Energy
25–40 kcal/kg/day, with extra 10 % in patients with ascites
Carbohydrate
• 50–60 % of total calories
• Blood glucose monitoring recommended especially in patients treated with corticosteroids
Protein
• 1.0–1.5 g/kg/day even in patients with mild encephalopathy
• In patients with grade 3 or 4 hepatic encephalopathy, 0.6–0.8 g/kg/day starting dose recommended with gradual increase
• Whole protein formula recommended except patients in grade 3 or 4 encephalopathy when use of branched chain amino acids is recommended
Fat
20–30 % of total energy intake
Fluids
40–50 mL/kg/day, with restriction to 1.0–1.5 L/day if serum sodium <125 mmol/L
Thiamine
100 mg/day, given for at least 2 weeks after hospitalization
Folic acid
1 mg/day, given for at least 2 weeks after hospitalization
Vitamin D
50,000 units three times per week
Vitamin A
10,000 units daily or 50,000 units three times per week
Vitamin E
400 units/day
Iron
Use only if iron deficiency
Calcium
1200–1500 mg/day
Zinc
220 mg twice daily
Corticosteroids
Corticosteroids (prednisone or methylprednisolone) represent the most intensely studied pharmacological treatment in AH. They are aimed at suppressing the hepatic inflammatory response through inhibition of nuclear factor κB transcriptional activity [70], which is thought to be a crucial signaling pathway activating innate immune response in alcoholic liver disease [71, 72]. In spite of limitations of available therapeutic trials, corticosteroids are probably the most effective mortality-reducing agents in certain subgroups of patients with AH. Their use was incorporated into the AASLD [3] and EASL (European Association for the Study of Liver) [12] guidelines that recommend to initiate treatment with steroids in patients with severe forms of AH (defined as Maddrey’s discriminant function (DF) >32) on the basis of two meta-analyses showing reduction in short-term mortality [73, 74]. There are strengths and limitations of the use of corticosteroids in AH indicated by the diverse selection criteria and study designs used in the evaluation of the therapeutic benefits of corticosteroids. The phrase from Dr. Charles Davidson, “prednisolone survival effect becomes evident only in a group of patients [with AH] neither so ill that their fate is already sealed nor yet so well that they would recover anyway,” well portrays the limited patient population where corticosteroids might be beneficial [75–77].
Review of Studies of Corticosteroids in Patients with AH
The therapeutic use of corticosteroids in AH has to be viewed from the perspective of trials in which their effect was evaluated. These trials have numerous limitations, including the heterogeneity of patients with AH, presence of nonresponders to corticosteroids, and exclusion of patients with infections, GI bleeding, or renal insufficiency.
The “Steroid Controversy”
AH is associated with significant inpatient mortality that can reach up to 80 % in the most severe forms [78]. The main causes of death are liver failure, sepsis, hepatorenal syndrome, and gastrointestinal bleeding. Evaluation of treatment effect on short-term survival requires identification of patients with significant risk of death. Until the Discriminant Function became available [78, 79], no objective and reproducible criterion existed to predict early mortality. The heterogeneity of patients with AH that were enrolled in corticosteroid trials in pre-DF era resulted in a wide range of survival in the placebo-treated arms, ranging from 81 % if patients with mild AH were included to 0 % survival in trials with patients with the most severe AH [76–83].
The DF described by Maddrey et al. identifies patients with a high risk of early (1–2-month) mortality [79, 81]. It is calculated as 4.6 (prothrombin time − control prothrombin time [in seconds] + bilirubin [in μmol/L]/17). The presence of DF > 32 and/or encephalopathy is used as a definition criteria for acute AH, which, in the absence of treatment, has spontaneous survival between 50 and 65 % [78, 79, 81]. After acceptance of DF > 32 as inclusion criteria of patients with AH to the clinical trials, the short-term survival of glucocorticoid-treated patients has remained remarkably constant, with a 2-month survival rate of approximately 80 % [73, 79, 84–86].
The variable severity of AH in patients enrolled into clinical trials was likely the strongest contributor to the “controversial evidence” of the efficacy of steroids in AH, advocated by some authors [87–89]. This skepticism was further deepened by the widespread belief in the 1970s and 1980s that corticosteroids caused duodenal ulcers [90] and by the belief that corticosteroids increase the risk of infection [91]. The latter myths have been recently disproved by two randomized controlled trials ([91, 92] and below), and the “steroid controversy” has been the subject of multiple meta-analyses [73, 74, 89, 93, 94] and of an ongoing prospective trial [88].
