CHAPTER 94 Tumors and Cysts of the Liver
PRIMARY MALIGNANT TUMORS
Among primary malignant tumors of the liver, hepatocellular carcinoma is by far the most common.
HEPATOCELLULAR CARCINOMA
Epidemiology
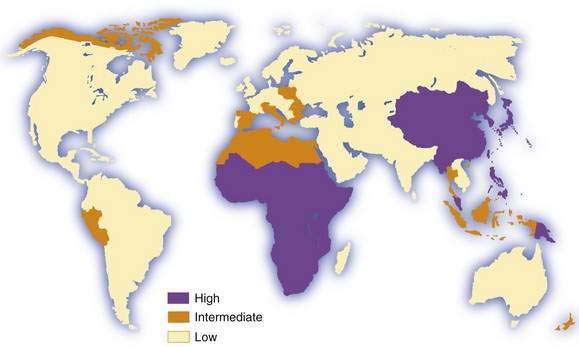
Hepatocellular carcinoma is the commonest primary malignant tumor of the liver. It is the fifth most common cancer in men and the eighth most common in women, and it ranks fourth in annual cancer mortality rates.1,2 Information on incidence is derived from an increasing but still limited number of cancer registries, and it is possible to classify countries into broad risk categories only. Moreover, in low-income (developing) countries, especially in sub-Saharan Africa, hepatocellular carcinoma is underdiagnosed and underreported, in some cases by as much as 50%. Despite these sources of inaccuracy, hepatocellular carcinoma clearly has an unusual geographic distribution (Fig. 94-1). Moreover, the tumor is not necessarily uniformly common throughout countries with a high incidence, such as China3 and Mozambique.4 The incidence of hepatocellular carcinoma has increased considerably in Japan since the 1980s, and lesser increases have been recorded in developed Western countries, including North America and Western Europe.5 Interestingly, a study from Japan has shown that the rate of hepatocellular carcinoma began to decline in 2000, presumably because of the aging of the cohort of persons infected with hepatitis C virus (HCV).6 A similar downward trend has been noted in some European countries, including France and Italy.7 By contrast, in the United States, hepatocellular carcinoma is the cancer that has been increasing in incidence most rapidly since 2000, at a time when other major cancers such as cancers of the lung, breast, prostate, and colon are decreasing.8 Considerable racial and ethnic variation exits in the incidence of hepatocellular carcinoma in the United States. The incidence among Asians is highest, almost double that of white Hispanics and more than four times higher than that of whites.9
Migrants from countries with a low incidence to areas with a high incidence of hepatocellular carcinoma usually retain the low risk of their country of origin, even after several generations in the new environment. The consequences for migrants from countries with a high incidence to those with a low incidence differ, depending on the major risk factors for the tumor in their country of origin and whether chronic hepatitis B virus (HBV) infection, if this is the major risk factor, is acquired predominantly by the perinatal or horizontal route (see later and Chapter 78).2,10,11
Men are generally more susceptible than women to hepatocellular carcinoma. Male predominance is, however, more obvious in populations at high risk for the tumor (mean male-to-female ratio, 3.7 : 1.0) than in those at low or intermediate risk (2.4 : 1.0).1,2 In industrialized countries, the number of men and number of women with hepatocellular carcinoma in the absence of cirrhosis are almost equal.
The incidence of hepatocellular carcinoma increases progressively with advancing age in all populations, although it tends to level off in the oldest age groups.1,2 In Chinese and particularly in black African populations, however, the mean age of patients with the tumor is appreciably younger than in other populations. This finding is in sharp contrast to the age distribution in Japan, where the incidence of hepatocellular carcinoma is highest in the cohort of men ages 70 to 79 years.6 Hepatocellular carcinoma is rare in children.12,13
Clinical Features
Although the typical clinical features of hepatocellular carcinoma are well recognized (including abdominal pain and weight loss in patients with cirrhosis), more patients are now being diagnosed at an early stage, when they have no specific symptoms or signs. This trend toward earlier diagnosis is probably the result of surveillance programs in patients with chronic liver disease (see later). In far-advanced disease, patients with hepatocellular carcinoma usually present with typical symptoms and signs, and diagnosis is easy. In addition, hepatocellular carcinoma often coexists with cirrhosis,14 and the onset of hepatocellular carcinoma is marked by a sudden unexplained change in the patient’s condition.
Patients with hepatocellular carcinoma often are unaware of its presence until the tumor has reached an advanced stage. The most common, and frequently first, symptom is right hypochondrial or epigastric pain. Other symptoms are listed in Table 94-1.
Table 94-1 Symptoms and Signs of Hepatocellular Carcinoma
| SYMPTOM | FREQUENCY (%) |
|---|---|
| Abdominal pain | 59-95 |
| Weight loss | 34-71 |
| Weakness | 22-53 |
| Abdominal swelling | 28-43 |
| Nonspecific gastrointestinal symptoms | 25-28 |
| Jaundice | 5-26 |
| SIGN | |
| Hepatomegaly | 54-98 |
| Ascites | 35-61 |
| Fever | 11-54 |
| Splenomegaly | 27-42 |
| Wasting | 25-41 |
| Jaundice | 4-35 |
| Hepatic bruit | 6-25 |
Physical findings vary with the stage of disease. Early in the course, evidence of cirrhosis alone may be present, or abnormal findings may be absent (see Table 94-1). When the tumor is advanced at the time of the patient’s first medical visit, the liver is almost always enlarged, sometimes massively. Hepatic tenderness is common and may be profound, especially in the later stages. The surface of the enlarged liver is smooth, irregular, or frankly nodular. An arterial bruit may be heard over the tumor15; the bruit is heard in systole, rough in character, and not affected by changing the position of the patient. Although not pathognomonic, a bruit is a useful clue to the diagnosis of hepatocellular carcinoma. Less often, a friction rub may be heard over the tumor, but this sign is more characteristic of hepatic metastases or abscesses.
