General Features
The studies of Rocha and Kokko
177 and Burg and Green
178 were the first to investigate salt absorption in the thick ascending limb, and their work defined several key features of this unique epithelium. First, salt absorption in the medullary and cortical TAL generates a lumen-positive transepithelial voltage, which is sensitive to furosemide. Second, the transport of Cl
– occurs against both electrical and chemical gradients and involves an active transport process that is dependent on intact basolateral Na
+,K
+-ATPase activity.
179 A final important feature of the TAL is that this segment consists of a tight epithelium, which despite its high ionic conductance, possesses a very low permeability to water. The apical membrane of the TAL constitutes the major barrier to transcellular and paracellular water flow.
180 The high
ionic conductance and low water permeability effectively further dilutes fluid entering the TAL from the ascending thin limb.
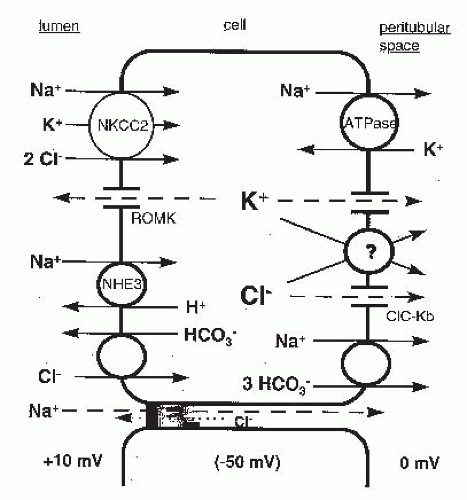
Figure 5.6 integrates the results of several electrophysiologic and biochemical studies to provide a model of salt reabsorption in the thick ascending limb. According to this model, net Cl
– absorption by the TAL is a secondary active transport process. Luminal Cl
– entry into the cell is mediated by an electroneutral Na
+-K
+-2Cl
– cotransport process driven predominantly by the favorable electrochemical gradient for Na
+ entry.
181 Because the Na
+ gradient is maintained by the continuous operation of the basolateral membrane Na
+,K
+-ATPase pump, the apical entry of Cl
– via the cotransporter ultimately depends on the operation of the basolateral Na
+,K
+-ATPase.
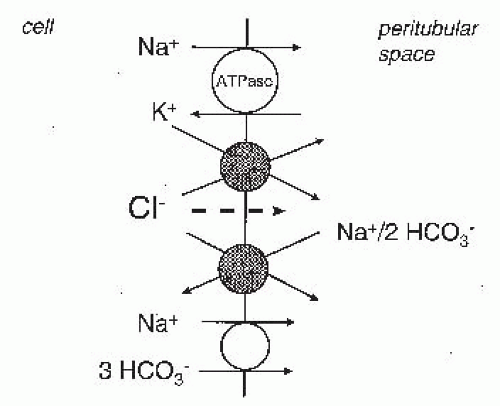
In contrast to the electroneutral entry of Cl
– across the apical membrane, the majority of Cl
– efflux across the basolateral membrane proceeds through conductive pathways.
182,
183 A favorable electrochemical gradient for Cl
– efflux through dissipative pathways has been demonstrated by Greger et al.
184 in the rabbit cTAL. Intracellular Cl
– is maintained at concentrations above electrochemical equilibrium by the continued entry of Cl
– via the apical Na
+-K
+-2Cl
– cotransporter.
185According to the model in
Figure 5.6, K
+ that enters TAL cells via the Na
+-K
+-2Cl
– cotransporter recycles, to a large extent, across the apical membrane via a K
+-conductive pathway. This apical K
+ recycling serves several purposes. First, it ensures a continued supply of luminal K
+ to sustain Na
+-K
+-2Cl
– cotransport. Without recycling, the luminal K
+ concentration would fall rapidly as a consequence of K
+ entry via Na
+-K
+-2Cl
– cotransport and would limit net NaCl absorption. Second, the apical membrane K
+ current provides a pathway for net K
+ secretion by the TAL. In mouse TAL, for example, the rate of K
+ secretion amounts to about 10% of the rate of net Cl
– absorption.
182 K
+ secretion in this segment is an active process, ultimately driven by the Na
+,K
+-ATPase, proceeding in the face of a lumen-positive transepithelial potential. Third, under open circuit conditions, the transcellular and paracellular pathways form a current loop in which the currents traversing the two pathways are of equal size, but which traverse in the opposite direction. The potassium current from cell to lumen polarizes the lumen and causes an equivalent current to flow from the lumen to the bath through the paracellular pathway.