The attempts to clarify whether corticosteroids improve survival in AH followed two approaches. The first approach relied on the classical methodology of meta-analysis utilizing published results of randomized controlled trials [89, 93, 94]. The second approach was based on a meta-analysis of primary, raw data derived from randomized controlled trials but restricted only to patients with severe alcoholic hepatitis (i.e., those having DF > 32 and/or encephalopathy) [73, 74].
In 1990, Imperiale et al. [93] studied 11 randomized trials from 1966 to 1989 and found that patients with alcoholic hepatitis treated with steroids were associated with a 36 % (CI 95 %, 28–50 %) reduction in mortality. In this meta-analysis, the mortality benefit was only evident in patients with hepatic encephalopathy, and the influence of admission DF on therapeutic effect of steroids was not evaluated in this study [93]. In 1994, Christensen et al. [94] examined 13 randomized controlled trials published between 1971 and 1992 and found no survival benefit of therapy of corticosteroids. In this meta-analysis, Christensen et al. did not perform a subgroup analysis of the data [94], but a review of the studies included in that meta-analysis indicates the survival benefit of steroids in all three studies in which Maddrey’s DF was used as inclusion criteria (studies [78, 79, 81] within meta-analysis [94]). This study was followed by another meta-analysis from the same group, published in 2008 under the label of the Cochrane Hepato-Biliary consortium [89] that examined 15 randomized trials with a total of 721 patients. The study found that corticosteroids did not reduce mortality of the entire population of patients with alcoholic hepatitis, compared with placebo, but mortality was statistically reduced in subgroups that were either experiencing hepatic encephalopathy or could be characterized as having severe AH with DF > 32 [89]. In spite of the beneficial effect of corticosteroids in patients with severe AH (as opposed to patients with mild or moderate AH), both meta-analyses [89, 94] lead Christensen et al. to conclude that corticosteroids are ineffective in patients with AH [94].The appropriateness of this conclusion was challenged by Imperiale, O’Connor, and McCullough [95] who emphasized the beneficial effect of corticosteroids in patients with severe alcoholic hepatitis.
In 2001, Mathurin et al. [73] used pooled primary data from three placebo-controlled trials to compare corticosteroids with placebo in patients, all of whom had severe AH (DF > 32). In this group of patients, at 28 days, patients who received corticosteroids had a significantly higher survival (85 % vs. 65 %) compared to those who received placebo, revealing that steroid treatment of severe alcoholic hepatitis is associated with a survival advantage, with a number needed to treat of 5. Increasing age and serum creatinine were found to be independent prognostic factors for death from severe AH in this study [73]. Nine years later, Mathurin et al. published another meta-analysis [74] based on individual patient data by adding two additional randomized controlled trials [65, 96] to their previous analysis [73]. Again, only data for patients with DF > 32 on admission were included in meta-analysis. Combination of all five RCTs [65, 79, 81, 96, 97] confirmed beneficial effects of corticosteroids on short-term survival, demonstrating an 80 % survival of patients treated with steroids at one month, compared to 65 % survival in patients treated with placebo [74].
Nonresponders to Corticosteroid Treatment
About one-fourth of patients with severe AH (DF > 32) will not respond to corticosteroids [85]. These patients have a grim prognosis, with fewer than 25 % surviving 6 months. The benefits of early identification of nonresponders to steroid treatment would be that these patients could be spared of side effects of unnecessary corticosteroid treatment and that alternative therapies, such as biological treatment or liver transplant, advocated by some authors [98, 99], could be considered.
Nonresponsiveness to steroids can be predicted in an assay that tests responsiveness to prednisone in peripheral blood mononuclear cells isolated from patients with AH [100]. In this assay, termed DILPA (dexamethasone suppression of lymphocyte proliferation test), lymphocytes isolated from the blood of patients with severe AH are stimulated with phytohemagglutinin in vitro with or without the presence of dexamethasone [100]. The assay duration is about 48 h and requires 3H thymidine for the testing of lymphocyte proliferation. A lack of response to steroids is defined as suppression of lymphocyte proliferation by less than 60 % of the maximal proliferation count. The accuracy of the DILPA assay in predicting 6-month survival in patients with severe AH is 0.86 (as determined by area under the ROC) [101]. The DILPA assay has not been widely accepted in clinical practice.