Ascites may be present when the patient is first seen or may appear with progression of the tumor. In most patients, ascites is the result of long-standing cirrhosis and portal hypertension (see Chapter 91), but in some cases it is caused by invasion of the peritoneum by the primary tumor or metastases. The ascitic fluid may be blood-stained. In a small proportion of patients, hepatocellular carcinoma invades the hepatic veins, thereby causing Budd-Chiari syndrome, and tense ascites results (see Chapter 83).16 Splenomegaly, if present, reflects coexisting cirrhosis and portal hypertension.
Physical evidence of cirrhosis may also be noted. Severe pitting edema of the lower extremities extending up to the groins occurs when hepatocellular carcinoma has invaded the hepatic veins and propagates into and obstructs the inferior vena cava.16 A Virchow-Trosier (supraclavicular) node, Sister Mary Joseph’s (periumbilical) nodule, or enlarged axillary lymph node is rarely present.
Paraneoplastic Manifestations
Some of the deleterious effects of hepatocellular carcinoma are not caused by local effects of the tumor or metastases (Table 94-2). Each of the paraneoplastic syndromes in hepatocellular carcinoma is rare or uncommon. One of the more important is type B hypoglycemia, which occurs in less than 5% of patients, manifests as severe hypoglycemia early in the course of the disease,16 and is believed to result from the defective processing by malignant hepatocytes of the precursor to insulin-like growth factor II (pre-IGF II).17 By contrast, type A hypoglycemia is a milder form of glycopenia that occurs in the terminal stages of hepatocellular carcinoma (and other malignant tumors of the liver). It results from the inability of a liver extensively infiltrated by tumor, and often cirrhotic, to satisfy the demands for glucose by a large, often rapidly growing tumor and by the other tissues of the body.
Table 94-2 Paraneoplastic Syndromes Associated with Hepatocellular Carcinoma
Another important paraneoplastic syndrome is polycythemia (erythrocytosis), which occurs in less than 10% of patients with hepatocellular carcinoma.18 This syndrome appears to be caused by the synthesis of erythropoietin or an erythropoietin-like substance by malignant hepatocytes.
Patients with hepatocellular carcinoma, especially the sclerosing variety, may present with hypercalcemia in the absence of osteolytic metastases. When hypercalcemia is severe, it may result in the typical complications of hypercalcemia, including drowsiness and lethargy. The probable cause is secretion of parathyroid hormone–related protein (PTHrP) by the tumor.19
Cutaneous paraneoplastic manifestations of hepatocellular carcinoma are rare except for pityriasis rotunda (circumscripta), which may be a useful marker of the tumor in black Africans. The rash consists of single or multiple, round or oval, hyperpigmented, scaly lesions on the trunk and thighs that range in diameter from 0.5 to 25 cm.20
Diagnosis
The gold standard for the diagnosis of hepatocellular carcinoma is pathology. For practical purposes (i.e., to apply treatment), hepatocellular carcinoma can only be diagnosed in the presence of an abnormality on imaging of the liver. The development of hepatocellular carcinoma is thought to occur as a result of a multistep sequential process from a dysplastic focus of hepatocytes to a low-grade dysplastic nodule to a high-grade dysplastic nodule to early well-differentiated hepatocellular carcinoma, and then to less differentiated states.21,22 In early hepatocellular carcinoma, particularly when a needle biopsy specimen is examined, controversy may exist among pathologists as to whether a particular specimen is consistent with dysplasia or carcinoma. Dysplastic nodules and even regenerative cirrhotic nodules can be seen on imaging studies and are potentially confused with hepatocellular carcinoma. Although there are specific imaging features based on the enhancement patterns with dynamic imaging of dysplastic nodules and hepatocellular carcinoma (see later), some overlap occurs.23,24 Nevertheless, there is a growing consensus, based on guidelines from the major European and American liver societies and now backed up by published experience, that the diagnosis of hepatocellular carcinoma can be made in the appropriate clinical setting based on specific imaging characteristics, with or without an elevated serum alpha fetoprotein (AFP) level.24–27
Serum Tumor Markers
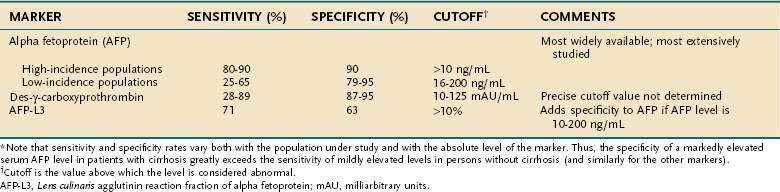
Many of the substances synthesized and secreted by hepatocellular carcinoma are not biologically active. Nevertheless, a few are produced by a sufficiently large proportion of tumors to warrant their use as serum markers for hepatocellular carcinoma. The most helpful of these markers is AFP (Table 94-3).
Alpha Fetoprotein
AFP is an α1-globulin normally present in high concentrations in fetal serum but in only minute amounts thereafter. Reappearance of high serum levels of AFP strongly suggests the presence of hepatocellular carcinoma (or hepatoblastoma [see later]),28 especially in populations in which hepatocellular carcinoma is most prevalent: The great majority of Chinese and black African patients have a raised serum concentration of AFP (>10 ng/mL), and approximately 75% have a diagnostic level (>500 ng/mL). These percentages are lower in populations at low or intermediate risk for the tumor, in which the sensitivity ranges from 25% to 65%, with a specificity of 79% to 95% and cutoff values for an elevated and diagnostic level of 16 and 200 ng/mL, respectively.29–35 With higher levels of AFP, the confidence in the diagnosis of hepatocellular carcinoma is greater. Although levels higher than 500 ng/mL usually indicate hepatocellular carcinoma, they sometimes can be seen in patients with active viral hepatitis. In the setting of a cirrhotic patient with a hepatic mass lesion larger than 2 cm in diameter and suggestive features of hepatocellular carcinoma, an AFP level higher than 200 ng/mL is considered diagnostic for hepatocellular carcinoma.25,26,33,36,37 The mean serum value of AFP in affected patients in regions with a high incidence of hepatocellular carcinoma is 60,000 to 80,000 ng/mL, compared with approximately 8,000 ng/mL in regions with a low or intermediate incidence of the tumor. Raised serum values range over six orders of magnitude, although an AFP concentration higher than 1 million ng/mL is rare. False-positive results also may occur in patients with tumors of endodermal origin, nonseminomatous germ cell tumors, and pregnancy. A progressively rising serum AFP concentration is highly suggestive of hepatocellular carcinoma.
