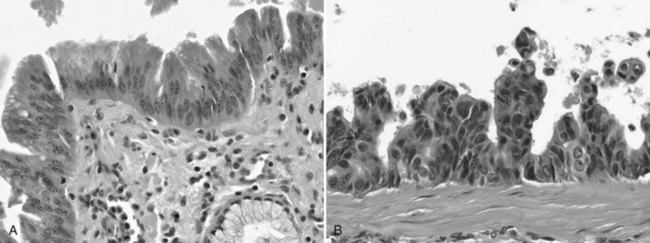
The biliary tract is lined by simple columnar epithelial cells of foregut origin (Fig. 47.1; Frierson, 1989; 1997). Most biliary neoplasms are related to this cell type and hence show major similarities to other foregut tumors, particularly those of pancreatic ductal origin. By far the most common neoplasm of the biliary tract is adenocarcinoma, which is referred to collectively with pancreatic ductal adenocarcinoma as a pancreatobiliary-type adenocarcinoma (Figs. 47.2 through 47.5), reflecting the similarities between these two neoplasms.
Tumors arising from the gallbladder and the different segments of the bile ducts have overlapping histologic characteristics; therefore their pathologic classification is also very similar (Adsay, 2004; Albores-Saavedra et al, 2000a, 2010; Lack, 2003). On the other hand, the risk factors, clinical findings, and biologic behavior may vary (see Chapters 49, 50A, and 50B). For instance, although gallstones are the main risk factor for gallbladder adenocarcinoma, adenocarcinomas of proximal bile ducts have a much stronger association with primary sclerosing cholangitis (PSC) (see Chapter 41) or anomalous pancreatobiliary duct junction (see Chapter 46). This variability also is partially reflected in the molecular alterations, which although not specific for the histologic subtype of the tumor, may affect the management and prognosis (see Chapter 8B). The different presentations of biliary adenocarcinoma also influence the mode by which a specimen is obtained for pathologic examination. For example, many gallbladder adenocarcinomas are currently identified in “routine” cholecystectomy specimens in primary care facilities, performed with a preoperative diagnosis of chronic cholecystitis or cholelithiasis, whereas extrahepatic bile duct lesions are seldom resected without a strong preoperative suspicion of carcinoma, and this is usually performed in tertiary care facilities.
This chapter discusses the pathologic aspects of biliary tract neoplasia, with special emphasis on adenocarcinomas. The tumor types are discussed by viewing the biliary system as a whole; site-specific characteristics are mentioned only when pertinent to pathologic classification. For the details of clinical findings, risk factors, and site-specific management, the reader is referred to other chapters of this book (see Chapters 49, 50A, and 50B).
Invasive Carcinomas Of The Biliary Tract
Most “biliary tract cancers” are conventional adenocarcinomas (pancreatobiliary type), which are morphologically very similar to pancreatic ductal adenocarcinoma (Adsay, 2004; Albores-Saavedra et al, 2000a, 2010; Lack, 2003). This tumor is referred to as gallbladder adenocarcinoma in the gallbladder, cholangiocarcinoma in the intrahepatic biliary tract, and adenocarcinoma of the extrahepatic bile ducts in the extrahepatic biliary tract. As in adenocarcinomas of other organs, these are usually tumors of the elderly. The association of biliary adenocarcinomas with preceding chronic inflammation has been well established (Herzog & Goldblum, 1996; Sheth et al, 2000), mostly by epidemiologic data showing the high incidence of gallbladder cancer in areas with a high prevalence of gallstones and by the pathologic observation that many individual carcinomas have associated gallstones or cholecystitis (see Chapter 49). Also, the risk of adenocarcinoma is relatively high in patients with PSC and indirectly in those with ulcerative colitis (see Chapter 41). The association of biliary cancers with parasites (see Chapter 45; Carriaga & Henson, 1995) and choledochal cysts (see Chapter 46) is also presumably related to the potential for chronic inflammation from constant epithelial injury and repair that leads to neoplastic alterations (Parkin et al, 1993).