Out of clinical parameters that would predict responsive to corticosteroids, the early change in bilirubin levels (ECBL, defined as bilirubin level at 7 days lower than bilirubin level on the first day of treatment) has shown high predictive value [85]. In their 2003 prospective study involving 238 patients, Mathurin et al. [85] set out to identify clinical and laboratory markers that would predict lack of response to corticosteroids in patients with severe AH (DF > 32). They used 6-month survival as an end point because of the rule requiring 6 months for listing alcoholic patients for liver transplantation. All patients were treated with corticosteroids. The overall 1-month survival was 85 %, consistent with previous data on survival in corticosteroid-treated patients with AH [73, 74], and the 6-month survival was 64 %. An ECBL at 7 days was observed in 75 % of patients. At 6 months, survival of patients with ECBL (83 %) was significantly higher than in patients without ECBL (23 %). ECBL was by far the strongest predictor of 6-month survival (odds ratio 7.1 [95 % confidence interval 4.0–11.1]) for 6-month survival compared to patients with no ECBL. The study suggested that evolution of bilirubin level at 7 days may be useful in identification of severe AH patients with poor prognosis, and the predictive value of ECBL was higher than that of PT or DF.
The ECBL has been incorporated as a dynamic component into the Lille model predicting the 6-month survival in patients with severe AH [102]. Other clinical predictors included in the Lille model are age, the presence of renal insufficiency, albumin level, and prothrombin time. The Lille score calculator (available at http://www.lillemodel.com/score.asp) generates a score that ranges between 0 and 1. The ideal cutoff of 0.45 can be used to define responders to corticosteroids (Lille score < 0.45) and nonresponders (Lille score > 0.45). The therapeutic response to steroids can be further refined by defining three populations: complete responders (Lille score < 0.16), partial responders (Lille score 0.16–0.56), and null responders (Lille score > 0.56) [98]. This sub-stratification has prognostic value, as demonstrated in two subsequent studies by Mathurin’s group [92, 103]: the 6-month survival is about 90 % in complete responders, 45–78 % for partial responders, and less than 45 % in null responders. DF, MELD, or Glasgow score have significantly worse accuracy to predict 6-month survival, compared to the Lille score (are under the ROC of 0.73, 0.72, and 0.67, respectively, vs. 0.85 for Lille score) [102]. In patients with Lille score > 0.45 (nonresponders), the EASL guidelines recommend to discontinue corticosteroids after 7 days of treatment [12].
Steroids in AH Patients with Infection: Timing Matters
Infection has long been considered a contraindication to glucocorticoid therapy in patients with severe alcoholic hepatitis. It is estimated that the presence of infection as exclusion criterion in patients with severe AH may have barred 25 % of otherwise eligible patients from consideration of treatment [91]. It is thought that liver failure with resulting immune paralysis is a major factor driving increased susceptibility to bacterial infections in patients with advanced liver cirrhosis or severe AH [104, 105].
In their prospective study, Louvet et al. [91] performed screening for infection in 246 patients admitted for severe AH. All patients underwent chest X-ray and their blood, urine, and ascites were cultured. Out of all patients, 63 (25 %) were found to have an infection (spontaneous bacterial peritonitis, urinary tract infection, and pneumonia being the most prevalent infections) and received antibiotic treatment. Corticosteroids were started only after a priori defined criteria for antibiotic treatment response were met. Patients with severe AH and with infection responding to antibiotic treatment had similar 2-month survival compared to patients with severe AH and no infection (71 % vs. 72 %). Data on 6-month survival were not provided in the study [91].
However, there was a significant difference in survival when patients with infection treated prior to initiation of steroids (above) were compared to patients who developed infection while on treatment with steroids. After initiation of corticosteroids, 57 patients (24 %) developed an infection after a median time of 14 days, and there has been substantial proportional increase in pneumonia (40 % of infected patients). Patients infected after corticosteroid treatment had significantly lower 2-month survival (46 %) compared to noninfected patients (78 %). Only the Lille score and MELD score were independent predictors of survival in these patients, whereas ascites, encephalopathy, Maddrey DF, leukocytosis, or C-reactive protein levels were not. Importantly, the presence of infection after corticosteroid initiation proved to be a negative determinant of survival only in patients who responded to corticosteroids (i.e., Lille score < 0.45), but not in nonresponders (Lille score > 0.45) where it is thought that it was the lack of steroid response but not infection determining unfavorable prognosis in nonresponders [91].