AFP is not essential to hepatocarcinogenesis, and thus not all hepatocellular carcinomas produce AFP. The levels of AFP appear to be affected by ethnicity, underlying cause of liver disease, and tumor stage.30,33 Synthesis of AFP by a tumor is permanent and age-related; the younger the patient, the more likely the serum value is to be raised and the higher the level attained. According to the American Association for the Study of Liver Diseases (AASLD) guidelines, hepatocellular carcinoma can be diagnosed with confidence in patients with a serum AFP level higher than 200 ng/mL and a mass in the liver.25 An AFP level higher than about 500 ng/mL predicts worse outcomes with liver transplantation compared with lower levels.38 Attempts to correlate the degree of differentiation of hepatocellular carcinoma with production of AFP have produced conflicting results.
Fucosylated Alpha Fetoprotein
AFP is heterogeneous in structure. Its microheterogeneity results from differences in the oligosaccharide side chain and accounts for the differential affinity of the glycoprotein for lectins. AFP secreted by malignant hepatocytes contains unusual and complex sugar chains that are not found in AFP produced by nontransformed hepatocytes. One variant, Lens culinaris agglutinin reactive fraction (AFP-L3), appears to improve the specificity of AFP, particularly AFP serum levels from 10 to 200 ng/mL.39,40 The recommended cutoff value for AFP-L3 to diagnose hepatocellular carcinoma is higher than 10%, although the specificity varies depending on the absolute level of AFP. A Western series has suggested that a cutoff value of 35% is necessary to achieve 100% specificity.40 Therefore, AFP-L3 is not sufficiently validated to confirm the diagnosis of hepatocellular carcinoma without other supporting findings, such as suggestive imaging.
Des-γ-Carboxy Prothrombin
Serum concentrations of des-γ-carboxy prothrombin (DCP) (also known as prothrombin produced by vitamin K absence or antagonist II [PIVKA II]) are raised in most patients with hepatocellular carcinoma.41 DCP is an abnormal prothrombin that is thought to result from a defect in the post-translational carboxylation of the prothrombin precursor in malignant cells.42 In Western populations, DCP may be a better marker than, or at least a complementary marker to, AFP.43–45 In black Africans, however, DCP is less sensitive and less specific than AFP.46 The appropriate cutoffs are not well established, and thus the precise role of DCP in the diagnosis of hepatocellular carcinoma requires validation.
Other Markers
Multiple other potential serum markers for hepatocellular carcinoma are in the exploratory phase of evaluation, including glypican 3, Golgi protein 73, hepatocyte growth factor, insulin growth factor 1, transforming growth factor-β1, and proteomic profiling using surface-enhanced laser desorption/ionization time-of-flight (SELDI-TOF) mass spectrometry.47–51 All these novel markers have been shown to be elevated in patients with hepatocellular carcinoma compared with those with only chronic liver disease, but clear cutoff values and comparisons with other markers have not been established. Some of these markers may be complementary to established markers, although none of them has an established high throughput method of measurement, as required for a clinical test. The roles of these markers in the diagnosis of hepatocellular carcinoma await further study.
Imaging
The diagnosis of hepatocellular carcinoma generally requires imaging evidence of a focal lesion in the liver, although large infiltrating lesions can also be diagnostic. Arterial hyperenhancement, particularly seen on dynamic contrast imaging of the liver, is observed because the blood supply of hepatocellular carcinoma comes from newly formed abnormal arteries (neoangiogenesis).23,52,53 As a nodule transforms from low- to high-grade dysplasia and then to hepatocellular carcinoma, the primary blood supply shifts from portal to arterial—especially new abnormal arterial branches that produce characteristic findings on dynamic contrast imaging of the liver.27
Ultrasonography
Ultrasonography detects most hepatocellular carcinomas but may not distinguish this tumor from other solid lesions in the liver. As with all imaging methods, the sensitivity increases with increasing size of the lesion. A systematic review of eight studies using histologic reviews of liver explants has shown that ultrasound has fair sensitivity (pooled estimate, 48%; 95% confidence interval [CI], 34% to 62%) with good specificity, estimated at 97% (95% CI, 95% to 98%).24 Advantages of ultrasonography include safety, availability, and cost-effectiveness, although it has the drawbacks of being nonstandardized and examiner-dependent. Body habitus, particularly obesity, may limit the sensitivity of this test. Approximately two thirds of symptomatic hepatocellular carcinomas are uniformly hyperechoic, whereas the remainder are partly hyperechoic and partly hypoechoic.54 Small tumors are uniformly hypoechoic. The ultrasonographic appearance is influenced by the presence of fat, calcium, and necrosis. Tumors located immediately under the right hemidiaphragm may be difficult to detect. In Japanese patients in particular, hepatocellular carcinoma may have a well-defined, even thick capsule, which can be seen on ultrasonography. Ultrasonography with Doppler technology is useful for assessing the patency of the inferior vena cava, portal vein and its larger branches, hepatic veins, and biliary tree.
Dynamic contrast-enhanced Doppler ultrasonography with intra-arterial infusion of CO2 microbubbles and intravenous enhanced color Doppler ultrasonography are refinements that, by characterizing hepatic arterial and portal venous flow in tumorous nodules, facilitate the diagnosis of malignant and benign hepatic nodules.55 These techniques are not often performed in the United States.