Growth Patterns and Macroscopic Features
Based on their macroscopic growth pattern, biliary carcinomas have been divided into four types: 1) polypoid, 2) nodular (nodular-sclerosing), 3) schirrous-constricting, and 4) diffusely infiltrative (Albores-Saavedra et al, 2000a; Todoroki et al, 1980; Van Heerden et al, 1967; Weinbren & Mutum, 1983). Polypoid growth typically is found in carcinomas with an associated component of intraductal papillary neoplasm (IPN), previously designated noninvasive papillary carcinoma (Albores-Saavedra et al, 2010), which are associated with a better prognosis. The nodular and schirrous types have a propensity to infiltrate surrounding tissues and are therefore difficult to resect. The diffusely infiltrating type tends to spread linearly along the ducts. The constricting and diffusely infiltrative patterns may be difficult to differentiate from chronic inflammatory conditions, especially PSC and autoimmune cholangitis (Corvera et al, 2005). Significant histologic overlaps occur among these different growth patterns, so their utility in tumor classification is limited.
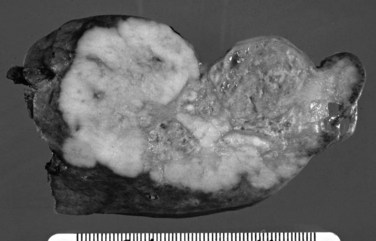
On cut sections, the intraluminal components of biliary carcinomas, especially those with a polypoid gross appearance, may appear more friable, soft, and tan; this reflects the presence of intraductal papillary neoplasm elements growing into the lumen, and ulceration and necrosis may be evident in larger tumors. The infiltrating components of adenocarcinomas are more schirrous (scarlike) with a firm, white, gritty appearance (see Fig. 47.2) because of the abundance of desmoplasia, a fibrotic tissue reaction associated with infiltrating carcinoma. Necrosis may also be evident.
When adenocarcinoma invades into the adjacent liver, the growth pattern often becomes more expansile, and the liver-carcinoma interface appears deceptively well demarcated. This allows easier detection of the boundaries of these carcinomas in hepatic resections. In contrast, the boundaries of carcinomas invading the hilar soft tissue are typically poorly defined and difficult to appreciate. Gallbladder carcinomas are often associated with gallstones. In patients with porcelain gallbladder (Stephen & Berger, 2001), the wall of the gallbladder may be entirely calcified.
Microscopic Features
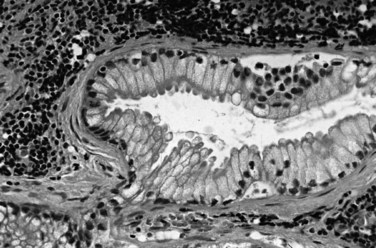
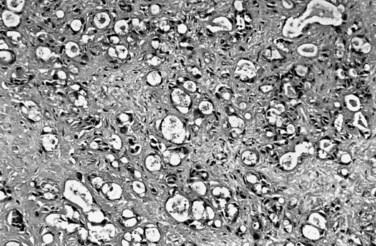
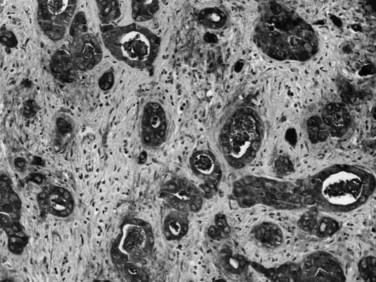
Most adenocarcinomas of gallbladder and bile ducts show the characteristic features of a pancreatobiliary-type adenocarcinoma (see Figs. 47.3 to 47.5): both simple and complex, irregularly shaped glands mixed with small clusters of cells, often with associated stromal desmoplasia (Adsay, 2004; Albores-Saavedra et al, 2000a, 2010; Lack, 2003). The glands often are well formed, lined by cuboidal cells, and show dilated lumina. Often the nuclear grade is unexpectedly high for the degree of glandular differentiation, and marked variation in nuclear size, shape, and intracellular location occurs between different cells within the individual glands. The cytoplasm may be acidophilic and granular in some cases and pale or clear in others. Variable amounts of intracytoplasmic and intraluminal mucin are present; in some cases, mucin is readily evident by routine histologic examination; in others, it is demonstrable by special stains.