This study suggested that it is safe to use corticosteroids in patients with severe AH if infection is identified and treated prior to initiations of corticosteroids. The study also showed that survival benefit of antibiotic therapy in patients who develop infection after initiation of corticosteroid treatment can be expected only in responders to steroids [91].
Steroids in AH Patients with GI Bleeding
Both AASLD and EASL guidelines specify that patients with severe AH with recent upper GI bleed are not ideal candidates for corticosteroid treatment [3, 12]. The reason for this may be a carryover from clinical trials performed in the 1970s and 1980s in which patients with upper GI bleeding were excluded due to the belief that corticosteroids caused gastroduodenal ulcers and also because no effective treatment of upper GI bleeding existed at that time [90]. Since then, however, much has changed, including the advent of proton pump inhibitors, endoscopic treatment of variceal bleeding, and transjugular portosystemic shunting [106]. Also, antibiotics given for 7 days after GI bleeding in patients with cirrhosis reduce infections and increase survival [107].
Rudler et al. [92] conducted a retrospective analysis of survival among patients with severe AH who presented to a hospital with upper GI bleed and compared them with patients with severe AH without GI bleeding. A total of 48 patients with upper GI bleed and 47 patients without GI bleed were analyzed. The two groups did not differ in the presence of AH on biopsy (approximately 80 %) and DF or MELD score. After stabilization and effective bleeding control per Baveno V recommendations [108], both groups were started on corticosteroids. The 6-month survival was similar in both groups (74 % vs. 70 %). The probability of developing an infection after starting corticosteroids was lower among subjects with upper GI bleed (24 %) as compared with subjects without upper GI bleed (45 %). This was attributable to antibiotic therapy mandated in patients with acute GI bleed and could have improved survival in GI bleeders in the study. If validated in prospective trials, this data indicate that GI bleed does not worsen survival in AH patients treated with corticosteroids [92].
Pentoxifylline
In a randomized controlled trial in severe AH, treatment with pentoxifylline improved survival compared to placebo [109]. Two other studies have evaluated pentoxifylline in AH, in smaller cohorts and without histological confirmation of AH. In a study comparing pentoxifylline with corticosteroids, a benefit in survival in the pentoxifylline group was observed, with 15 % 1-month mortality in pentoxifylline-treated patients vs. 35 % mortality in patients treated with corticosteroids [110]. In a second study, in which a combination of pentoxifylline and corticosteroids was compared with corticosteroids alone, no difference in survival was found; however, this study was not double-blind in design [111]. Experimental data have demonstrated the anti-inflammatory and antitumor necrosis factor alpha effects of pentoxifylline [112], although its benefit in severe alcoholic hepatitis seems to be related to the prevention of hepatorenal syndrome [103, 109]. In the subgroup of patients not responding to corticosteroids, defined as the lack of early change in bilirubin level on day 7, corticosteroids were discontinued and pentoxifylline was started; however, this proved to be an ineffective rescue strategy, resulting in 36 % 2-month survival in nonresponders in whom corticosteroids were discontinued, compared to 31 % 2-month survival in nonresponders transitioned from corticosteroids to pentoxifylline [113].
A large randomized controlled trial in 270 patients with biopsy-proven, severe AH did not show any benefit in survival with the combined administration of pentoxifylline and corticosteroids, compared to corticosteroids alone; a lower incidence of hepatorenal syndrome was noted in the combined treatment group (3 % vs. 12 % in corticosteroid group at 1 month, although this difference was no longer significant at 6 months) but did not result in improved survival compared to the prednisolone-alone group [103]. In the large randomized controlled trial STOPAH, which included more than 1000 patients [88], results with pentoxifylline were not better than placebo for short-term mortality [98, 114]. In summary, the data available to date does not provide strong evidence to support using pentoxifylline in ALD, and there is no role of pentoxifylline as adjunct or rescue therapy in patients treated with corticosteroids.