Computed Tomography
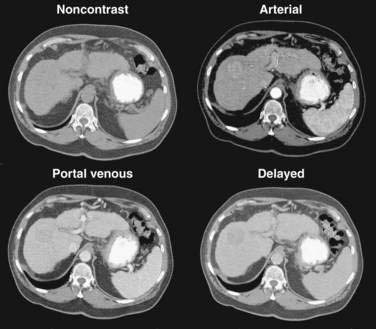
Multiphase, also called dynamic, helical computed tomography (CT) is the imaging technique of choice for the diagnosis of hepatocellular carcinoma.24,54,55 CT during arterial portography is also helpful but rarely done because it is invasive. Phases in dynamic contrast-enhanced CT can include noncontrast, arterial, portal venous, and delayed phases. The classic and most diagnostic pattern for hepatocellular carcinoma is a combination of enhancement in the arterial phase (with the uninvolved liver lacking enhancement), loss of central nodule enhancement compared with the uninvolved liver (washout), and capsular enhancement in the portal-venous and delayed phases (Fig. 94-2).25,56 When the lesion is larger than 2 cm in diameter, this pattern has almost 100% specificity for hepatocellular carcinoma.36,37,56 When the nodule is 1 to 2 cm, guidelines recommend a second type of dynamic imaging (magnetic resonance imaging [MRI] or contrast ultrasonography) to confirm the diagnosis of hepatocellular carcinoma, although the specificity of one dynamic study is higher than 90%.57 CT often finds so-called hypervascular-only lesions, which enhance in the arterial phase and become isodense to the surrounding liver in the portal-venous and delayed phases. These lesions may be dysplastic nodules, arterial portal shunts, atypical hemangiomas, hepatocellular carcinoma, confluent fibrosis, or aberrant venous drainage. When less than 2 cm in diameter, only about 30% are hepatocellular carcinomas, which grow over time. Other causes disappear or remain stable on follow-up studies. Current guidelines recommend biopsy of lesions larger than 1 cm if the serum AFP level is less than 200 ng/mL and serial imaging for lesions smaller than 1 cm.58 Hepatocellular carcinoma may also have other patterns on CT, such as washout only on delayed imaging, a hypovascular nodule, or a fat-containing nodule.27,58 Overall, the pooled estimate of sensitivity and specificity for detecting hepatocellular carcinoma by CT is 67.5% (95% CI, 55% to 80%) and 92.5% (95% CI, 89% to 96%), respectively. Dynamic CT is also useful for detecting invasion into the portal or hepatic veins and identifying the location and number of tumors; these findings are critical for planning treatment.
Magnetic Resonance Imaging
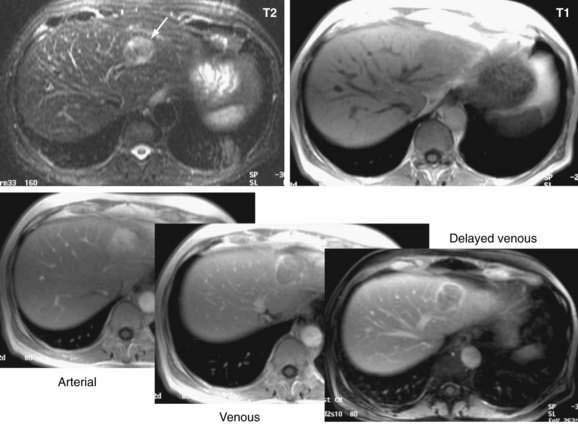
Dynamic MRI using gadolinium contrast agents provides another way of distinguishing hepatocellular carcinoma from normal liver tissue. The performance of MRI and the findings on multiphase contrast enhancement are similar to those described for CT (Fig. 94-3). Typically, the signal intensity on T1-weighted images is low.27,54 The pooled estimate of sensitivity and specificity for detecting hepatocellular carcinoma by MRI is 80.6% (95% CI, 70% to 91%) and 84.8% (95% CI, 77% to 93%), respectively.24 MRI may be slightly superior overall to CT, although local expertise should dictate the choice of imaging technique.
Hepatic Angiography
Since the advent of CT and MRI, the diagnostic role of hepatic angiography has decreased. Digital subtraction angiography is helpful for recognizing small hypervascular hepatocellular carcinomas but may miss early, well-differentiated hypovascular tumors. Hepatocellular carcinomas often are densely vascular, although multinodular tumors may be relatively avascular.59 The arteries in the tumor are irregular in caliber and do not taper in the usual way, and the smaller branches may show a bizarre pattern. The hepatic veins fill early, and retrograde filling of the portal veins results from the presence of arteriovenous anastomoses within the tumor. An additional finding is a delay in capillary emptying, which is seen as a blush. The center of some large tumors may be avascular as a result of necrosis or, less often, hemorrhage. Angiography is essential for delineating the hepatic arterial anatomy in planning embolization or chemoembolization of the tumor or infusion of cytotoxic drugs directly into the hepatic artery or its branches (see later).
Pathology
Microscopic Appearance
Hepatocellular carcinoma is classified histologically into well-differentiated, moderately differentiated, and undifferentiated (pleomorphic) forms.60
Progenitor Cell Hepatocellular Carcinoma
A class of primary liver cancer appears to have its origins in progenitor cells, the stem cells of the liver, located in association with the canals of Hering. Progenitor cell activation is seen in association with chronic viral hepatitis and cirrhosis, presumably relegated to senescence of hepatocytes. These tumors may appear morphologically like typical hepatocellular carcinoma or mixed cholangiohepatocellular carcinoma. Tumor cells stain positively for cytokeratin 19, and the tumor appears to have a more aggressive course than typical hepatocellular carcinoma.61
Metastases
Extrahepatic metastases are present at autopsy in 40% to 57% of patients with hepatocellular carcinomas.62 The most common sites are the lungs (up to 50% in some reports) and regional lymph nodes (approximately 20%). The adrenal glands are frequently involved.