186 Because the paracellular pathway is cation selective (P
Na/P
Cl = 2 to 6), the majority of the current through the paracellular pathway is carried by Na
+ moving from the lumen to the interstitium. This paracellular absorption of Na
+ increases the efficiency of Na
+ transport by the TAL.
187 With reference to
Figure 5.6, for each Na
+ transported through the cell and requiring the use of ATP, one Na
+ is transported through the paracellular pathway without any additional energy expenditure. Finally, the apical K
+ current satisfies the continuity requirement imposed by a high degree of conductive Cl
– efflux across basolateral membranes.
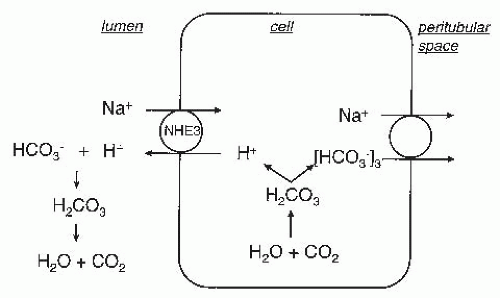
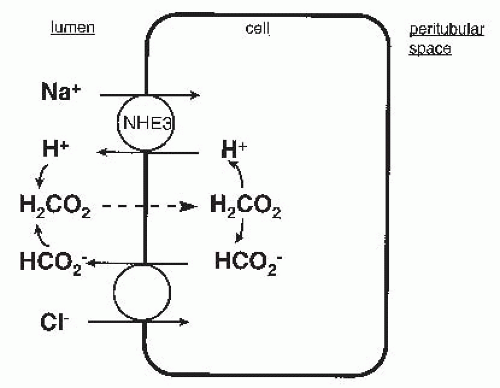
182A small component of Na
+ transport by the TAL is accounted for by NaHCO
3 absorption.
188 In the rat TAL, the rate of NaHCO
3 absorption is roughly 5% to 10% of that for NaCl absorption. NaHCO
3 absorption appears to be mediated by an apical membrane amiloride-sensitive Na
+/H
+ exchanger and a basolateral membrane electrogenic Na
+-3(HCO
3–) cotransporter.
188The following sections will describe the individual components of the mechanism for TAL salt transport (
Fig. 5.6) in greater detail.
Apical Na+-K+-2Cl– Cotransport
Studies of Cl
– transport across apical membranes of intact TAL segments
189 and in isolated membrane vesicle preparations
190 established that the predominant mode for Cl
– entry into the TAL cell is via a Na
+-K
+-2Cl
– cotransporter. A characteristic feature of this transporter is its sensitivity to inhibition by furosemide, bumetanide, and other 5-sulfamoylbenzoic acid derivatives.
191 The measurement of isotope flux into TAL cells or membrane vesicles prepared from the inner stripe of the outer medulla yielded a stoichiometry of 1 Na
+:1 K
+:2 Cl
– cotransport.
190 K
+-independent NaCl cotransport has also been described under certain conditions.
192The proteins that mediate the Na
+-K
+-2Cl
– cotransport have been cloned. An absorptive form of the Na
+-K
+-2Cl
– cotransporter, referred to as NKCC2 or BSC1, was initially cloned by Gamba et al.
193 based on sequence homology to the thiazide-sensitive Na
+-Cl
– cotransporter (see the following). A second Na
+-K
+-2Cl
– cotransporter, NKCC1, was cloned by Payne et al.
194 NKCC2 (BSC1) is the primary mediator of apical salt entry in the thick ascending limb. In situ hybridization and single-nephron reverse transcriptase polymerase
chain reaction (RT-PCR) studies demonstrated the expression of NKCC2 in the MTAL and CTAL,
195 and immunohistochemical studies indicate that NKCC2 is localized to the apical membrane of these nephron segments.
196 The importance of NKCC2 in mediating salt reabsorption in the TAL is illustrated by the fact that loss-of-function mutations of NKCC2 cause Bartter syndrome (
Table 5.1),
197 a Mendelian salt-wasting disorder characterized by hypokalemia, metabolic alkalosis, hyperaldosteronism, and normal-to-low blood pressure, results from a defect in salt absorption by the thick ascending limb.
The NKCC2 cDNA encodes a glycoprotein containing ˜1100 amino acids and having a predicted molecular weight of 115 to 120 kDa.
193 The full-length protein contains 12 transmembrane domains containing a sizable extracellular loop with N-glycosylation sites positioned between transmembrane segments 7 and 8, and large intracellular amino and carboxy termini flank the transmembrane regions. NKCC2 belongs to the SLC12A family of cation chloride cotransporters, which is part of the amino acid polyamine organocation cotransporter (APC) superfamily.
198 Based on the homology to other crystallized APC family members, the cotransporter structure probably consists of two clustered groups of five transmembrane helices that are positioned in a symmetric, inverted orientation.
199 The details regarding how this fold facilitates the three-ion cotransport remain obscure but surely will provide an initial framework for more detailed structure-function studies in the coming years.
The two cytoplasmic domains of NKCC2 mediate specific regulatory functions. The amino terminus is believed to be unstructured and contains several cytosol-exposed serines and threonines, which are phosphoacceptor sites. These residues are phosphorylated by at least three protein kinases that stimulate NKCC2 activity and/or plasma membrane expression (discussed in detail below).
200 The carboxy terminus is large and comprises ˜40% of the total NKCC2 sequence. It may also contain phosphorylation sites, although to date this has not been explored in detail. It is clear, however, that the NKCC2 C-terminus serves as a hub for interactions with proteins that regulate its trafficking, including the glycolytic enzyme aldolase
201 and secretory membrane carrier protein 2 (SCAMP2).
202 It also serves as the interface for the formation of NKCC2 homodimers,
203 which was confirmed recently when the crystal structure of the C-terminus of the related prokaryotic cation chloride cotransporter MaCCC was solved.
199At least six isoforms of NKCC2 have been identified.
200 These isoforms are the result of alternative splicing of two regions of the NKCC2 gene: the first region is a 96 base pair region that encodes part of the second transmembrane domain, whereas the second region encompasses the extreme C-terminus. Three variants of the 96 base pair region are encoded by different versions of exon 4 of the NKCC2 gene. These exons are differentially spliced into NKCC2 pre-mRNAs to generate three distinct isoforms (A, B, and F), which alter the amino acid composition of the second transmembrane domain. Each of the A, B, and F isoforms can have either a long C-terminus, or a truncated C-terminus; although to date, the short isoforms have only been described in the murine TAL.
204 In addition, several “tandem” transcripts have been described in the human kidney; these contain combinations of exons 4A, 4B, and 4F spliced alongside one another into the NKCC2 pre-mRNA.
205 Transcripts containing exons 4A/4F, 4B/4A, and 4B/4A/4F have been reported. Because these tandem transcripts contain redundant sequences encoding for the second transmembrane domain, they probably cause the misfolding of NKCC2, resulting in the formation of nonfunctional isoforms. Because these isoforms may still form oligomers with NKCC2, they likely exert a dominant-negative effect on NKCC2 function and inhibit its activity.
206The A, B, and F isoforms show differential expression within the thick ascending limb. In the rat nephron, the A isoform was found in both the cortical and medullary TAL, the B isoform is restricted to the cortical TAL, whereas the F isoform is present in the medullary, but not the cortical, the TAL, and to a lesser extent, in the outer medullary collecting duct. Although some interspecies discrepancies have been noted, similar findings have generally been observed in the embryonic mouse and human kidney.
205,
207,
208When the 4A, 4B, and 4F exons are spliced into transcripts containing a long C-terminus, all three products are capable of mediating Na
+-K
+-2Cl
– cotransport. However, the A, B, and F isoforms have different transport properties that may have physiologic relevance. Isoforms A and B have higher affinities for Na
+, K
+, and Cl
– than the F isoform. The A isoform possesses the highest transport capacity of all three isoforms. Based on the known distribution of the A, B, and F isoforms in the TAL, it is currently thought that the A isoform accounts for the high transport capacity of the medullary TAL, whereas the presence of the more active A and B isoforms in the cortical TAL allows for the continued reabsorption of salt to take place, even though the tubular fluid in this segment is more dilute than plasma. Supporting this is experimental evidence demonstrating that the NKCC2 A and B isoforms can both be strongly activated by Na
+, K
+, and Cl
– at concentrations that are much more dilute than the composition of tubular fluid in the cortical TAL.
208