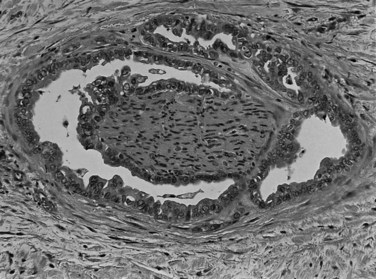
Perineural (see Fig. 47.4) and vascular invasion are common, and carcinoma cells may have a deceptively well-differentiated appearance, even when they invade these structures. In fact, the distinction of a well-differentiated adenocarcinoma from a benign reactive process in this region is one of the more challenging differential diagnoses in surgical pathology.
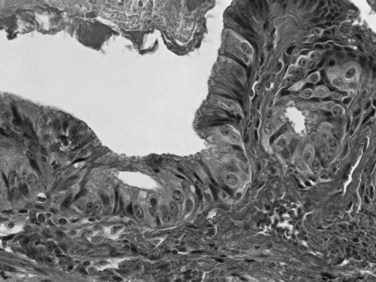
Dysplasia, or biliary intraepithelial neoplasia (BilIN; Fig. 47.6), is often present in the adjacent biliary epithelium (Zen et al, 2007). Sometimes the intraepithelial component comprises a papillary neoplasm (Fig. 47.7). In such cases, the noninvasive and invasive components of the tumor should be evaluated separately, and the extent of invasion should be quantified because those with “minimal invasion” have a better prognosis (Albores-Saavedra et al, 2000b).
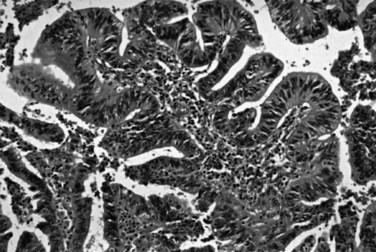
FIGURE 47.7 Intraductal papillary neoplasm. This polypoid lesion shows papillary fronds lined by neoplastic cells.
Anatomic Variations
For therapeutic and prognostic purposes, tumors of the extrahepatic biliary tract are separated by their anatomic distribution into upper third (above the cystic duct junction, including the both hepatic ducts, common hepatic duct, and the cystic duct), middle third (upper half of the common bile duct [CBD]), and lower third (distal half of the CBD) (Klatskin, 1965; Tompkins et al, 1981; Van Heerden et al, 1967; Weinbren & Mutum, 1983). The distinctive clinical characteristics of adenocarcinomas occurring in different segments of the biliary tract are discussed in other chapters of this book, and only a few issues pertinent to pathologic aspects are mentioned here. Most carcinomas arise in the upper third of the bile ducts and tend to be the schirrous-constricting and diffusely infiltrative types. Recent studies have shown that many originate within 5 mm of the cystic duct junction or within the cystic duct itself. Hilar carcinoma, located at the confluence of the right and left hepatic ducts, sometimes referred to as a Klatskin tumor (Bosma, 1990; Klatskin, 1965), has distinctive clinical features. Klatskin tumors usually grow into the liver rather than distally toward the duodenum (see Chapter 50B; Hayashi et al, 1994), and the component that invades the liver is often well demarcated. Carcinomas in the middle third tend to be the nodular-sclerosing type—thickened along a long segment, with a narrow lumen and inflammatory changes in the surrounding tissues; hence these are difficult to differentiate from sclerosing cholangitis. They have a very high propensity for perineural invasion and involvement of radial surfaces, making curative resection difficult (Bhuiya et al, 1993). Those in the distal third have the best prognosis, partly because of their resectability by pancreatoduodenectomy and partly because many, especially those close to the ampullary region, are composed predominantly of noninvasive papillary neoplasmic elements (Adsay, 2004; Albores-Saavedra et al, 2000a; Lack, 2003).
Pathologic Differential Diagnosis
The difficulty at the clinical level of distinguishing biliary adenocarcinomas from benign inflammatory conditions, such as sclerosing cholangitis, is also problematic at the microscopic level (Ludwig, 1989; Ludwig et al, 1992). Reactive changes in the accessory biliary ductules in the wall of the bile ducts can mimic adenocarcinomas, although nonneoplastic ductules may retain a lobular configuration, and they lack the density of cellularity of a carcinoma. As also discussed in Chapter 56, pancreatobiliary adenocarcinomas can be deceptively benign appearing, composed of well-formed glandular elements lined by fairly organized, cytologically bland glandular cells (see Figs. 47.3 and 47.4). The distinction of reactive changes in the surface epithelium from dysplasia (BilIN) often proves to be even more challenging, especially because any injury to the biliary epithelium, including instrumentation and stent placement, has a tendency to induce marked cytologic atypia that mimics the appearance of high-grade dysplasia (Fig. 47.8). Marked nuclear enlargement or irregularity, hyperchromasia, loss of polarity, mitotic figures, apoptotic cells, and intraluminal necrosis are findings in favor of a neoplastic process (see Fig. 47.6); however, overlaps are common, and at times this distinction may not be possible on the basis of biopsies or frozen sections.