Fibrolamellar Hepatocellular Carcinoma
The fibrolamellar variant of hepatocellular carcinoma typically occurs in young patients, has an approximately equal gender distribution, does not secrete AFP, is not caused by chronic hepatitis B or C, and almost always arises in a noncirrhotic liver.63–65 Fibrolamellar hepatocellular carcinoma is more often amenable to surgical treatment and therefore generally carries a better prognosis than that for conventional hepatocellular carcinoma. It does not, however, respond to chemotherapy any better than other forms of hepatocellular carcinoma. The hepatocytes are characteristically plump, deeply eosinophilic, and encompassed by abundant fibrous stroma composed of thin, parallel fibrous bands that separate the cells into trabeculae or nodules. The cytoplasm is packed with swollen mitochondria and, in approximately half of the tumors, contains pale or hyaline bodies. Nuclei are prominent, and mitoses are rare.
Staging
Accurate staging of hepatocellular carcinoma is necessary for prognostication and also to assist with selection of therapy. Determining the optimal staging system for hepatocellular carcinoma has been controversial, in part because it needs to take into account both the severity of the underlying liver disease and the size and degree of spread of the tumor. As with all cancers, the TNM (tumor-node-metastasis) system can be used to stage hepatocellular carcinoma, but this system does not factor in the underlying liver disease. A study66 comparing the usefulness of seven staging systems, including the Okuda, TNM, Cancer of the Liver Italian Program (CLIP), Barcelona Clinic Liver Cancer (BCLC), Chinese University Prognostic Index (CUPI), Japanese Integrated Staging (JIS), and Group d’Etude et Traitement du Carcinome Hépatocellulaire (GETCH) systems in a cohort of patients from the United States, has found the BCLC staging system to have the best independent predictive power for survival. The BCLC system has been adopted by the AASLD for use in its practice guidelines on management of hepatocellular carcinoma.25 This staging classification also includes a treatment schedule based on stage (Fig. 94-4).67
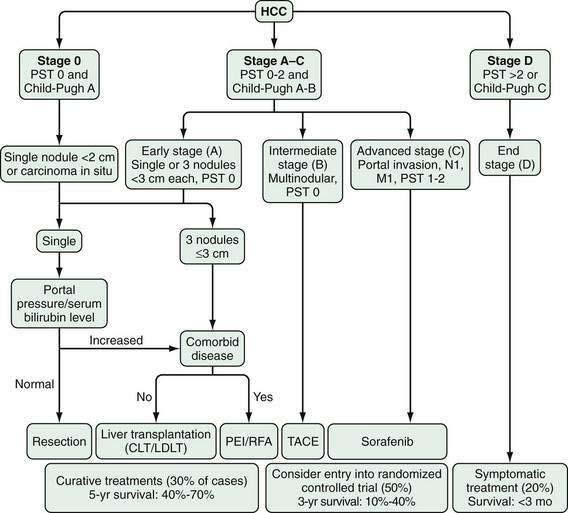
Figure 94-4. Barcelona Clinic Liver Cancer (BCLC) staging classification and treatment schedule. Staging is based on tumor size and spread, the patient’s performance status (PST) on a scale of 0 (good) to >2 (poor), and liver function as assessed by the Child-Pugh class (see Chapter 90). Patients with very early (stage 0) hepatocellular carcinoma (HCC) are optimal candidates for surgical resection. Patients with early (stage A) HCC are candidates for radical therapy (resection, cadaveric liver transplantation [CLT] or live-donor liver transplantation [LDLT], or local ablation via percutaneous ethanol injection [PEI] or radiofrequency ablation [RFA]). Patients with intermediate (stage B) HCC benefit from transarterial chemoembolization (TACE). Patients with advanced HCC, defined as the presence of macroscopic vascular invasion, extrahepatic spread, or cancer-related symptoms (PST 1 or 2) (stage C), benefit from sorafenib. Patients with end-stage disease (stage D) should receive symptomatic treatment. The treatment strategy will transition from one stage to another when treatment fails or is contraindicated. M, metastasis stage; N, nodal stage.
(Adapted from Llovet J, Di Bisceglie A, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst 2008; 100:698-711.)
Causes and Pathogenesis
In contrast to many other malignancies, for which risk factors can only sometimes be identified, the immediate cause of hepatocellular carcinoma can usually be identified and is most commonly chronic viral hepatitis or cirrhosis. Hepatocellular carcinoma is multifactorial in cause and complex in pathogenesis. Four major causative factors have been identified (Table 94-4). The differing blend of risk factors in various parts of the world may explain, in part, the diverse biologic characteristics of hepatocellular carcinoma in various populations.68
Table 94-4 Risk Factors for Hepatocellular Carcinoma
| Major Risk Factors |
| Other Liver Conditions |
| Inherited Conditions Not Associated with Liver Disease |
| Other Factors |
Hepatitis B Virus
Some 387 million carriers of HBV exist in the world today, and hepatocellular carcinoma will develop in as many as 25% of them (see Chapter 78). HBV accounts for up to 80% of hepatocellular carcinomas, which occur with high frequency in East Asian and African populations.68,69 Persistent HBV infection antedates the development of hepatocellular carcinoma by several to many years, an interval commensurate with a cause and effect relationship between the virus and the tumor. Indeed, in at-risk populations, the HBV carrier state is largely established in early childhood by perinatal or horizontal infection.70,71 Approximately 90% of children infected at this stage of life become chronic carriers of the virus, and these early-onset carriers face a lifetime relative risk for developing hepatocellular carcinoma of more than 100, compared with uninfected controls.72
An effective vaccine against HBV has been available since the early 1980s and, in countries in which this vaccine has been included in the expanded program of immunization for a sufficient length of time, the HBV carrier rate among children has decreased by 10-fold or more. Studies in Taiwan, where universal immunization was started in 1984 and where the rate of HBV carriage among children has decreased by more than 10-fold, have already shown a 70% reduction in the mortality rate from hepatocellular carcinoma in children in the vaccinated age groups.73 This finding gives promise for the ultimate eradication of HBV-induced hepatocellular carcinoma and provides further evidence of the causal role of the virus in the development of this tumor.