Liver Transplantation
According to UNOS, history of alcohol use as the etiology for cirrhosis in patients with end-stage liver disease represents 18.7 % of those who receive liver transplantation in the United States (http://optn.transplant.hrsa.gov/converge/data/default.asp) [115]. All of these patients on liver transplant lists are expected to demonstrate at least 6 months of alcohol-free period and a strong support system to prevent relapse in alcohol use. Survival of the transplanted patients and organs is excellent in this patient population [116]. Five-year patient survival of alcoholic hepatitis and alcoholic cirrhosis was 80 % and 78 %, respectively, and five-year graft survival was 75 % and 73 % [116]. In contrast to those who stopped alcohol, patients with acute alcoholic hepatitis who fail to respond to medical therapy are not considered for liver transplantation in most transplant centers. A recent study from Europe demonstrated that early liver transplantation resulted in improved cumulative 6-month survival and that benefit was maintained during the 2-years follow-up. Of the transplanted 26 patients, 3 had alcohol use relapse after transplantation [99]. Given the shortage of livers for transplantation and the addictive behavior of patients with alcoholic hepatitis, there are many ethical considerations regarding liver transplantation in patients with severe acute alcoholic hepatitis [117]. Investigation of the utility of liver transplantation for patients with severe steroid-resistant alcoholic hepatitis is an area where future clinical trials are needed.
Emerging and Novel Therapies in Alcoholic Liver Disease and Alcoholic Hepatitis
Standard of care in alcoholic liver disease and alcoholic hepatitis is very limited and are based on old studies and recommendations from decades ago. The lack of efficient treatment options clearly demonstrate that alcoholic liver disease has been a “neglected” disease with respect to clinical trials, drug development, and attention of the pharmaceutical industry and clinical research. This devastating picture has been somewhat modified by the recent initiative of the NIAAA that initiated clinical and translational research in alcoholic hepatitis.
When approaching treatment of alcoholic liver disease and/or alcoholic hepatitis, understanding critical elements of disease pathomechanisms is critical and could guide the design of disease-specific interventions. Studies in animal models indicate many potential checkpoints for targeting the development of alcoholic liver disease.
These include but are not limited to gut permeability, hepatocyte damage and cell death, mediators and cell types of inflammation, process of fibrosis, regeneration, and hepatocellular cancer. Emerging therapies will be discussed based on these categories below. There are several important considerations in designing new therapeutic interventions:
What is the status of the disease induced by alcohol? Treatment of moderate and acute severe alcoholic hepatitis will very likely require different approaches and interventions. Although the exact triggers and mechanisms that differentiate moderate from severe alcoholic hepatitis are yet to be delineated, experimental data suggest that the severity of alcoholic liver disease is determined not only by quantitative but also qualitative differences in the pathomechanism. For example, in severe acute alcoholic hepatitis, activation of innate immune responses and the pro-inflammatory cytokine cascade in severe alcoholic hepatitis represents a vicious cycle that might be very difficult to break without compromising fundamental elements of host defense.
Will one agent/drug be sufficient to interfere with the multifactorial process in the pathogenesis of acute alcoholic hepatitis? Consideration of therapeutic approaches that target different key components of acute alcoholic hepatitis may be more effective than a single therapy.
Targeting the Gut
Based on recent experimental evidence on the effect of alcohol on the gut, interventions that prevent alcohol-induced increase in gut permeability and microbial translocation and/or restore alcohol-induced disturbance of the gut microbiome could be reasonable interventions. It remains to be evaluated whether these components should be targeted individually or collectively to achieve improvement in alcoholic liver disease or in alcoholic hepatitis [118, 119]. Animal and human studies identified the benefit of zinc on gut permeability [120]. Targeting the microbiome composition by using VSL3 or lactobacillus GG may have benefits in alcoholic liver disease [121, 123]. Fecal transplantation has proven benefit in Clostridium difficile infection, and it is in early clinical trials in alcoholic liver disease [122]. Additional potential targets based on animal studies include miR-155 inhibition [124]. Farnsenoid receptor (FXR) activation inhibited inflammation and preserved the intestinal barrier in inflammatory bowel disease, and its potential role in alcoholic liver disease is yet to be explored [125] (Fig. 15.1).
