Ureteropelvic junction (UPJ) obstruction is a common anomaly, and presents clinically in all pediatric age groups. The past 3 decades have witnessed an evolution in the surgical correction of UPJ obstruction on several fronts, with open surgical techniques yielding way to endoscopic, laparoscopic, and robotic-assisted approaches. Robotic-assisted surgery has several advantages in complex laparoscopic reconstructive procedures such as pyeloplasty. Comparative studies of laparoscopic and robot-assisted repairs have demonstrated similar success rates. Laparoscopic pyeloplasty is here to stay because of its advantages of safety, efficacy, decreased morbidity, reduced hospital stay, and, perhaps most importantly, cost-effectiveness.
Key points
- •
Ureteropelvic junction (UPJ) obstruction is a common anomaly, and presents clinically in all pediatric age groups.
- •
The past 3 decades have witnessed an evolution in the surgical correction of UPJ obstruction on several fronts, with open surgical techniques yielding way to endoscopic, laparoscopic, and robotic-assisted approaches.
- •
Robotic-assisted surgery has several advantages in complex laparoscopic reconstructive procedures such as pyeloplasty.
- •
Comparative studies of laparoscopic and robot-assisted repairs have demonstrated similar success rates.
- •
Laparoscopic pyeloplasty is here to stay because of its advantages of safety, efficacy, decreased morbidity, reduced hospital stay, and, perhaps most importantly, cost-effectiveness.
Introduction
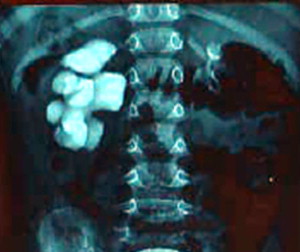
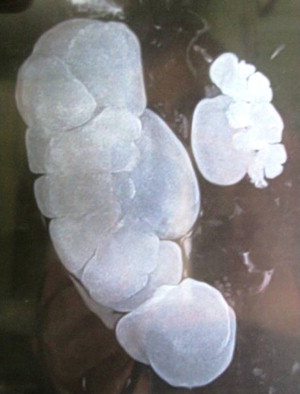
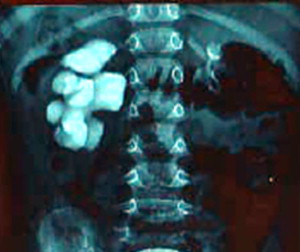
Ureteropelvic junction (UPJ) obstruction is a common anomaly, and presents clinically in all pediatric age groups ( Figs. 1 and 2 ). There tends to be a clustering in the neonatal period because of the detection of antenatal hydronephrosis, and again later in life because of symptomatic occurrence. Today most cases are identified and diagnosed in the perinatal period.
The past 3 decades have witnessed an evolution in the surgical correction of UPJ obstruction on several fronts, with open surgical techniques yielding way to endoscopic, laparoscopic, and robotic-assisted approaches. Among the various open surgical techniques, the Anderson-Hynes pyeloplasty has become the most commonly used open surgical procedure for the repair of UPJ. The principal reasons for the universal acceptance of the Anderson-Hynes dismembered pyeloplasty have been (1) broad applicability, including preservation of lower pole or crossing vessels, (2) excision of the pathologic UPJ with appropriate repositioning, and (3) successful reduction pyeloplasty. This operation is generally easy to perform and can be accomplished by several surgical approaches, including anterior subcostal, flank, and posterior lumbotomy. Recent advances in equipment and surgical techniques have made minimally invasive surgery (MIS) a well-tolerated and efficient option in the management of UPJ obstruction.
Introduction
Ureteropelvic junction (UPJ) obstruction is a common anomaly, and presents clinically in all pediatric age groups ( Figs. 1 and 2 ). There tends to be a clustering in the neonatal period because of the detection of antenatal hydronephrosis, and again later in life because of symptomatic occurrence. Today most cases are identified and diagnosed in the perinatal period.
The past 3 decades have witnessed an evolution in the surgical correction of UPJ obstruction on several fronts, with open surgical techniques yielding way to endoscopic, laparoscopic, and robotic-assisted approaches. Among the various open surgical techniques, the Anderson-Hynes pyeloplasty has become the most commonly used open surgical procedure for the repair of UPJ. The principal reasons for the universal acceptance of the Anderson-Hynes dismembered pyeloplasty have been (1) broad applicability, including preservation of lower pole or crossing vessels, (2) excision of the pathologic UPJ with appropriate repositioning, and (3) successful reduction pyeloplasty. This operation is generally easy to perform and can be accomplished by several surgical approaches, including anterior subcostal, flank, and posterior lumbotomy. Recent advances in equipment and surgical techniques have made minimally invasive surgery (MIS) a well-tolerated and efficient option in the management of UPJ obstruction.
Laparoscopic pyeloplasty
History
Laparoscopic pyeloplasty was first reported in the adult population in 1993 by Kavoussi and Peters and Schuessler and colleagues. Tan reported the first pediatric series of transperitoneal laparoscopic dismembered pyeloplasty in 18 children aged 3 months to 15 years. Yeung and colleagues described their initial experience with laparoscopic retroperitoneal dismembered pyeloplasty in 13 children, 1 of whom required open conversion. El Ghoneimi and colleagues reported their experience of 50 retroperitoneal laparoscopic pyeloplasties in children aged 22 months to 15 years. Similarly, Reddy and colleagues performed laparoscopic pyeloplasties in 16 children, 5 months to 11 years old. During the early 2000s many such small series reported the feasibility of laparoscopic pyeloplasty in children.
Technique
Transperitoneal access
An enema is administered the night before surgery to ensure that the colon is empty. Intraoperative antibiotics are administered to minimize the risk of infection. Patients are catheterized before surgery and the catheter left on free drainage during the operation. Patients are positioned in the lateral position and secured by placing a sand bag to support the back. Patients are further stabilized by strapping the iliac crest to the operating table with an adhesive bandage. Patients are placed as close as possible to the edge of the operating table. The first port is created by open laparoscopy using a blunt Hasson cannula through the umbilical skin crease. A purse-string suture is secured tightly around the Hasson trocar. The abdomen is insufflated with CO 2 to 10 to 12 mm Hg. A single 5-mm instrument port and a single 3-mm instrument port are required. Correct placement of these ports is critical to the ease of performing the anastomosis. Occasionally an extra 5-mm port is placed for retraction purposes. The peritoneum overlying the exposed kidney is incised just lateral to and above the colonic flexure. The loose adventitia around the kidney is detached from the renal capsule. Once the correct plane is identified, the renal capsule is traced into the renal sinus until the renal pelvis is identified. The renal pelvis is further dissected free from the medial side. The UPJ and proximal ureter are identified ( Fig. 3 A). The adventitia around the proximal ureter and UPJ is cleared. The ureter is dismembered with a small cuff of renal pelvis, leaving a 1.5- to 2.0-cm pyelotomy to reanastomose to the ureter (see Fig. 3 C). The lateral wall of the ureter is opened longitudinally and spatulated (see Fig. 3 D) for about 1.5 to 2.0 cm along its lateral margin. The UPJ and proximal ureter attached at this point to the spatulated ureter are then excised. The ureteropelvic anastomosis is performed with an 18-cm 6-0 polyglactin suture on a three-eighths round-bodied needle. The first suture is placed at the apex of the spatulated ureter from outside in, then driven through the most dependent part of the pyelotomy. The posterior anastomosis is completed running up the length of the spatulated ureter and pelvis. A 0.025-inch guide wire is then passed through the proximal ureter into the bladder. A 3F multilength double-pigtail catheter is passed over the guide wire into the bladder. Next, the proximal end of the double-pigtail stent is placed within the renal pelvis. The anterior anastomosis is then completed as a continuous layer (see Fig. 3 E). Postoperatively, the drain is removed when less than 5 mL per 24 hours is draining. The catheter is removed the next day. Oral fluids and feeding are started at the appearance of peristaltic sounds. Children are followed for urinary tract infection, and renography is repeated at 3 months.
Retroperitoneal approach
The child is positioned in a lateral or semilateral decubitus position, and 3 to 4 laparoscopic ports are inserted at the surgeon’s discretion. Retroperitoneal access is achieved through the first trocar incision 15 mm long and 10 mm from the lower border of the tip of the 12th rib. The Gerota fascia is approached by a muscle-splitting blunt dissection, then opened under direct vision. An index finger is introduced to push the peritoneum forward, thus creating a retroperitoneal cavity. A modification of Gaur balloon technique is used to dissect the retroperitoneum. Two index fingers of a powder-free surgical glove are placed, one inside the other and ligated onto the 5/10-mm trocar sheath. The dissection is then performed by instilling 500 mL of warm saline through the insufflation channel of the trocar. After completed dissection, the trocar is reinserted without the balloon and pneumoperitoneum established (maximum pressure 12 mm Hg). The first trocar is fixed with a purse-string suture applied around the deep fascia, to ensure an airtight seal. A 5/10-mm telescope is inserted through the first trocar. A second 3/5-mm trocar is inserted posteriorly near the costovertebral angle, while the third 3/5-mm trocar is inserted 1 cm above the top of the iliac crest at the anterior axillary line. To avoid transperitoneal insertion of this trocar, the working space is fully developed and the deep surface of the anterior wall muscles identified before the trocar is inserted. The insufflation pressure is maintained at less than 12 mm Hg and the flow rate of CO 2 is progressively increased from 1 to 3 L/min. The Gerota fascia is incised parallel to the psoas muscle and the perirenal fat is dissected to reveal the lower pole of the kidney, which is then mobilized. The UPJ is identified ( Fig. 4 A) and minimal dissection is used to free the UPJ from connective tissue; small vessels are divided after bipolar electrocoagulation. If needed, a fourth 3/5-mm trocar is inserted lateral to the lumbosacral muscles near the iliac crest. A stay suture of 5-0 absorbable suture material is placed at the UPJ for traction. The anterior surface of the UPJ is cleared to identify any crossing vessels. The renal pelvis (see Fig. 4 B) is partly divided by scissors at the most dependent part, while light traction on the stay suture is helpful for manipulating the UPJ maintaining under mild traction. The ureter is partly divided and incised vertically for spatulation (see Fig. 4 D). The traction suture helps to mobilize the ureter so that the scissors can be in the axis of the ureter. The anterior surface of the kidney is left adherent to the peritoneum so that the kidney is retracted medially with no need for individual kidney retraction. The pelviureteric anastomosis ( Fig. 5 A) begins using 6-0 absorbable sutures and a tapered 3/8 circular needle. The first suture is placed from the most dependent portion of the pelvis to the most inferior point or vertex of the ureteric spatulation. The suture is tied using the intracorporeal technique with the knots placed outside the lumen. The same suture is used on the anterior wall of the anastomosis. The UPJ is maintained on traction and the suture line stabilized. A 4.5F polyurethrane double-J stent (see Fig. 5 B) is inserted through the suture line to the bladder at the end of the anterior layer reconstruction (see Fig. 5 C). The pelvis is trimmed if needed. The UPJ and the trimmed part of the pelvis remain undismembered and are removed only after the last suture is placed, thus maintaining stability and decreasing tension on the suture line. The stent is left indwelling for 4 to 6 weeks. The Foley catheter is removed 24 hours after surgery. The children are covered with prophylactic antibiotics.
Results
It is well known that adhering to sound surgical principles, minimal handling of the ureter at the time of repair, and judicious use of internal stenting or nephrostomy tube drainage ensures a successful outcome. A successful pyeloplasty has been defined as improvement in hydronephrosis and stabilization or improvement in function on renal scan along with a decrease in washout time. In those situations where the child presents with symptoms, resolution of flank/abdominal pain or vomiting should also occur. Several series have reported good results following laparoscopic pyeloplasty ( Table 1 ).