HBV DNA is integrated into cellular DNA in approximately 90% of HBV-related hepatocellular carcinomas.74 The sites of chromosomal insertion appear to be random, and whether viral integration is essential for hepatocarcinogenesis is still uncertain. The virus appears to be directly and indirectly carcinogenic.75 Possible direct carcinogenic effects include cis-activation of cellular genes as a result of viral integration, changes in the DNA sequences flanking the integrated viral DNA, transcriptional activation of remote cellular genes by HBV-encoded proteins, particularly the X protein, and effects resulting from viral mutations. The transcriptional activity of the HBV X protein may be mediated by interaction with specific transcription factors, activation of the mitogen-activated protein (MAP) kinase and Janus kinase–signal transducer and activator of transcription (JAK/STAT) pathways, an effect on apoptosis, and modulation of DNA repair. Studies have shown a clear link between the amount of HBV replication (measured as serum level of HBV DNA [viral load]) and subsequent risk of hepatocellular carcinoma. The long-term risk of hepatocellular carcinoma increases markedly in patients with serum HBV DNA levels higher than 104 copies/mL.76 A randomized controlled trial of antiviral therapy has also shown a reduction in the incidence of hepatocellular carcinoma in association with reductions in serum levels of HBV DNA on therapy.77
Indirect carcinogenic effects are the result of the chronic necroinflammatory hepatic disease, in particular cirrhosis, induced by the virus. The increased hepatocyte turnover rate resulting from continuous or recurring cycles of cell necrosis and regeneration acts as a potent tumor promoter. In addition, the distorted architecture characteristic of cirrhosis contributes to the loss of control of hepatocyte growth, and hepatic inflammation generates mutagenic reactive oxygen species. The transgenic mouse model of Chisari and coworkers has provided indirect support for the role of prolonged hepatocyte injury in hepatocarcinogenesis.78
Hepatitis C Virus
Approximately 170 million people in the world today are chronically infected with HCV and are at greatly increased risk for the development of hepatocellular carcinoma. In Japan, Italy, and Spain, HCV is the cause of about 75% of hepatocellular carcinomas, and, in other industrialized countries, HCV infection, often in combination with alcohol abuse, is emerging as a major cause of the tumor.68,79 Patients with HCV-induced hepatocellular carcinoma generally are older than those with HBV-related tumors, and it is likely that the HCV infection is acquired mainly in adult life.
Cirrhosis
In all parts of the world, hepatocellular carcinoma frequently coexists with cirrhosis.80 All causative forms of cirrhosis may be complicated by tumor formation. A long-term follow-up study of 2126 U.S. military veterans with cirrhosis found that hepatocellular carcinoma developed in 100 (4.7%) over an average period of 3.6 years.80 The calculated rate was 1.3/100 patient-years. Risk factors for hepatocellular carcinoma included obesity, low platelet count, and the presence of antibody to hepatitis B core antigen. A similar study from Italy found an incidence of hepatocellular carcinoma of 3.7/100 patient-years among persons with HCV infection and 2.0/100 patient-years among persons with HBV infection. Older age and male gender were confirmed as risk factors among patients with cirrhosis.81
Aflatoxin B1
Dietary exposure to aflatoxin B1, derived from the fungi Aspergillus flavus and Aspergillus parasiticus, is an important risk factor for hepatocellular carcinoma in parts of Africa and Asia. These molds are ubiquitous in nature and contaminate a number of staple foodstuffs in tropical and subtropical regions (see Chapter 87). Epidemiologic studies have shown a strong correlation between the dietary intake of aflatoxin B1 and incidence of hepatocellular carcinoma.82 Moreover, aflatoxin B1 and HBV interact synergistically in the pathogenesis of hepatocellular carcinoma. Heavy dietary exposure to aflatoxin B1 may contribute to hepatocarcinogenesis through an inactivating mutation of the third base of codon 249 of the TP53 tumor suppressor gene.83,84
Other Liver Conditions
Hepatocellular carcinoma develops in as many as 45% of patients with hemochromatosis (see Chapter 74).85 Malignant transformation was thought previously to occur only in the presence of cirrhosis (and is certainly more likely to do so), but this complication also has been reported in patients without cirrhosis.86 Excessive free iron in tissues may be carcinogenic, perhaps by generating mutagenic reactive oxygen species.87 Further support for this theory comes from the observations that black Africans with dietary iron overload are at increased risk of hepatocellular carcinoma88 and that rats fed a diet high in iron develop iron-free dysplastic foci and hepatocellular carcinoma in the absence of cirrhosis.89 Hepatocellular carcinoma develops occasionally in patients with Wilson disease, but only in the presence of cirrhosis (see Chapter 75).90 Malignant transformation has been attributed to the cirrhosis but also may result from oxidant stress secondary to the accumulation of copper in the liver.91 Hepatocellular carcinoma also may develop in patients with other inherited metabolic disorders that are complicated by cirrhosis, such as α1-antitrypsin deficiency and type 1 hereditary tyrosinemia, and in patients with certain inherited diseases in the absence of cirrhosis—for example, type 1 glycogen storage disease (see Chapter 76). Hepatocellular carcinoma develops in approximately 40% of patients with membranous obstruction of the inferior vena cava, a rare congenital or acquired anomaly (see Chapter 83). Continuous cycles of hepatocyte necrosis followed by regeneration resulting from the severe and unremitting hepatic venous congestion render the cells susceptible to environmental mutagens, as well as to spontaneous mutations.92
More recently, the role of obesity, diabetes mellitus, and fatty liver disease have come to be recognized in the causation of hepatocellular carcinoma,93–95 although the mechanisms whereby these overlapping conditions contribute to causing hepatocellular carcinoma is unknown. Certainly, cirrhosis caused by nonalcoholic steatohepatitis may give rise to hepatocellular carcinoma, but it appears that these risk factors may also be additive to chronic viral hepatitis and cirrhosis.
A statistically significant correlation between the use of oral contraceptive steroids and the occurrence of hepatocellular carcinoma has been demonstrated in countries in which the incidence of hepatocellular carcinoma is low and no overriding risk factor for development of the tumor is present.96 Epidemiologic evidence of a link between cigarette smoking and the occurrence of hepatocellular carcinoma is conflicting, although most of the evidence suggests that smoking is a minor risk factor.97 Heavy smokers have an approximately 50% higher risk than nonsmokers. The incidence of hepatocellular carcinoma is increased in patients with human immunodeficiency virus (HIV) infection compared with controls in the general population, presumably because of the increased rate of chronic viral hepatitis in the HIV-positive population.98
Although the aforementioned risk factors have been identified, the precise mechanisms whereby they lead to hepatocellular carcinoma still need to be elucidated. Multiple cellular pathways are involved in causing unconstrained proliferation of hepatocytes and increased angiogenesis against a background of chronic liver disease. These pathways have become the targets for new molecular therapies against hepatocellular carcinoma (Table 94-5).99
Table 94-5 Key Molecular Pathways Involved in Hepatocarcinogenesis
JAK/STAT, janus kinase/signal transducers and activators of transcription; mTOR, mammalian target of rapamycin.
Adapted from Roberts L. Emerging experimental therapies for hepatocellular carcinoma: What if you can’t cure? In: McCullough A, ed. AASLD Postgraduate Course, 2007. Boston: AASLD, 2007; p 185.
Natural History and Prognosis
Symptomatic hepatocellular carcinoma carries a grave prognosis; in fact, the annual incidence and mortality rates for the tumor are almost identical. The main reasons for the poor outcome are the extent of tumor burden when the patient is first seen and the frequent presence of coexisting cirrhosis and hepatic dysfunction. The natural history of hepatocellular carcinoma in its florid form is one of rapid progression, with increasing hepatomegaly, abdominal pain, wasting, and deepening jaundice, and with death ensuing in two to four months. In industrialized countries, however, the tumor appears to run a more indolent course with longer survival times.100 Rare cases of spontaneous tumor regression have been reported.
Treatment
Important advances in the treatment of hepatocellular carcinoma have occurred since the 1980s; these advances include randomized controlled trials that support the benefits of certain treatments such as chemoembolization and the multikinase inhibitor sorafenib. Overwhelming evidence supports the superiority of liver transplantation over other therapies for patients with portal hypertension and cirrhosis. Because hepatocellular carcinoma is usually a combination of two diseases—the underlying liver disease (usually cirrhosis with varying degrees of decompensation) and the cancer itself—both factors must be taken into account when selecting treatment. When presented with a patient with hepatocellular carcinoma, the clinician should decide which is the best initial therapy: surgical resection or liver transplantation, if the patient is a candidate for either; ethanol or radiofrequency ablation, if possible based on the size of the tumor; chemoembolization; and, if the tumor is too advanced, sorafenib or a clinical trial. Table 94-6 describes the treatment options for hepatocellular carcinoma. The BCLC staging classification and treatment schedule can help guide the clinician in choosing the most appropriate treatment (see Fig. 94-4).
Table 94-6 Treatment Options for Hepatocellular Carcinoma
| MODALITY | COMMENTS |
|---|---|
| Surgical resection | Curative but limited to noncirrhotic patients and cirrhotic patients without portal hypertension |
| May be technically difficult | |
| High recurrence rate | |
| Liver transplantation | Successful in selected patients (Milan criteria; see text and Chapter 95) |
| Requires lifelong immunosuppression | |
| Expensive and not available worldwide | |
| Alcohol injection and radiofrequency ablation | Potentially curative for small tumors, including multiple tumors |
| High recurrence rate | |
| Chemoembolization | Prolongs survival in unresectable tumors if hepatic function is preserved |
| Chemotherapy | Palliative only; can be used as an adjunct to surgical resection or transplantation |
| Drug toxicity common | |
| Targeted molecular therapies | Sorafenib is the first such agent shown to improve patient survival |
Surgical Resection
Surgical therapy, whether by tumor resection or liver transplantation, offers the best chance of cure for hepatocellular carcinoma. For resection to be considered, the tumor should be confined to one lobe of the liver, favorably located, and, ideally, the nontumorous liver tissue should not be cirrhotic. Expert surgical centers can achieve five- and ten-year survival rates of 40% and 26%, respectively, with a mean tumor diameter of 8.8 cm in noncirrhotic patients.101 Unfortunately, these patients represent less than 5% of Western cases.102,103 Resection is also effective if the tumor is limited to the left lobe or a portion of the right lobe, thereby permitting a segmental resection if the patient has Child (Child-Pugh) class A cirrhosis, the serum bilirubin level is normal, and portal hypertension is not present (based on imaging, a normal platelet count, and lack of varices on endoscopy or on direct measurement of the hepatic venous pressure gradient). Using these criteria, five-year survival rates of 50% can be achieved. In parts of the world where liver transplantation is not available, surgical resection is a viable option, particularly for Child class A patients without portal hypertension and with a Model for End-stage Liver Disease (MELD) score of 9 (see Chapter 90). All the tumor nodules need to be removed with negative margins, and the patient needs to be left with enough functional liver volume (usually defined as ≅40%) to survive the postoperative period.104–106 Overall, resection is feasible in only approximately 15% of patients. Resection performed at expert surgical centers carries an operative mortality rate of less than 5%, but at low volume centers the mortality rate is almost three times greater.107 Unfortunately, recurrence after resection occurs in more than 50% in the long term, and salvage liver transplantation is rarely possible.108
Liver Transplantation
Liver transplantation is performed in patients in whom the tumor is not resectable but is confined to the liver or in whom advanced cirrhosis and poor liver function preclude resection (see Chapter 95).25 Liver transplantation is the ideal therapy for hepatocellular carcinoma because it provides the largest possible resection margin, removes the remaining liver, which is at high risk for de novo tumors, and replaces the dysfunctioning liver. Liver transplantation can fail in patients with extrahepatic tumor, which tends to grow rapidly under the influence of post-transplantation immunosuppression. Because the availability of donor livers is limited, the consensus is that the outcomes of liver transplantation for hepatocellular carcinoma should be similar to those for other indications for liver transplantation and superior to those for other treatments for hepatocellular carcinoma. Several large series have demonstrated that if one selects candidates based on the Milan criteria—a single lesion up to 5 cm in size or two to three lesions, each up to 3 cm, with no large-vessel vascular invasion or metastasis—the five-year survival rate is 70% to 75%, and the tumor recurrence rate is 10% to 15%.102,109–111 These criteria led to the hepatocellular carcinoma MELD exception pathway, which was adopted in the United States in 2002. As a result of the change, the frequency of hepatocellular carcinoma as an indication for liver transplantation rose from 4.6% to 26% of the total adult liver transplant population. Additionally, progression of the tumor beyond the Milan criteria before a patient undergoes transplantation has largely been eliminated.38,112 In other parts of the world, waiting times before transplantation remain critical, and when the waiting time increases to one year, as many as one half of patients will not receive a transplant.102 An analysis of four-year survival rates for all patients transplanted in the United States has confirmed that overall outcomes for those transplanted with hepatocellular carcinoma are only minimally worse than for those transplanted for other indications.38 Certain subgroups of patients do worse, including those with nodules 3 to 5 cm in diameter, a MELD score of 20, and a serum AFP level of ≥455 ng/mL.
