Fig. 13.1
Lateral tangential bovine patch venoplasty
If at all possible we prefer maximal attempts at full venous mobilization in order to construct a primary end-to-end anastomosis (Fig. 13.2). This can be performed with gaps up to 5 cm, and potentially more, depending on the specific patient anatomy. We perform full hepatic release and mobilization with right and left portal trunk dissection to gain additional length proximally and complete mobilization of the mesenteric root distally. The right colon should be completely mobilized inferiorly and medially from the retroperitoneal attachments of the anterior surface of the right kidney. This is continued dissecting the right and transverse mesocolon off the duodenum moving medially towards the groove between the uncinate of the pancreas and the mesenteric root. Our standard practice is to divide the splenic vein at the confluence not only to gain access to the SMA for the retroperitoneal dissection by allowing mobilization of the tumor and vein laterally, but also for gaining additional centimeters of length for venous reconstruction. Such maneuvers allow significant added length and in most cases allow approximation of the distal and proximal resection vein margins without tension.
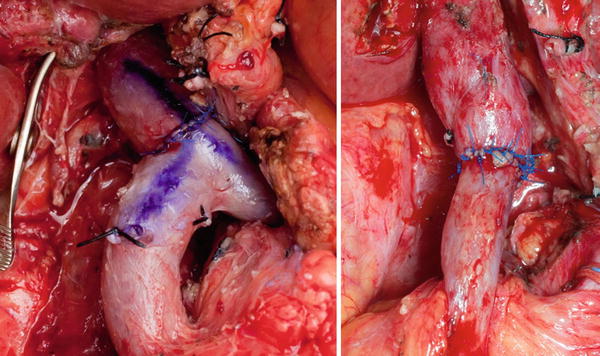

Fig. 13.2
Primary end-to-end venous anastomoses
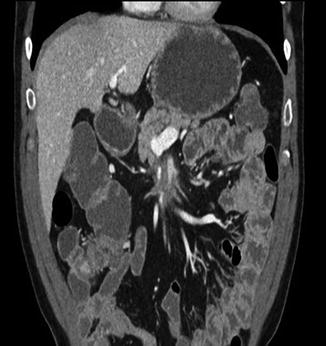
Reconstruction options for interposition grafts are variable and dependent on surgeon experience and comfort. Vein grafts such as left renal vein, internal jugular vein, saphenous vein, and deep femoral vein have all been described and the choice is surgeon- and experience-dependent (Fig. 13.3). We would caution the routine use of synthetic grafts, particularly in those patients predicted to have intermediate to high-risk postoperative pancreatic fistula, due to the life-threatening potential for post-pancreatectomy hemorrhage and/or difficult-to-treat long-lasting graft infection [42]. As pancreatectomy has a high risk of abdominal infection the use of synthetic venous prostheses might increase this complication [43]. One of the long-term risks of mesenteric venous reconstruction is subsequent thrombosis and occlusion with resulting complications and the use of synthetic grafts is a described risk factor for postoperative thrombosis [44].