Biliary carcinomas that grow into the liver must be distinguished from primary hepatocellular carcinomas (see Chapter 78). The presence of true glandular elements and mucin are common findings in biliary carcinomas and typically are lacking in hepatocellular carcinomas. In contrast, hepatocellular carcinomas may have intracellular bile and generally lack significant stromal fibrosis and desmoplasia. Other distinctive features of hepatocellular carcinomas—the solid and trabecular growth pattern, centrally located nuclei with prominent nucleoli, and abundant eosinophilic cytoplasm—usually are identifiable; immunohistochemical demonstration of hepatocellular differentiation with markers such as hepatocyte-1 or glypican-3 can be used in problematic cases.
Biliary carcinomas metastatic to other sites may mimic the primary tumors of those organs. In particular, metastases to the ovary often become cystic and are mistaken for primary ovarian mucinous cystic neoplasms (Young & Hart, 1989), and lung metastases can resemble mucinous bronchioloalveolar carcinomas. Metastases to the peripheral liver from biliary carcinomas can be nearly impossible to distinguish from metastatic pancreatic ductal adenocarcinoma.
Immunohistochemical and Molecular Characteristics
By immunohistochemistry, biliary adenocarcinomas typically express CK7, CK19, CA19-9, CEA , MUC1 (Fig. 47.9), and MUC5AC (Adsay, 2004; Albores-Saavedra et al, 2000a; Lack, 2003). On occasion, these markers can be helpful in distinguishing certain other carcinomas that do not express these markers, such as hepatocellular carcinoma. However, none of these markers is specific enough to prove biliary origin for an adenocarcinoma when a metastasis from another organ is under consideration. Biliary adenocarcinomas generally lack expression of CK20 and CDX2 typically found in intestinal adenocarcinomas; TTF1 and napsin, found in pulmonary primaries; and hormone receptors. Mutations at codon 12 of the KRAS oncogene, seen in more than 90% of pancreatic ductal adenocarcinomas, are much less common in biliary adenocarcinomas (Rashid et al, 2002); the frequency of KRAS mutation appears to decrease from distal to proximal along the biliary tree. Similarly, loss of SMAD4 is also less common in biliary than pancreatic adenocarcinomas (Argani et al, 2001). More than half of the cases have abnormal expression of TP53 and loss of heterozygosity at 8p, 9q, and 18q; amplification of ERBB2 is also reported in more than half.
Other Types of Carcinomas in the Biliary Tract
Other carcinomas of glandular epithelial origin, in the gallbladder and biliary tract are classified separately from pancreatobiliary adenocarcinomas (Adsay, 2004; Albores-Saavedra et al, 1996, 2000a, 2010; Lack, 2003). Intestinal-type adenocarcinomas (Albores-Saavedra et al, 1986) are morphologically similar to their counterparts in the gastrointestinal tract. Signet ring cell carcinomas are characterized by a diffusely infiltrative pattern of individual cells, often with signet ring morphology because of intracellular mucin; a cordlike growth pattern may also occur in the biliary tract but is exceedingly uncommon. Mucinous adenocarcinomas may be seen in some cases, with extensive mucin production associated with stromal mucin deposition (Fig. 47.10), usually mixed with conventional adenocarcinomas; this subtype can occur with intraductal papillary neoplasms. Some studies suggest that the prognosis of mucinous adenocarcinomas may be more favorable than that of conventional pancreatobiliary-type adenocarcinoma (Bosma, 1990). Adenosquamous carcinomas (Nishihara et al, 1994) are rare tumors in which a mixture of glandular and squamous differentiation is seen in variable amounts. Clear-cell carcinomas (Vardaman & Albores-Saavedra, 1995) are described, in which the morphologic features resemble those of renal cell carcinoma.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree