Fig. 1.1
Conceptual model. As alcoholic liver disease progresses from fatty liver to cirrhosis, the risk of complications increases. Death related to these complications can develop at any stage of the disease but is more likely in the advanced stages. Thus mortality related to ALD does not always imply the presence of cirrhosis. Alcohol can be viewed conceptually as a risk factor for progression of disease as well as a cause of liver disease
A major barrier for the characterization and study of ALD is the limited set of biomarkers or other diagnostic criteria for different stages of the disease other than liver biopsy. Simply relying on biopsy is not feasible most of the times, in particular in epidemiological studies. Recently developed noninvasive methods (i.e., ultrasound or MR elastography) are providing encouraging results; however, more research is needed to fully characterize their use as diagnostic criteria for stages of ALD.
Worldwide Burden of Alcoholic Liver Disease
In 2010, the Global Burden of Disease Study estimated that alcohol-attributable cirrhosis was responsible for 493,300 deaths in the world (roughly 157,000 among women and 336,400 among men), representing 0.9 % of all the deaths. In addition, it estimated that alcohol accounted for 48 % of all deaths due to cirrhosis. The estimated number of deaths from alcohol-attributable liver cancer was 80,600, with a strikingly higher burden among men (65,900 deaths) than among women (14,800) [4] These estimates have been widely used by the WHO for the preparation of the Global Status Report on Alcohol and Health 2014 [5] and for their emphatic recommendation of interventions to reduce alcohol consumption as a priority for public health agencies. This recommendation was based on their conclusion that even small amounts of alcohol may contribute to development of liver disease or deaths due to liver disease in a vulnerable host. However, the conclusion has been challenged on the basis that consumption of low to moderate amounts of alcohol may have different effects, including potential benefits, than those observed in heavier drinkers.
In the next few paragraphs, we will discuss the main sources of data for that type of studies and the methods used to obtain these estimates.
Mortality Statistics
Figure 1.2 shows the cirrhosis-related mortality rates in several countries by sex in 2000 and 2010, illustrating the trends over time and geographical variations (Fig. 1.2). Similar data have been reported elsewhere [4, 6]. While these data are widely used by public health agencies to define the burden of ALD in the population, it is very important to realize that the mortality statistics underestimate the true burden of the disease. The use of the international classification of disease (ICD) has attempted to standardize the completion of death certificates throughout the world. Although this effort has been very important, changes in the ICD classification codes and level of detail have made the evaluation of trends more difficult.
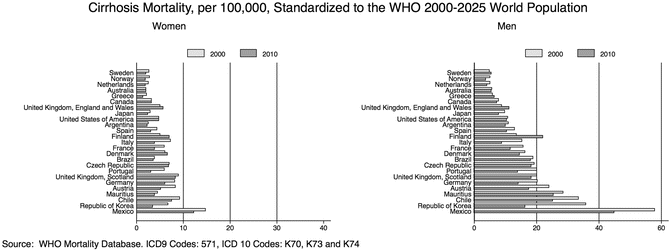

Fig. 1.2
Trends in cirrhosis mortality 2000–2010 in selected countries by sex. Cirrhosis mortality rates among different countries vary considerably. In some countries, the mortality rate decreased between 2000 and 2010, whereas in others such as the UK and Finland, the rate increased
In addition, two important issues must be considered in the interpretation of these types of studies: (1) Clinicians are well aware of the difficulty in establishing cirrhosis as an underlying cause of death. (2) These trends fail to distinguish between alcoholic versus other cause of cirrhosis. For death certificate and other data using ICD codes, the attribution of alcohol versus other causes is highly subjective and may be biased. Studies validating death certificates with data from hospital records and other sources have estimated the number of deaths from cirrhosis with no mention of alcohol reassigned to alcohol related more than doubles [7].
Finally, it is important to recognize that it is highly likely that misclassification in death certificates has significant social, regional, and temporal trends .
National Estimates of Alcohol Consumption
The WHO has established a surveillance system throughout the world to record per capita consumption of ethanol and to estimate the prevalence of lifetime abstainers, former drinkers, and current drinkers (http://www.who.int/gho/alcohol/en/).
These estimates are largely based on representative surveys in each of the countries. While misclassification can be expected to occur more frequently among those with heavy consumption, broadly speaking, self-reported alcohol consumption can be considered a valid measure of the population on average.
Most of these ecological studies have countries as unit of analysis as opposed to individuals. Their inferences are particularly useful to characterize population level determinants of the disease. There is an intrinsic relationship between diseased individuals and the whole population from which they come. In the context of the association between alcohol and liver disease, this observation has a long history. In 1961, Dr. Gerald Klatskin highlighted the strong significant linear correlation between death rate from cirrhosis and the average per capita consumption of alcohol in 1939 [8]. That remarkably high correlation has been replicated over time (Fig. 1.3) .
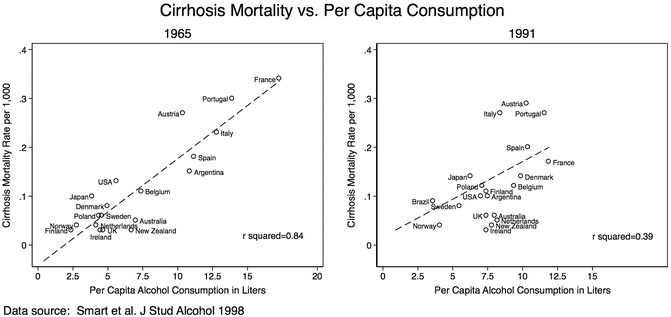

Fig. 1.3
Ecological studies: Relationship between cirrhosis mortality and per capita alcohol consumption, 1965 and 1991. The relationship between per capita alcohol consumption and cirrhosis mortality rates was more linear in 1965 than in 1991. The differences are more likely related to changes in classification of cirrhosis than to estimation of per capita alcohol consumption
Risk Factors for Alcoholic Fatty Liver Disease (AFLD)
Fatty liver is likely a metabolic consequence of ingesting moderately large amounts of ethanol. Studies have demonstrated that alcohol decreases beta oxidation and synthesis of fatty acids in the liver thus increasing the supply of fatty acids [9]. Studies in human volunteers demonstrated unequivocally that feeding 68–130 g of ethanol daily with a diet of 16 % protein and 36 % fat or 25 % protein and 25 % fat resulted in a significant increase in hepatic triglycerides and changes within the ultrastructure of mitochondria in all subjects within 6–14 days [10–12]. Similar changes were observed in volunteers after drinking 270 g of ethanol in addition to a standard diet for only 2 days [10, 11]. None of the volunteers had blood alcohol concentrations (BACs) above 80 mg/dl during the time of the study. These findings suggest that heavy binge drinking rapidly results in fatty infiltration of the liver, even in the absence of a high BAC.
A number of epidemiological studies have demonstrated the strong association between excessive alcohol consumption and hepatic steatosis [13]. A case-control autopsy study of individuals who died in automobile car crashes in the USA showed a prevalence of fatty liver (56 %) in those who had a blood alcohol concentration > 0.08 % at the time of death and were presumably heavy drinkers based on reports from family members [14]. Many other studies have reported a similar prevalence of fatty liver in alcoholics admitted to detox centers [15, 16]. Selection bias is a common threat to the validity of this type of studies since evaluation of subjects is not performed in a random fashion.
Some of the best US population-based studies that assessed hepatic steatosis include the Dallas Heart Study (DHS) [17] and the Third National and Nutrition Examination Survey (NHANES III) [18]. While in the NHANES III, a significant association between elevated alcohol consumption and hepatic steatosis was observed (Fig. 1.4); the DHS did not demonstrate such association. It is possible that the number of individuals with heavy alcohol consumption in the DHS was not sufficient to examine this association.
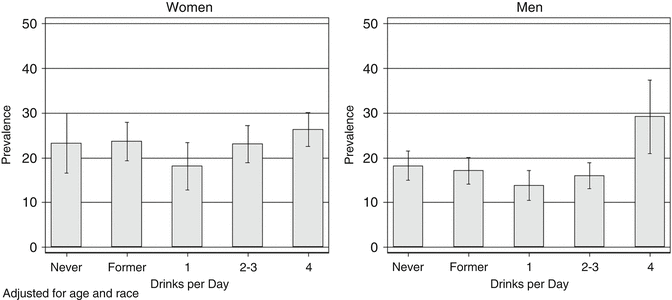

Fig. 1.4
Age- and race-adjusted prevalence of steatosis by usual number of drinks per day. US Adult Population, 1988–1994. (NHANES III). Steatosis in the NHANES III cohort was evaluated using hepatic ultrasound. As noted above, the prevalence of steatosis in men drinking ≥ 4 drinks daily was greater than in never or former drinkers, while the prevalence of steatosis was slightly lower in those drinking one drink/day compared to either never drinkers or those consuming more than three drinks daily. This U-shaped or J-shaped relationship between alcohol consumption and disease has also been observed for coronary heart disease and several other outcomes
Similar studies from Europe examined hepatic steatosis in relationship to both alcohol consumption and obesity. A population-based sample of individuals in Northern Italy, the Dionysos study, estimated that the prevalence of fatty liver determined by ultrasound imaging was 46 % in nonobese, heavy drinkers (>60 g daily intake), 76 % in obese individuals with BMI > 30, and 95 % in obese, heavy drinkers [19]. Another study conducted in France identified obesity as a significant risk factor for steatosis (OR = 2.5, 95 % confidence interval 1.0–6.6), in a sample of patients with heavy alcohol consumption (>50 g daily) even after adjustment for age, sex, duration of alcohol abuse, and amount of alcohol consumption [16]. Of note, both Italy and France are predominantly wine-drinking countries in which the pattern of consumption of alcohol as well as the preferred beverages may differ from North America.
As opposed to the consistent positive association between heavy alcohol consumption and hepatic steatosis, more recent studies suggest a protective effect of low to moderate amounts on the prevalence of hepatic steatosis (Fig. 1.4) [20–22]. One possibility is that low doses of alcohol may exert beneficial effects on insulin resistance and other metabolic parameters that are important in nonalcoholic fatty liver disease (NAFLD). Observational studies have identified a significant body of evidence suggesting that consumption of moderate amounts of alcohol is associated with decreased risk of diabetes [23]. It is therefore plausible that the small improvements in insulin resistance (in particular hepatic insulin resistance) counterbalance the pro-steatotic effect of alcohol [20, 21]. However, the possibility that the epidemiological results are confounded by differences in diet or other aspects of a healthy lifestyle pattern frequently observed among moderate drinkers is difficult to exclude as an explanation.
The interaction between alcohol and other components of the diet in development of fatty liver has been examined in small metabolic studies [10–12, 24]. Even though fat accumulation occurred in almost all subjects who were fed large amounts of ethanol, the extent of fatty liver was greater in those subjects fed a high-fat diet with ethanol. Although a number of large cohort studies have examined the relationship between diet, alcohol, and heart disease, there are relatively few that have reported the relationship between ethanol and fat intake in the development of fatty liver disease [22, 25, 26].
Risk Factors for Alcoholic Hepatitis and Cirrhosis
Alcohol Consumption
Before 1960, protein malnutrition was widely believed to be the cause of cirrhosis in heavy drinkers. Although nutritional deficiencies are common in these individuals, there is now agreement that alcohol rather than generalized malnutrition is the most important factor causing serious liver injury in heavy drinkers [27]. Many studies have shown a relationship between per capita consumption of alcohol and the prevalence of cirrhosis across years and countries (Figs. 1.3 and 1.5) [8, 28, 29]. However, in population and case-control studies, the absolute risk of serious liver injury in heavy drinkers is surprisingly low, ranging from 6 to 15 % [30–32]. Data from the Dionysos study estimated the prevalence of alcoholic cirrhosis to be 0.43 % in the population, accounting for approximately 38 % of all cases of cirrhosis in the study population [31]. The absolute risk of developing alcoholic cirrhosis was calculated to be 9.8 % in those consuming more than 60 g of ethanol daily, which is similar to studies from Copenhagen that reported an absolute risk of 6 % [30] in subjects drinking more than 35 drinks/week (~60 g daily). By contrast, a population study of 1270 Chinese drinkers estimated an absolute risk of 14.6 % in those drinking more than 40 g daily. Two prospective studies in Chinese showed an increased risk of cirrhosis with a threshold of 20 g daily drinking [32–34], whereas the threshold appears to be at least 30–60 g in Western studies [30, 35, 36]. Whether the difference between these two populations is related to ethnic or genetic differences in susceptibility or to differences in accurate reporting of alcohol consumption is unclear.
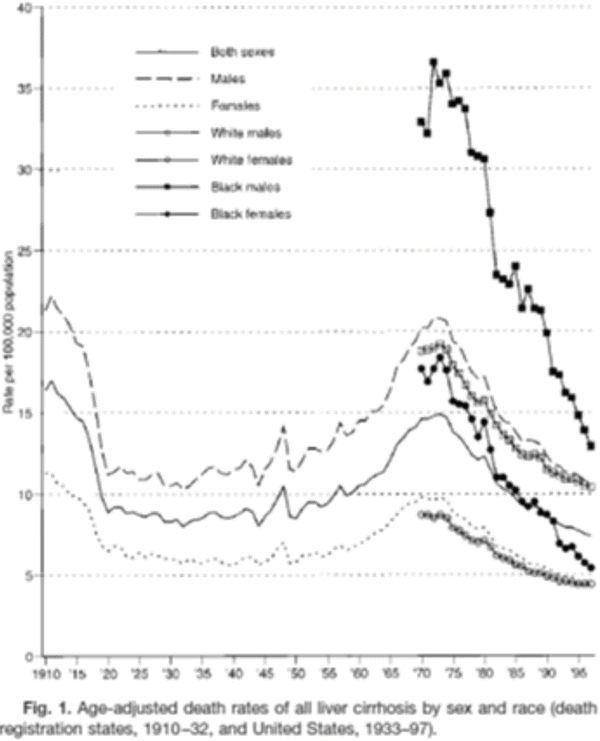

Fig. 1.5
Age-adjusted cirrhosis mortality in the USA by sex and race, 1910–1995. From Stinson et al. Alcoholism Clinical and Experimental Res, 2001, p. 1181. Cirrhosis mortality rates in the USA decreased substantially between 1910 and 1920 presumably as a result of prohibition that ended in 1919. Although an increase was observed between 1950 and 1970, the rate returned in 1995 to levels observed during prohibition
In almost all studies, the risk of more serious forms of ALD and cirrhosis mortality increases with higher daily consumption of alcohol [8, 35, 36] (Fig. 1.6). However, very heavy drinkers and those who are sick with acute alcoholic hepatitis are often underrepresented in prospective population studies making it difficult to determine the true increase in risk. Patients admitted to hospital with alcoholic hepatitis and cirrhosis reported mean daily alcohol intake of 170–220 g [15, 37]. Finding a dose–response relationship is consistent with a direct causal effect of alcohol, but the relatively low prevalence of advanced disease even in heavy drinkers indicates that additional factors such as genetics, patterns of consumption, diet, and other factors are important contributors to the overall risk.
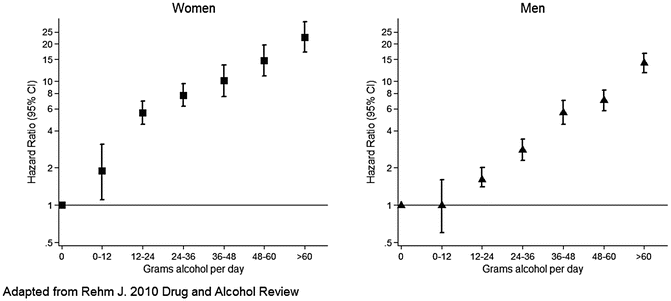

Fig. 1.6
Dose–response relationship between alcohol consumption and cirrhosis mortality by sex. Data from a meta-analysis illustrate the nearly linear relationship between dose and cirrhosis mortality rate. The cirrhosis mortality rate for women is increased compared to that for men and is particularly evident at lower levels of consumption, between 12 and 24 g daily
Duration of Heavy Drinking
Most patients with serious alcoholic liver disease manifest signs and symptoms in the 4th to 5th decades of life, although occasionally, subjects in their 20s may present with florid alcoholic hepatitis. Determining the duration of heavy consumption is problematic since many heavy drinkers vary their intake over the course of the lifetime. The mean duration of heavy drinking averaged 20 years in patients with alcoholic liver disease compared to 13 years in those with alcoholic pancreatitis [37]. A steady increase in the cumulative incidence of cirrhosis and non-cirrhotic liver disease was observed beginning around age 45–50 in the Dionysos study [31]. Changes in cirrhosis mortality rates were correlated with changes in per capita alcohol consumption during prohibition in the USA (Fig. 1.5) [8, 28, 38]. Cirrhosis mortality rates were also associated with changes in alcohol consumption in Europe during the 1980s–1990s but the temporal relationship was not sufficiently clear to define a specific lag period [39]. While it is clear that fatty liver can develop within a relatively few days of heavy consumption, more serious stages of ALD such as alcoholic hepatitis and cirrhosis develop over a much longer interval of time. Since serious alcoholic liver injury such as alcoholic hepatitis and cirrhosis occurs infrequently even in those who drink very heavily for many years, a number of other factors likely contribute to the overall risk.
Patterns of Consumption and Beverage Type
Whether alcoholic hepatitis and cirrhosis develop more often in binge drinkers or in those who are daily drinkers is unclear. Drinking outside mealtime was associated with a higher risk of cirrhosis and non-cirrhotic liver disease in the Dionysos study [31]. Danish drinkers who drank only wine had a lower risk of cirrhosis than beer and spirit drinkers which is of interest since in Denmark, wine is consumed primarily at mealtime [35]. These results must be interpreted cautiously since cirrhosis mortality rates are relatively high in France, a predominantly wine-drinking country (Fig. 1.2), making it likely that the pattern of consumption rather than beverage type is key. The duration of alcohol consumption (16–17 years) was similar in Spanish drinkers with alcoholic steatosis, hepatitis, or cirrhosis, but an irregular pattern of drinking (mealtime and between mealtimes) was far more likely in patients with alcoholic hepatitis with or without cirrhosis than in those with either cirrhosis alone or steatosis alone [40]. The authors also noted that patients with severe alcohol withdrawal syndrome (SAWS) on admission to the hospital were more likely to have irregular patterns of consumption than those without SAWS. Although some have speculated that the substantial rates of binge drinking both in North America and in Europe may eventually lead to an increase in the risk of developing more severe ALD, so far, the data are inconclusive. Clearly more screening and surveillance efforts are needed to detect any trend; in addition, preventive efforts to decrease the level of alcohol consumption in the whole population (including heavy drinkers) seem warranted.
Gender
Women are at greater risk for development of serious liver injury including alcoholic hepatitis and cirrhosis than men based on equivalent intake of alcohol. Both prospective, population-based and case-control studies have identified a threshold of 30–80 g of ethanol daily in men and 20–40 g in women above which the relative risk of serious alcoholic liver disease is significantly increased [31, 32, 37, 41]. A meta-analysis by Rehm and colleagues illustrates the lower threshold for increased risk of ALD in women compared to men (Fig. 1.6) [36]. Other studies noted similar mortality rates from alcoholic cirrhosis in women and men but the relative risk of hospitalization for alcoholic cirrhosis was higher in women than in men [8]. Anecdotally, the frequency of severe, acute alcoholic hepatitis seems to be higher in women than in men. Many potential mechanisms have been proposed to explain the increased risk in women. The lower threshold of drinking required to develop alcoholic hepatitis and cirrhosis in women may, in part, be related to differences in body weight and/or composition.
The increased risk may also result from differences in metabolism of ethanol in women compared to men. ADH activity is influenced by sex hormones [42]. Although gastric metabolism of ethanol may be lower in women than in men, it is unlikely that the difference in metabolism accounts for the increased risk of alcoholic liver disease in women.
Nutritional Factors: Protein, Vitamins, and Minerals
Although protein/calorie malnutrition was once believed to be the cause of liver disease in heavy drinkers, there are no compelling data to support this conclusion [27]. Surveys of patients with advanced liver disease showed adequate dietary protein intake above the recommended daily requirements in the vast majority. In one study, a high-fat diet was correlated with the risk of cirrhosis in heavy drinkers [41]. Other studies noted differences in the incidence of cirrhosis that were related to the source of animal protein most often consumed [43]; however, this observation remains unconfirmed.
Many specific nutrient deficiencies are common in patients with alcoholic cirrhosis including B vitamins and some minerals, particularly zinc [44]. Zinc deficiency increases gut mucosal permeability by disrupting tight junctions, allowing translocation of bacteria and other pathogen-associated molecular patterns [45]. Recent studies have noted differences in gut mucosal permeability in patients with serious alcoholic liver injury, particularly alcoholic hepatitis, suggesting a role for the “leaky gut” in the pathogenesis of alcoholic hepatitis. To our knowledge, no RCT has evaluated the role of zinc among people with ALD for the prevention of cirrhosis.
Vitamin A deficiency is often associated with zinc deficiency. Both are common in heavy drinkers particularly those with liver disease [46]. Unfortunately, large doses of vitamin A are hepatotoxic and the combination of ethanol and vitamin A supplementation is more toxic, making guidelines about replacement of vitamin A challenging in heavy drinkers [46]. Although high intake of vitamin A was associated with a higher risk of cirrhosis in an Italian case-control study, there was no additive effect of alcohol and vitamin A intake [47]. By contrast, lower intakes of riboflavin and vitamin B12 were associated with an increased risk of cirrhosis in the same population .
Interaction Between Alcohol Consumption and Obesity
Fatty liver, steatohepatitis, and cirrhosis can all occur in obese individuals who do not drink any alcoholic beverages, usually referred to as nonalcoholic fatty liver disease (NAFLD) . Accumulating evidence has linked obesity to an increased risk of all the hepatic manifestations of ALD (namely, steatosis, hepatitis, cirrhosis), cirrhosis mortality, and decompensation of cirrhosis [16, 48–50]. However, studying the interaction between alcohol and obesity on liver disease is complex.
From an operational perspective, many studies linking obesity with cirrhosis (presumably due to NAFLD) have segregated the study population based on the level of alcohol consumption. The studies of NAFLD by definition exclude excessive drinking. Therefore, very few studies with wide ranges of obesity and alcohol consumption have examined any manifestation of liver disease. National surveys that include BMI, alcohol, and liver enzymes, liver steatosis by ultrasound, or liver-related mortality probably provide the best evidence for an association [49, 51, 52].
In the ALD literature, a large study of heavy drinkers, who underwent biopsies upon admission to hospital, identified obesity as one of the five variables independently associated with alcoholic cirrhosis [16]. All subjects were consuming at least 50 g of ethanol daily. Of the 1604 subjects included, 411 had cirrhosis with or without acute alcoholic hepatitis. In addition to obesity, the duration of heavy consumption and female gender were identified as independent risk factors for both acute alcoholic hepatitis and cirrhosis. Other studies have also identified obesity as an independent risk factor for cirrhosis in heavy drinkers [49, 51].
Although the risk of steatosis is increased in obese, heavy drinkers, it has been suggested that the risk of fibrosis may not be greater in obese subjects who drink small amounts of alcohol [20]. These findings, however, are in disagreement with another study [53]. Clearly, the relationship between alcohol consumption and obesity in the risk of advanced liver disease and cirrhosis is complex, since light to moderate consumption of alcohol may improve insulin sensitivity and other metabolic parameters that reduce the development of NAFLD, whereas heavier consumption may be additive with NAFLD in the risk of progression to cirrhosis.
Alcohol and Iron Overload
Iron overload predicted mortality in patients with alcoholic cirrhosis but not cirrhosis from hepatitis C [59]. However, the relationship between iron overload and alcoholic cirrhosis is complex. Studies of a well-characterized population of patients with genetic hemochromatosis (C282Y homozygotes) showed that patients whose alcohol intake was > 40–60 g daily had a ninefold higher than expected risk of cirrhosis [60]. Since there was not a control group of subjects without iron overload, comparing the risk in this population to the risk of cirrhosis in subjects consuming similar amounts of alcohol without genetic hemochromatosis is difficult. Similar findings were observed in another population of patients with genetic hemochromatosis [61]. Interestingly, high intake of dietary iron was associated with cirrhosis but was independent of alcohol intake [47].
In animal studies, feeding a high-iron diet with alcohol increases lipid peroxidation and fibrosis [58]. Non-transferrin-bound iron levels are significantly elevated in active drinkers but not in those who have advanced alcoholic liver disease who are abstinent [62], while serum transferrin levels are elevated in subjects with fatty liver due to heavy drinking but low in patients with alcoholic cirrhosis [63]. Serum ferritin levels are increased even in moderate drinkers but serum ferritin poorly predicts hepatic iron concentration even in heavy drinkers [58, 64]. The mechanism underlying so-called secondary iron overload in heavy drinkers is unclear.
While there is good evidence to show that any alcohol intake above 40 g daily in men leads to an increased risk of cirrhosis, there is relatively little compelling evidence that heterozygosity for C282Y or H63D increases the risk of alcoholic cirrhosis [60]. Although secondary iron overload is common in patients with alcoholic cirrhosis, the extent of hepatic iron overload is far less than is seen in genetic hemochromatosis and probably does not contribute significantly to the development of liver injury.
Alcohol and Hepatitis C
Both heavy alcohol consumption and hepatitis C are common causes of cirrhosis in Europe and North America. Although hepatitis C can lead to cirrhosis even in nondrinkers, heavy alcohol consumption is a significant risk factor in the progression of fibrosis and development of cirrhosis in patients with hepatitis C infection [65–71]. Studies have also shown a higher prevalence of advanced fibrosis or cirrhosis in patients with hepatitis C infection who drink more than 50–80 g of ethanol daily [72]. Both the lifetime consumption of alcohol and the amount of alcohol consumed following infection with HCV were associated with the increased risk of cirrhosis in HCV patients, whereas the mean daily intake at the time of the study was not related [67]. Although data from Italy did not show an increased risk of cirrhosis in those with lifetime daily drinking less than 50 g daily, the authors reported that higher daily intake increased the odds of cirrhosis in those with HCV and that the effects of heavy alcohol consumption and HCV infection are synergistic rather than additive (Table 1.1) [71]. One meta-analysis provided an estimated pooled relative risk for cirrhosis of 2.33 in those drinking more than 30–80 g of ethanol daily [73]. The increased risk for cirrhosis in patients with hepatitis C is more evident in men than in women, a finding that is somewhat surprising since women are at higher risk of cirrhosis due to heavy drinking compared to men, as described before. One explanation for the difference is that fewer heavy drinking women were represented in the studies of patients infected with HCV.
Table 1.1
Odds ratios for cirrhosis according to lifetime daily alcohol intake and anti-HCV status
Lifetime daily alcohol intake | Anti-HCV negative | Anti-HCV positive | S index |
|---|---|---|---|
None | 1.0 | 9.2 (2.0–43.2) | |
25 or 50 g/day | 0.9 (0.5–1.6) | 9.5 (4.3–21.2)* | 1.1 (<0–2.4) |
75 or 100 g/day | 4.5 (2.1–9.3)* | 26.1 (8.7–78.2)* | 2.2 (0.6–3.7) |
125 or 150 g/day | 13.0 (5.7–29.4)* | 133.4 (37.6–473)* | 6.5 (4.4–8.7)* |
≥175 g/day | 15.0 (7.1–31.7)* | 147.2 (42.1–514)* | 6.6 (4.5–8.7)* |
Some studies have shown that the risk of progression of chronic hepatitis C is increased when there is fat seen on liver biopsy. Obesity and diabetes are risk factors for progression of chronic hepatitis C to cirrhosis. Genotype 3 hepatitis C is associated with hepatic steatosis as well as an increase in the risk of developing type 2 diabetes independently of BMI. Therefore, it is highly plausible that fatty liver and inflammation due to hepatitis C may act in a synergistic manner in development of fibrosis and cirrhosis in these patients.
While most guidelines for management of chronic hepatitis C recommend abstinence, whether the risk of cirrhosis in moderate drinkers with chronic hepatitis C infection is increased is a subject of controversy [74]. One study of 78 patients with paired liver biopsies showed increased progression of fibrosis in patients drinking less than 40 g daily [75]. However, several larger studies have shown that the risk of cirrhosis in patients with HCV infection is not increased in light to moderate drinkers (<30 g daily) [47, 76, 77]. Data from these large studies casts doubt on whether progression is more likely to occur in light to moderate drinkers, particularly those who drink less than once weekly.
Alcohol and Hepatitis B Infection
Even though heavy alcohol consumption increases the progression of chronic hepatitis C to cirrhosis, there is very little evidence that alcohol consumption has a similar effect on the progression of hepatitis B. Unlike chronic hepatitis C, the pathogenesis of inflammation in chronic hepatitis B is closely related to the host immune response to the infection. This difference may be important in explaining the difference between the effects of alcohol consumption on hepatitis B and C. Most studies have been carried out in Asia where the prevalence of chronic hepatitis B is high and the consumption of alcoholic beverages is relatively low. These cultural differences may account for the lack of evidence of an interaction. Even though consumption of alcoholic beverages is increasing in Asia, widespread vaccination and subsequent reduction in the incidence of hepatitis B infection may make demonstrating a potential association more challenging in the future.
Genetic Factors
Differences in susceptibility to complex diseases among individuals are often due to inherited traits. Early studies showed an increase in the rate of concordance for alcoholic cirrhosis in monozygotic versus dizygotic twins [78]. Tremendous advances in understanding how single-nucleotide polymorphisms influence risk of disease or response to exogenous factors now help to explain why some individuals are more susceptible while others are inherently resistant. Although genetic risk factors are unlikely to explain all of the individual differences in the incidence of chronic diseases such as alcoholic liver disease, they are undoubtedly important risk factors [79]. Ultimately, the interaction between genes that increase risk for ALD and environmental exposure (consumption) to ethanol is likely responsible. Both genes that increase the probability of heavy consumption (alcohol use disorders) and genes that increase the probability of liver injury in those who drink heavily are important contributors to the overall risk for an individual.
Alcohol-Metabolizing Enzymes: ADH, ALDH, and Cytochrome P450 IIE1
Alcohol dehydrogenase (ADH) , the primary family of enzymes responsible for metabolism of ethanol, was one of the first enzymes purified. Five classes of ADH as well as genetic polymorphisms that determine the rate of metabolism and elimination of ethanol have been identified [80]. Furthermore, genetic polymorphisms in the rate of metabolism of ethanol and acetaldehyde are associated with differences in consumption of alcoholic beverages. For example, some East Asians have an aversive reaction to drinking alcoholic beverages characterized by flushing and nausea. These individuals have a high frequency of ADH3*1, an isoenzyme that rapidly metabolizes ethanol to acetaldehyde and a high frequency of a catalytically inactive form of aldehyde dehydrogenase, ALDH2*2 [81, 82]. Homozygosity for ALDH2*2 results in moderately severe intolerance for alcohol with flushing above a minimal level of consumption. Asians with this phenotype have a low incidence of alcohol use disorders, perhaps because of the aversive effects of acetaldehyde accumulation after drinking alcohol. In one European study, ADH2*2 was associated with a lower risk of alcoholism [83], whereas another study, also from Europe, failed to show any association of ADH polymorphisms with either alcoholism or ALD [84]. A subsequent meta-analysis showed an increased risk of alcoholism associated with ADH2*1 and ADH3*2 [85]. The effects were most pronounced in East Asians but were also noted in Caucasians. These alleles code for isoenzymes with a lower catalytic rate than ADH2*2 and ADH3*1, suggesting that a high rate of elimination of alcohol may protect against developing alcohol use disorders, particularly in East Asians with the ALDH2*2 allele.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