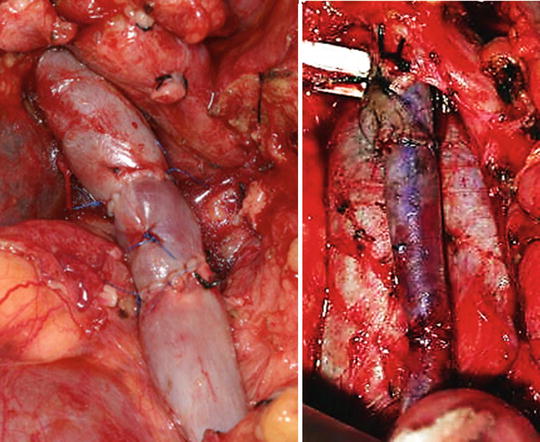

Fig. 13.3
Autologous vein interposition grafts (left renal vein and internal jugular conduits)
We have recently increased the use of custom-fashioned bovine pericardial tube grafts created over a 28–32 Fr chest tube with an endovascular stapler to create tube grafts of various lengths (Fig. 13.4). These grafts are not only resistant to infection but this technique allows individual case tapering of the graft to the appropriate proximal and distal PV/SMV diameters. We have significant experience with such customized grafts with no significant detriment in patency or complications and this avoids harvest of other vascular conduits or use of synthetics for longer reconstructions. We orient the tube graft with the staple line either at the 12 or 6 o’clock position which allows the tube graft to assume a near perfect circular dimension once the viscera resumes normal position overlying the reconstruction. This provides ideal flow dynamics without the elliptical compression that might occur if oriented otherwise. In all cases of interposition grafts, care must be taken to avoid excess length and possible kinking of the graft or vein above and below that can lead to postoperative thrombosis and may require early operative revision.
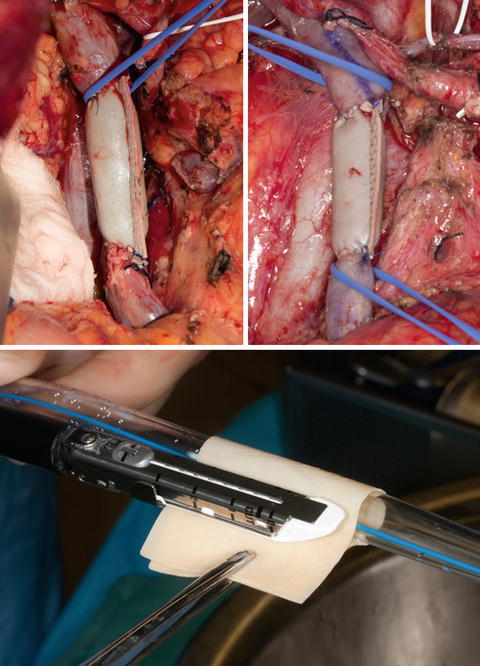

Fig. 13.4
Custom-fashioned bovine pericardial tube interposition grafts
Although the bulk of the literature and practice of venous resection in pancreatectomy is during pancreaticoduodenectomy, venous procedures may also be required during distal or total pancreatectomy. Tumors in the left neck or body of the pancreas can undergo subtotal extended distal pancreatic resection with the limits of proximal pancreatic resection determined at the level of the gastroduodenal artery (GDA), a natural anatomic landmark. This allows preservation of the duodenum and head of the pancreas. Care must be taken however as any resections beyond the GDA carry risk of inadvertent bile duct injury. In such extended resections with tumors arising in the pancreatic neck/body, often the splenic vein is occluded up to or involves the confluence and may extend into the PV/SMV. Such cases are reconstructed with either lateral patch grafting or formal segmental resection and anastomoses, either primarily or with conduit as described earlier.
In my practice I perform temporary SMA inflow occlusion during the venous reconstruction to prevent bowel wall congestion and edema that will hinder subsequent anastomoses. Soft plastic atraumatic bulldogs clamps are recommended to avoid intimal injury. Rummel tourniquet occlusion has also led to cases of arterial injury and is generally avoided. If the venous reconstruction can be performed in a rapid fashion, temporary SMA occlusion is optional. We prefer use of systemic heparin at the time of venous resection without reversal and continue postoperative heparin prophylaxis for 30 days. Finally we highly advocate the use of duplex ultrasound after every reconstruction to confirm patency and normal flow dynamics.
Sinistral Hypertension and Shunting Procedures
One of the most often unappreciated aspects of venous resection in pancreatectomy is the maintenance or re-establishment of gastrosplenic venous outflow. If the confluence including the splenic vein requires resection or is ligated for additional venous length and/or due to need for direct access to the SMA for the retroperitoneal dissection, postoperative acute sinistral hypertension may develop if adequate gastrosplenic retrograde outflow collaterals (IMV, coronary vein, gastroepiploic vein via gastrocolic trunk) are either anatomically unavailable or have been ligated as a result of the resection. In such circumstances our practice is to construct a distal splenorenal shunt (DSRS) to avoid the possibility of abrupt segmental left-sided venous hypertension that can result in splenomegaly with resultant acute hypersplenism, hypertensive gastropathy, varices, and subsequent postoperative hemorrhage that has occurred in several patients.
Recent reports have provided a proof of concept for the safety and efficacy of such venous decompressive techniques [41, 45, 46]. In the majority of cases the IMV terminates proximally into the inferior border of the splenic vein at its midpoint or near the splenoportal angle. The presence of this natural anatomic outflow pathway provides sufficient venous drainage of the spleen and gastric remnant and should be safely preserved and left in situ to provide retrograde sinistral outflow after splenic vein division. However, in up to one-third of patients, the IMV drains into the SMV as a separate trunk. Acute postoperative sinistral hypertension can thus develop after splenic vein division or resection. For oncological necessity, particularly with microscopically invasive pancreatic adenocarcinoma, wide vascular resection of the portal venous confluence including the IMV is often necessary. Furthermore, ligation of the left gastric vein (coronary vein) performed during lymphadenectomy may also limit gastric remnant venous drainage. In such cases, splenic vein shunting can be particularly useful and may mitigate the risks of sinistral hypertension. The need for splenic venous shunting can be predicted preoperatively on coronal imaging based on the anatomical variant of IMV insertion, as well as intraoperatively estimated after splenic vein division by identification of dilated gastric veins, a dusky, boggy appearance to the stomach, and turgor in the divided splenic vein itself.
Construction of the anastomosis technically requires adequate visualization of the left renal vein, which is identified underneath and to the left of the SMA. The renal vein can be further mobilized, if additional length is needed, by ligation of the left gonadal and/or adrenal vein. We do not advocate reimplantation of the splenic vein to the newly created portomesenteric venous reconstruction as this may result in flow dynamic changes as a result of kinking of the anastomosis, and subsequent thrombosis can propagate from the splenic vein into the newly reconstructed PV/SMV and result in mesenteric outflow obstruction with resultant bowel congestion and possible venous ischemia and liver dysfunction in addition to gastrosplenic hypertension.
In patients undergoing total pancreatectomy, in whom the short gastric venous collaterals are typically divided as part of splenectomy, venous resection may lead to severe venous congestion of the remaining stomach that may require extended gastric resection to avoid ischemic complications. In these cases careful preservation of the coronary vein may allow adequate gastric venous drainage without need for formal gastric resection.
In patients with preoperative SMV/PV occlusion secondary to tumor infiltration or thrombosis, numerous high-pressure, thin-walled venous collaterals develop around the pancreatic head and neck in order to decompress the mesenteric venous system. Pancreatic resection and concurrent venous reconstruction in these cases is considerably high risk as they are often complicated by significant venous hemorrhage. Furthermore, the ligation of such collaterals during the course of the operation further contributes to mesenteric hypertension and bowel congestion. In an effort to minimize intraoperative bleeding and simultaneously allow adequate hepatopetal outflow, the use of a temporary mesocaval shunt (MCS) can be utilized. This procedure is performed early on in the operation before the resectional procedure and portal dissection to avoid injury to these high-pressure, high-flow collaterals. Our preference is to use autologous internal jugular vein as the interposition graft as it is pliable enough and of adequate length to initially bring towards to the anterior surface of the inferior vena cava for temporary intraoperative mesenteric outflow shunting during the resection portion of the case. Once the specimen is removed it is a straightforward procedure to then subsequently transpose this graft to the proximal portal vein for completion of the portomesenteric reconstruction following resection. Temporary PTFE grafts can also be utilized in this setting if additional length is needed for shunting and after resection can be removed with either primary end-to-end venous anastomosis or with interposition grafting. The need for construction of a concomitant DSRS during mesocaval shunting is best anticipated before splenic vein ligation, when venous pressure is lowest, in order to dissect an adequate length of splenic vein from the undersurface of the remnant pancreas to reach the left renal vein.
Arterial Resection
Arterial resection for pancreatic cancer has historically been considered contraindicated due to its associated operative morbidity, high margin positive resection rate, and dubious survival advantage [29]. Although complex arterial resections have been performed in selected patients, it is still regarded as an extraordinary approach as arterial infiltration is typically a surrogate of a biologically aggressive tumor with high likelihood of occult disseminated disease rather than just a function of tumor location. Although anatomically borderline resectable criteria include isolated common hepatic artery involvement and partial SMA abutment, an initial resection, even if it can be technically performed, is currently not recommended in the absence of preoperative treatment and appropriate patient selection. Even greater caution is advised in proceeding with arterial resection in those tumors classified as anatomically locally advanced/unresectable.
However, this dogma has now been challenged with the introduction of effective modern therapeutics: the current anatomic arterial classification of locally advanced tumors does not categorically imply unresectable disease per se. As surgical resection remains the only hope for cure, more aggressive surgical approaches may be advocated to increase resection rates and institutions have released data on their experience with pancreatectomy and simultaneous arterial resections. Data from several small series of arterial en bloc resections suggest that such aggressive operations can result in relatively comparable overall survival to standard resections and thus can be justified in highly selected patients [47, 48]. The best available data comes from a recent meta-analysis of 26 studies of 366 and 2243 patients who underwent pancreatectomy with and without arterial resection. The cumulative data reveal that arterial resections are associated with longer operative times, increased intraoperative blood loss, prolonged length of stay, increased morbidity (median 53.6 %) (with a significant proportion of patients [17 %] suffering from bleeding, thrombotic, or ischemic complications), and increased perioperative mortality (median 11.8 %) when compared to those patients without arterial resections. Overall survival rates were similarly worse among patients who underwent arterial resection. However, these data did suggest improved long-term survival compared to patients with locally advanced disease who did not undergo resection [49].
With the use of improved systemic and locoregional therapies , aggressive operations with arterial resection may offer substantial benefit after extensive preoperative treatment, albeit with significant perioperative risk. Our own large experience with arterial resections in patients with a locally dominant phenotype has confirmed this conclusion. The administration preoperative therapy prior to consideration of arterial resection has been widely accepted [33]. All patients with any degree of arterial involvement should be considered for neoadjuvant therapy which in our opinion should invariably include: induction systemic chemotherapy—treatment of occult metastases, potential downstaging of primary tumor; and locoregional irradiation—treatment of primary tumor and surrounding at risk structures for local tumor control and to maximize possibility of a potential margin negative resection. Only after such standardized treatment should consideration of surgical resection be entertained as results of nonoperative therapy using this sequencing suggests nearly equivalent outcomes compared to surgery alone for such advanced cases [7]. As a disclaimer the arterial procedures that will be described are currently not recommended and should only be considered in highly selected patients at experienced and specialized centers ideally under protocol-based or clinical trials settings.
The arterial structures that are at risk for locoregional tumor involvement include the celiac, hepatic, and superior mesenteric arteries. In addition variant hepatic arterial anatomy places such vessels at risk, most commonly a replaced right hepatic artery [33]. Celiac stenosis caused by atherosclerotic disease or median arcuate ligament compression is another potential indication for arterial procedures. Types of arterial procedures include primary repair or angioplasty, resection and/or ligation alone without reconstruction, resection with primary anastomosis, and resection with interposition grafting, and complex revascularization. Simply stated, the more extensive the arterial involvement the more technically complex the required procedures are in order to render a negative margin resection and the more ensuing attendant morbidity and mortality. Therefore, patient selection for such procedures is of paramount importance and just as critical as technical expertise taking into consideration patient age and expected life-expectancy, grade of comorbidities, performance status, and anticipated quality of life. In our experience, the ideal patients for such aggressive operations are relatively young, fit, sophisticated to understand the risks and potential for limited oncologic benefit, and have undergone extensive preoperative therapy with some objective measure of efficacy. Such exceptional procedures are definitively not widely recommended but may have a role in highly experienced and specialized centers.
Critical in cases requiring arterial resection is the establishment and maintenance of adequate hepatic, gastric, and visceral perfusion. The potential anatomic limits of arterial resection extend distally from the right and left hepatic artery bifurcation of the proper hepatic artery to the celiac axis, its branches, and the proximal SMA. Tumor infiltration into the porta hepatis beyond 1–2 cm above the proximal sectoral hepatic artery bifurcation implies unresectability as these vessels are often small in caliber and resulting anastomotic failure will have significant hepatic and biliary consequences. As the biliary system relies on this arterial inflow, failure to accomplish this either technically or due to postoperative occlusion/thrombosis can lead to anastomotic breakdown and leak, stricture, or intrahepatic abscesses that can be extremely difficult to manage.
Tumors in the pancreatic head may extend medially along the common hepatic artery towards the celiac. Hepatic artery resection up to the proximal common hepatic artery root is possible with graft conduits. Simultaneous resections of celiac axis and hepatic arteries with complex revascularization have been performed with oncologic success. However, such cases also may also require total pancreatectomy and gastrectomy. The extent of arterial involvement that needs to be resected determines the extent of pancreatic resection and other organs required to accomplish this. Such multivisceral resections are required due to ischemic consequences of these procedures and may further increase the resultant risks [50, 51].
En bloc celiac artery resections are almost exclusively performed as part of distal pancreatic resections for anatomically locally advanced body tumors and have been shown to be feasible while allowing a reasonably acceptable margin negative resection rate and the potential to achieve significant local tumor control in selected patients [52, 53]. Due to the extensive arterial collateral circulation via pancreaticoduodenal arcades from the SMA through the GDA, hepatic and gastric perfusion can be maintained in most cases as long the tumor spares the proper hepatic artery distal to the GDA (Fig. 13.5). These cases commonly require some form of venous resection due to venous infiltration.
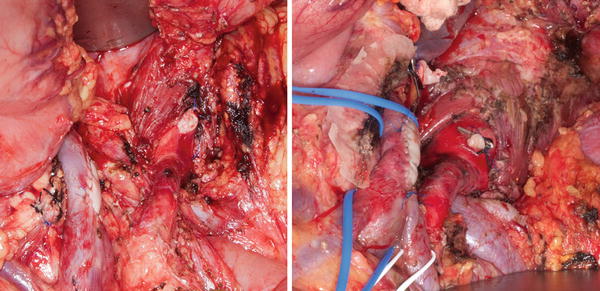

Fig. 13.5
R0 extended distal pancreatectomies with en bloc resections of tumor involved celiac arteries without arterial revascularization and concomitant bovine pericardial patch venoplasties . Patients underwent extensive preoperative induction systemic chemotherapy and consolidative chemoradiation prior to resection
If hepatic or gastric perfusion is determined to be insufficient following temporary occlusion of the common hepatic artery or if the GDA and proper hepatic artery need to be resected for more extensive tumors, then conduit bypass grafting needs to be performed to avoid ischemic complications. This can be performed with a variety of conduits. We prefer the superficial femoral artery (SFA), which is of adequate length and diameter and is also thick enough to resist complications from postoperative pancreatic fistula. SFA is harvested from the lower extremity and replaced with a PTFE graft. SFA jump grafts to the distal hepatic artery can be anastomosed to the stump of the celiac artery, the supraceliac aorta, or the lateral SMA (Fig. 13.6) . Intraoperative perfusion of the stomach should be carefully inspected as the left gastric and short gastric vessels via the splenic artery are resected en bloc with such resections. More complex advanced resections include extended distal pancreatectomy with en bloc celiac and SMA resections and revascularization for body tumors (Fig. 13.7). The higher incidence of POPF in patients undergoing distal pancreatic resection can severely comprise celiac procedures; thus all methods to decrease the incidence and severity of fistula should be employed.
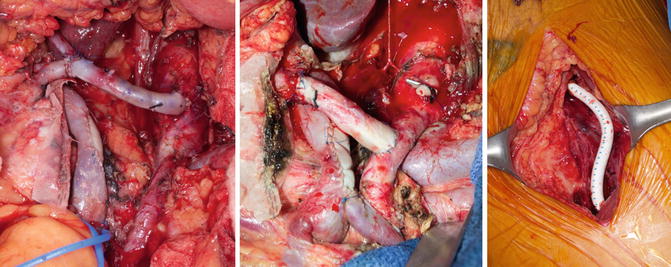
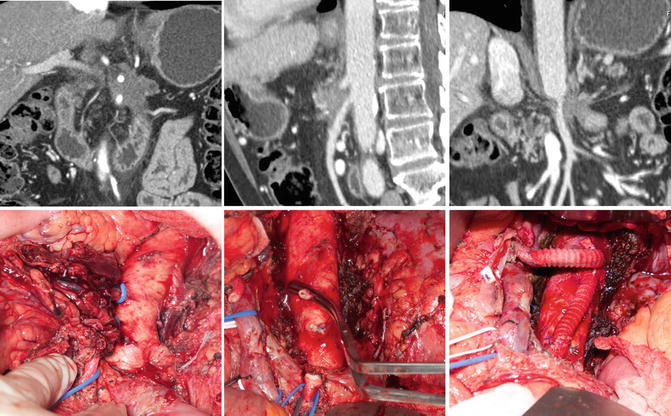

Fig. 13.6
R0 extended distal pancreatectomies with en bloc resections of tumor involved celiac arteries with arterial revascularization via SFA jump grafts from celiac stump or lateral SMA and concomitant bovine pericardial patch venoplasties. SFA harvest site is reconstructed with PTFE graft in lower extremity. Patients received extensive preoperative induction systemic chemotherapy and consolidative chemoradiation prior to resection

Fig. 13.7
R0 extended distal pancreatectomy with en bloc resection of tumor involved celiac and SMA with arterial revascularization via bifurcated rifampin-soaked Dacron graft from supraceliac aorta to distal HA and SMA and concomitant bovine pericardial patch venoplasty. Patient underwent extensive preoperative induction systemic chemotherapy and consolidative chemoradiation prior to resection without radiographic response; however, no viable tumor in resected specimen. Currently NED 29 months from diagnosis
For those cases where there is potential for SMA involvement, approaches to delineating resectability prior to resectional commitment include various artery-first strategies including left and right-sided dissections and infra- and supracolic approaches. After significant preoperative therapy including radiation, the residual soft tissue involving the SMA has been found in several cases to contain only fibros is and treated nonviable tumor on final pathologic processing; thus an argument for a planned R1 resection may exist in certain cases. The problem with this approach is that such a dissection is significantly difficult and may result in formal arterial injury thus not recommended unless performed with experienced hands capable of performing a repair if necessary. In some cases, there may exist extension of tumor infiltration deep into the mesenteric root involving multiple jejunal inflow vessels (Fig. 13.8). Furthermore it is exceedingly rare to not simultaneously require extensive venous confluence resection that often is the limiting anatomical factor to resection. Despite our group’s highly aggressive approach to tumors with extensive vascular involvement, simultaneous segmental PV/SMV and SMA resection carries with it prohibitive risk as complications with either vessel reconstruction can lead to fatal consequences and is not currently pursued.