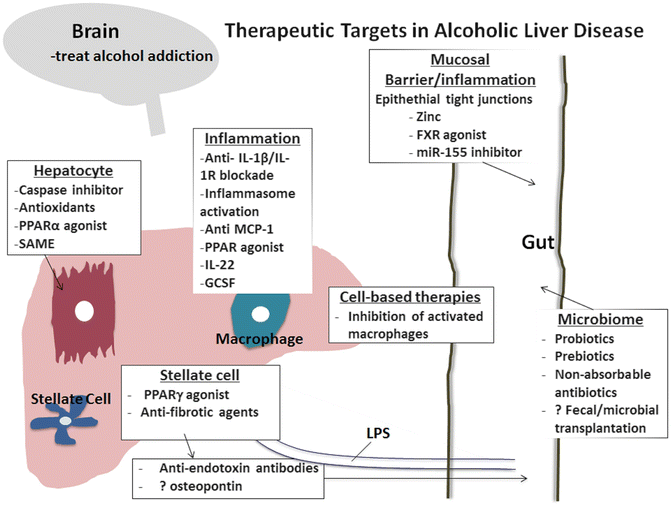

Fig. 15.1
Emerging and novel targets in treatment of alcoholic liver disease and/or alcoholic hepatitis
Modulation of Steatosis
Alcohol-induced steatosis in hepatocytes is an early effect of alcohol that is sustained during chronic alcohol use. Of the many signaling mechanisms regulating hepatocytes fat accumulation, activation of the PPAR-alpha was shown to have benefits on murine alcohol-induced liver disease [126]. PPAR-gamma and PPAR-delta agonist treatment improved hepatic insulin resistance in another study [126].
Modulation of Inflammation and Immunity
Recruitment of macrophages and neutrophils to the liver in alcoholic liver disease is mediated by chemokines [127]. MCP-1 (also named CXCL2) is produced by alcohol-exposed hepatocytes and immune cells are increased in early alcoholic liver disease and they recruit monocytic cells into the liver. In addition, MCP-1 induces fat accumulation in hepatocytes [128, 129]. In mice deficient of MCP-1, chronic alcohol feeding resulted in attenuated steatosis and inflammation suggesting that inhibition of MCP-1 may provide benefits in ALD. A dual CCR2/CCR5 antagonist cenicriviroc, is currently in clinical trial in human nonalcoholic steatohepatitis and testing of this antibody in ALD awaits investigation.
Targeting pro-inflammatory cytokines in acute alcoholic hepatitis has been the target of previous and current investigations. Clinical trials using anti-TNF-α blocking antibodies or inhibiting TNF receptor I had unsuccessful outcomes due to increased infections [130–133]. While TNF has complex effects on the immune system and the liver, studies with anti-TNF-α are perfect reminders about the impaired immune system of patients with alcoholic liver disease. Alcohol compromises both innate and adaptive immunity and the increased pro-inflammatory cascade activation in acute alcoholic hepatitis occurs together with impaired immune responses in these patients [134]. It remains to be seen whether anti-inflammatory strategies alone or in combination with other treatment could be beneficial. Currently, clinical trials have been initiated with the combination of recombinant IL-1 receptor antagonist combined with zinc and pentoxifylline in severe acute alcoholic hepatitis. Another study will test the effects of an anti-IL-1 antibody in combination with steroids in severe alcoholic hepatitis (Table 15.2).
Table 15.2
Alcoholic hepatitis clinical trials data
Title | Recruitment phase | Conditions |
|---|---|---|
Effect of Probiotics on Gut-Liver Axis of Alcoholic Hepatitis | Active, not recruiting | Alcoholic liver disease |
Pharmacokinetic and Pharmacodynamic Study of IDN-6556 in ACLF | Active, not recruiting | Acute-on-chronic hepatic failure | acute liver failure | liver cirrhosis | acute alcoholic hepatitis |
Assess Safety and Efficacy of ELAD (Extracorporeal Liver Assist System) in Subjects With Alcohol-Induced Liver Failure | Active, not recruiting | Acute alcoholic hepatitis |
Short-term Survival in Patients With Severe Alcoholic Hepatitis Treated With Steroid Versus Pentoxifylline | Enrolling by invitation | Alcoholic hepatitis |
FGL2/Fibroleukin and Hepatitis C Virus Recurrence Post Liver Transplantation | Enrolling by invitation | Liver transplantation | hepatitis C |
Efficacy of Antibiotic Therapy in Severe Alcoholic Hepatitis Treated With Prednisolone | Not yet recruiting | Alcoholic hepatitis | alcoholic liver disease |
Randomised Open-label Multicenter Study Evaluating Ciprofloxacin in Severe Alcoholic Hepatitis | Not yet recruiting | Alcoholic hepatitis | alcoholic cirrhosis |
A Safety and Efficacy Study of Mycophenolate Mofetil and Rilonacept in Patients With Alcoholic Hepatitis | Not yet recruiting | Alcoholic hepatitis |
Safety and Efficacy of IMM 124-E for Patients With Severe Alcoholic Hepatitis | Not yet recruiting | Alcoholic hepatitis |
Validation of the Procedure of Early Liver Transplantation in Alcoholic Hepatitis Resisting to Medical Treatment | Recruiting | Alcoholic hepatitis | alcoholic cirrhosis |
Protective Immune Mechanisms in Alcoholic Hepatitis | Recruiting | Alcoholic hepatitis |
Effects of Prednisolone and Pentoxifylline on the Regulation of Urea Synthesis in Alcoholic Hepatitis | Recruiting | Alcoholic hepatitis |
Study to Assess Safety and Efficacy of ELAD in Subjects With Severe Acute Alcoholic Hepatitis (sAAH) and Lille Score Failure | Recruiting | Severe acute alcoholic hepatitis |
Novel Therapies in Moderately Severe Acute Alcoholic Hepatitis | Recruiting | Acute alcoholic hepatitis |
Effects of Rifaximin in Patients With Acute Alcoholic Hepatitis | Recruiting | Alcoholic hepatitis |
Integrated Approaches for Identifying Molecular Targets in Alcoholic Hepatitis | Recruiting | Alcoholic hepatitis |
Efficacy of G-CSF in the Management of Steroid Non-responsive Severe Alcoholic Hepatitis | Recruiting | Severe alcoholic hepatitis |
Immune Cell Dysfunction in Severe Alcoholic Hepatitis | Recruiting | Hepatitis |
Alcohol Diet and Drug Use Preceding Alcoholic Hepatitis | Recruiting | Alcoholic hepatitis |
Alcoholic Hepatitis: A Multicenter, Observational Study by the TREAT Consortium | Recruiting | Alcoholic hepatitis |
Trial of Obeticholic Acid in Patients With Moderately Severe Alcoholic Hepatitis (AH) | Recruiting | Alcoholic hepatitis |
Efficacy and Safety of MG in the Patients With Alcoholic Fatty Liver Disease and Alcoholic Hepatitis | Recruiting | Alcoholic fatty liver disease | alcoholic hepatitis |
Granulocyte Colony Stimulating Factor (G-CSF) in Acute Liver Failure and Alcoholic Hepatitis | Recruiting | Acute liver failure |
Efficacy Study of Anakinra, Pentoxifylline, and Zinc Compared to Methylprednisolone in Severe Acute Alcoholic Hepatitis | Recruiting | Acute alcoholic hepatitis |
Efficacy and Safety of S-adenosyl-l-methionine in Treatment of Alcoholic Hepatitis With Cholestasis | Recruiting | Alcoholic hepatitis |
The Effect of High Dose Vitamin C on the Liver Function in Chronic Hepatitis Patients | Recruiting | Chronic hepatitis | chronic hepatitis C | chronic alcoholic hepatitis |
National Cohort of Uncomplicated Alcoholic Cirrhosis | Recruiting | Alcoholic cirrhosis |
Acoustic Liver Biopsy in Normals and in Patients With Cirrhosis Using Endoscopic Ultrasound | Recruiting | Normal | alcoholism | cirrhosis | hepatitis C |
Integrated Stepped Care for Unhealthy Alcohol Use in HIV | Recruiting | Liver diseases, alcoholic | alcoholism | HIV | hepatitis C |
Transient Elastography in the Determination of Advanced Fibrosis in Alcoholic Liver Disease | Recruiting | Alcoholism | liver disease | liver fibrosis |
N-Acetylcysteine in Severe Acute Alcoholic Hepatitis | Completed | Alcoholic hepatitis |
Inflammation, Immune Activation and Portal Hypertension in Alcoholic Hepatitis | Completed | Alcoholic hepatitis |
Metadoxine as a Therapy for Severe Alcoholic Hepatitis | Completed | Severe alcoholic hepatitis |
Treatment of Severe Alcoholic Hepatitis With Corticoids Plus N Acetyl Cysteine Versus Corticoids Alone | Completed | Alcoholic hepatitis |
Efficacy of Combination Therapy of Glucocorticoids and Bovine Colostrum in Treatment of Severe Alcoholic Hepatitis | Completed | Severe alcoholic hepatitis in “extremis”—defined by mDF>54 |
Intensive Enteral Nutrition and Acute Alcoholic Hepatitis | Completed | Severe alcoholic hepatitis |
Double-blind Randomized Controlled Trial in Severe Alcoholic Hepatitis | Completed | Alcoholic hepatitis | alcoholic liver disease
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|