Some authorities have advocated a modest expansion of the Milan criteria, with use of the so-called University of California, San Francisco (UCSF) criteria (a single lesion up to 6.5 cm in diameter or two or three lesions up to 4.5 cm each, with a total tumor diameter of 8 cm), based on excellent prospective outcomes from a small, single-center series, but these patients generally need a special exception from the regional review board in the United States.113 Other groups who use similar criteria have shown similarly good results.114 A larger multicenter study is needed before these criteria can be widely adopted.
Local Ablation
Local ablative therapies are potentially curative treatments for patients with small tumors, usually smaller than 3 to 5 cm in diameter, that are not amenable to resection or liver transplantation because of patient preference, the number and location of lesions, or significant hepatic dysfunction (Child class B or C; see Fig. 94-4).25,115 The first of these techniques available was percutaneous ethanol injection (PEI), a relatively effective and safe method that is still used and is most effective for lesions smaller than 2 to 3 cm in diameter.116 PEI requires multiple sessions and, in patients with small tumors and intact hepatic function, can lead to survival rates similar to those for surgical resection, although no randomized studies have been performed to demonstrate equivalent outcomes.117 Complications are rare but include tumor seeding of the needle track. More recently, radiofrequency ablation (RFA) has supplanted PEI, because it is more effective, particularly with larger tumors (up to 3 to 5 cm), requires fewer sessions, and has similar complication rates.118 RFA can be performed percutaneously or by a laparoscopic or open surgical approach. Survival rates are similar to those for surgical resection, although recurrence rates are higher and complications are uncommon.117,119 PEI is generally favored over RFA for lesions adjacent to a major vessel or large bile ducts. A randomized study has suggested that a combination of RFA and chemoembolization for tumors larger than 3 cm in diameter produces a survival benefit when compared with either treatment alone.120 PEI and RFA have been used to stabilize tumor growth in patients awaiting liver transplantation, but their use for this purpose is controversial and probably unnecessary, unless the waiting time for transplantation is more than six months or the tumor burden is near the limits of acceptability for transplantation.102,121–123
Chemoembolization
Transarterial chemoembolization (TACE) is a palliative treatment reserved for patients with relatively intact hepatic function (Child class A or B) and tumors not amenable to local ablative treatments because of size, number, or location (see Fig. 94-4).25,121 Six randomized trials and a meta-analysis have compared embolization or chemoembolization with supportive care and have shown overall improved survival with treatment.124–130 The effectiveness of TACE before liver transplantation has not been fully elucidated, but TACE can be considered if the waiting time for transplantation is more than six months or the tumor size is near the acceptable limit.131,132 Theoretically, TACE can be used to reduce the size of the tumor to make resection or transplantation possible (downstaging) or to allow a more conservative resection, although study results are mixed as to whether this approach is effective.133,134
Chemotherapy
A large number of anticancer drugs, including alkylating agents, antitumor antibiotics, antimetabolites, plant alkaloids, platinum derivatives, procarbazine, estrogen receptor modulators, and somatostatin, have been tried alone and in various combinations and by different routes of administration for the treatment of hepatocellular carcinoma, but response rates have invariably been less than 20%.126,135 Several small-molecule, targeted anticancer agents have been developed and studied for the treatment of hepatocellular carcinoma. Sorafenib, an inhibitor of Raf kinase and the tyrosine kinase activity of vascular endothelial growth factor receptors (VEGFRs) and platelet-derived growth factor receptor (PDGFR), is the first of these new agents to show modest improvement in survival compared with supportive care.136 The drug should be considered for patients with intact hepatic function (Child class A) and portal vein thrombosis, extrahepatic tumor, or failures of other therapies (see Fig. 94-4). Other targeted agents, alone and in combination with each other and with traditional chemotherapy, are being studied. Patients with advanced hepatic dysfunction (Child class C) or advanced tumor symptoms (Eastern Cooperative Oncology Group [ECOG] performance status > 2) have such a poor prognosis that only supportive care should be offered (see Fig. 94-4).25
Screening
Because symptomatic hepatocellular carcinoma seldom is amenable to surgical cure and responds poorly to conservative treatments, a pressing need exists to prevent the tumor or detect it at a presymptomatic stage when surgical intervention is still possible. Programs for detecting subclinical hepatocellular carcinomas are of two types: (1) screening whole populations with a high incidence of the tumor; and (2) case finding and long-term surveillance of persons at high risk for the development of hepatocellular carcinoma. Mass population screening has rarely been attempted, whereas case finding and surveillance of high-risk persons are more feasible25 and have been shown to be cost-effective in countries with a high incidence of the tumor.
An AASLD practice guideline published in 2005 provides recommendations for screening (Table 94-7).25
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree