The optimal management approach for children with ureterocele and complete pyeloureteral duplication, especially in the setting of high-grade ipsilateral vesicoureteral reflux, remains unclear. Trends in surgical management reflect a shift from single-stage open reconstruction toward conservative management and minimally invasive approaches. This article reviews lower tract approaches (endoscopic ureterocele incision and ipsilateral ureteroureterostomy), and upper tract approaches (ureterocele moiety heminephrectomy) in terms of selected operative techniques, patient selection, published outcomes, postoperative care, and follow-up. Current data support endoscopic puncture as a safe and effective treatment of symptomatic children with single-system intravesical ureteroceles.
Key points
- •
Evidence-based management for children with ureterocele and complete pyeloureteral duplication is not possible and treatment should be individualized.
- •
Management requires a tailored approach that involves consideration of renal function, severity of hydronephrosis and obstruction, drainage of the contralateral ureter and bladder outlet, and associated vesicoureteral reflux.
- •
Endoscopic decompression is best suited for (1) prompt decompression of ureteroceles in the setting of infection or obstruction, and (2) elective treatment of intravesical ureteroceles.
- •
For children with ectopic ureterocele undergoing heminephrectomy, the presence or absence of preoperative reflux is the key.
- •
Current data indicate that laparoscopic retroperitoneal heminephrectomy carries a higher risk of open conversion, significant urine leak, and innocent pole loss compared with a laparoscopic intraperitoneal approach.
Introduction
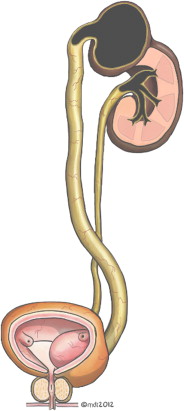
A ureterocele is a congenital cystic dilatation of the intravesical ureter that may occur as an isolated anomaly, but most commonly affects the superior moiety (SM) of a complete pyeloureteral duplication ( Fig. 1 ). Classification of ureteroceles is shown in Box 1 . Associated anatomic and pathophysiologic features ( Table 1 ) include intravesical ureteral obstruction, dysplasia or obstructive nephropathy of the ureterocele-associated moiety (40%–70%), and vesicoureteral reflux (VUR) to the ipsilateral inferior moiety (IM) (50%) or contralateral renal unit (25%). A tense ureterocele may mechanically obstruct the ipsilateral IM ureter, contralateral ureteral orifice, or bladder neck, although this is rare.
- •
Intravesical ureteral obstruction
- •
Dysplasia or obstructive nephropathy of the ureterocele-associated moiety (40%–70%)
- •
Vesicoureteral reflux (VUR) to the ipsilateral inferior moiety (IM) (50%) or contralateral renal unit (25%)
- •
Mechanical obstruction of the ipsilateral lower pole ureter, contralateral ureteral orifice, or bladder neck
| Intravesical | Cyst contained completely within bladder |
| Extravesical | Any portion extends into urethra or bladder neck |
| Cecoureterocele | Ureteral orifice in bladder, some tissue bulges beyond bladder neck |
| Ectopic | Orifice caudal to normal position of insertion on trigone |
| Stenotic | Small orifice |
| Sphincteric | Orifice within sphincter |
In the past, children with ureteroceles presented in early infancy with febrile urinary tract infection (UTI). Most cases are now discovered in the antenatal or neonatal period during routine ultrasonography evaluation, and the natural history of patients diagnosed in the modern era is largely undefined.
The goals of management for children with ureteroceles and pyeloureteral duplication ( Box 2 ) are clear and include prevention of renal damage associated with obstruction or VUR and UTI, promotion of continence, and minimization of surgical morbidity. However, the means of accomplishing these objectives remain a significant challenge in modern pediatric urology. Practice patterns are widely variable and no randomized controlled trials exist to guide management decisions. Selection of a treatment modality can therefore only be based on the balance between potential risks inherent to the condition and the summation of published results for a multitude of therapeutic alternatives.
- •
Prompt decompression of obstruction with infection
- •
Elimination of recurrent infection
- •
Relief of obstruction
- •
Elimination of clinically significant reflux
- •
Preservation of renal function (including functional moiety of a duplex system)
- •
Restoration and maintenance of continence
- •
Minimize surgical morbidity/minimize number of surgical procedures
There is particular disagreement regarding the optimal management of patients with duplex system ureteroceles, especially in the presence of significant vesicoureteral reflux. Current trends are away from single-stage open reconstruction (SM heminephrectomy, ureterocele excision, bladder base/neck reconstruction, and IM ureteral reimplantation) and toward conservative management and minimally invasive approaches.
This article discusses minimally invasive approaches for treatment of children with ureterocele and ectopic ureter. It addresses (1) nonoperative management before detailed discussion of (2) lower tract approaches (endoscopic ureterocele incision and ipsilateral ureteroureterostomy [IUU]) and (3) upper tract approaches (ureterocele moiety heminephrectomy) in terms of selected operative techniques, patient selection, published outcomes, postoperative care, and follow-up.
Introduction
A ureterocele is a congenital cystic dilatation of the intravesical ureter that may occur as an isolated anomaly, but most commonly affects the superior moiety (SM) of a complete pyeloureteral duplication ( Fig. 1 ). Classification of ureteroceles is shown in Box 1 . Associated anatomic and pathophysiologic features ( Table 1 ) include intravesical ureteral obstruction, dysplasia or obstructive nephropathy of the ureterocele-associated moiety (40%–70%), and vesicoureteral reflux (VUR) to the ipsilateral inferior moiety (IM) (50%) or contralateral renal unit (25%). A tense ureterocele may mechanically obstruct the ipsilateral IM ureter, contralateral ureteral orifice, or bladder neck, although this is rare.
- •
Intravesical ureteral obstruction
- •
Dysplasia or obstructive nephropathy of the ureterocele-associated moiety (40%–70%)
- •
Vesicoureteral reflux (VUR) to the ipsilateral inferior moiety (IM) (50%) or contralateral renal unit (25%)
- •
Mechanical obstruction of the ipsilateral lower pole ureter, contralateral ureteral orifice, or bladder neck
| Intravesical | Cyst contained completely within bladder |
| Extravesical | Any portion extends into urethra or bladder neck |
| Cecoureterocele | Ureteral orifice in bladder, some tissue bulges beyond bladder neck |
| Ectopic | Orifice caudal to normal position of insertion on trigone |
| Stenotic | Small orifice |
| Sphincteric | Orifice within sphincter |
In the past, children with ureteroceles presented in early infancy with febrile urinary tract infection (UTI). Most cases are now discovered in the antenatal or neonatal period during routine ultrasonography evaluation, and the natural history of patients diagnosed in the modern era is largely undefined.
The goals of management for children with ureteroceles and pyeloureteral duplication ( Box 2 ) are clear and include prevention of renal damage associated with obstruction or VUR and UTI, promotion of continence, and minimization of surgical morbidity. However, the means of accomplishing these objectives remain a significant challenge in modern pediatric urology. Practice patterns are widely variable and no randomized controlled trials exist to guide management decisions. Selection of a treatment modality can therefore only be based on the balance between potential risks inherent to the condition and the summation of published results for a multitude of therapeutic alternatives.
- •
Prompt decompression of obstruction with infection
- •
Elimination of recurrent infection
- •
Relief of obstruction
- •
Elimination of clinically significant reflux
- •
Preservation of renal function (including functional moiety of a duplex system)
- •
Restoration and maintenance of continence
- •
Minimize surgical morbidity/minimize number of surgical procedures
There is particular disagreement regarding the optimal management of patients with duplex system ureteroceles, especially in the presence of significant vesicoureteral reflux. Current trends are away from single-stage open reconstruction (SM heminephrectomy, ureterocele excision, bladder base/neck reconstruction, and IM ureteral reimplantation) and toward conservative management and minimally invasive approaches.
This article discusses minimally invasive approaches for treatment of children with ureterocele and ectopic ureter. It addresses (1) nonoperative management before detailed discussion of (2) lower tract approaches (endoscopic ureterocele incision and ipsilateral ureteroureterostomy [IUU]) and (3) upper tract approaches (ureterocele moiety heminephrectomy) in terms of selected operative techniques, patient selection, published outcomes, postoperative care, and follow-up.
Conservative management
The presence of ureterocele and duplication anomalies does not necessarily result in the development of clinically significant disease sequelae. Many investigators have begun to express concern about overtreatment and unnecessary surgery in this population. Several studies have shown that, if carefully selected, many asymptomatic children with ureteroceles can be safely managed initially without surgical intervention.
Shankar and colleagues observed 52 consecutive patients for 15 years with nonobstructed IMs, absence of bladder outlet obstruction (BOO), nonfunctioning SMs, and less than grade III VUR. No patients underwent surgery or had UTIs at median follow-up of 8 years. Direnna and Leonard similarly used a nonoperative approach in 6 patients without high-grade (III or IV) VUR, IM nonrefluxing hydroureteronephrosis, or BOO. Patients had either SM function less than 10% or greater than 10% with no obstruction. No patients developed UTIs or symptoms at 5-year follow-up. Hydronephrosis resolved in 4 of 6 patients and improved or remained stable in others, and VUR resolved in 4 of 6 patients and improved or remained stable in others.
Han and colleagues showed that a nonoperative approach can be successful irrespective of ureterocele moiety functionality or presence of high-grade VUR given absence of high-grade obstruction on renal scan. They reported a series of 13 patients with either single-system ureterocele or complete pyeloureteral duplication in which 6 patients had good function in the ureterocele-associated moiety and 5 patients had ipsilateral grade III or IV VUR. All 5 patients with high-grade reflux had spontaneous resolution at 3-year to 4-year follow-up. Only 4 of 13 patients required surgical intervention for UTI or progressive dilatation. The investigators reported no significant difference between the nonoperative and operative groups with regard to hydronephrosis grade, reflux grade, or ureterocele size. Merguerian and colleagues successfully observed a series of children with only minor SM dilatation and no evidence of obstruction on renal scan. None of these patients had experienced onset of symptoms or UTI at 5-year follow-up. Dilatation was stable in 6 patients and resolved in 4.
Patients with multicystic dysplasia in the ureterocele moiety may also be candidates for observation given absence of ureteral dilatation, BOO, and high-grade reflux. Coplen and Austin reported a series of 4 such patients, 3 of whom had associated grade I or II VUR. They noted ureterocele collapse in 2 of 4 (50%), resolution of VUR in 2 of 3 (67%), and spontaneous involution of multicystic dysplastic moieties in 4 of 4 (100%) by 18 months ( Box 3 ).
- •
Asymptomatic
- •
Good or absent function in ureterocele moiety
- •
Absence of grade III or IV VUR
- •
Absence of IM obstruction a
- •
Absence of BOO
a As shown by scintographic data or presence of marked nonrefluxing hydroureteronephrosis.
Current data suggest that a conservative approach is a favorable option for patients with small, asymptomatic, nonobstructive intravesical ureteroceles even if low-grade reflux is present. Patients with extravesical ureteroceles or multicystic dysplastic moieties may also be candidates in the absence of high-grade VUR or BOO. In any patient being managed conservatively, the development of BOO, symptomatic UTI, or significant worsening of upper tract obstruction should prompt consideration of operative intervention.
Lower tract approach: endoscopic ureterocele incision
Endoscopic ureterocele decompression is a widely used, minimally invasive method of effectively achieving timely ureterocele decompression and decreasing the risk of UTI while avoiding extensive trigonal surgery in infants. In recent years, endoscopic puncture has supplanted incision as the preferred technique. Details of the procedure are shown in Box 4 . In general, a larger incision offers more effective decompression with a potentially higher risk of de-novo VUR ( Fig. 2 ).
- •
Typically performed with an 8-Fr or 10-Fr endoscope and flexible 3-Fr monopolar wire electrode.
- •
The cutting current should be set high enough to ensure a clean puncture.
- •
In older children, a pediatric resectoscope and Collins knife can also be used.
- •
The bladder should be incompletely filled to achieve maximal ureterocele distension for incision. Incising distally on the ureterocele close to the bladder floor may prevent postoperative reflux. For intravesical ureteroceles, the puncture can be low on the front of the ureterocele, allowing collapsed tissue to establish an antireflux valve.
- •
For ectopic ureterocele, a single puncture of the intravesical portion of the ureterocele can be made just proximal to the bladder neck.
- •
Decompression may be difficult to achieve if the Bugbee electrode displaces the inner mucosal coat of the ureterocele away from the outer layer.
Alternative techniques include laser incision, percutaneously assisted incision, and concomitant ureterocele double puncture with intraureterocele fulguration. Although initial data suggest success that is comparable with traditional puncture, outcomes likely reflect the anatomic and functional characteristics of the urinary system rather than the technique used.
Patient Selection and Published Outcomes
Endoscopic puncture represents the treatment of choice for patients with ureterocele resulting in systemic infection, azotemia, or high-grade obstruction.
In the elective setting, essential preoperative considerations include ureterocele type and position, upper tract anatomy, and presence of associated ipsilateral VUR. In the past, management has been based primarily on ureterocele position with endoscopic intervention preferred for intravesical ureteroceles, and upper tract approach or complete reconstruction used for ectopic, duplex system ureteroceles ( Boxes 5 and 6 ).
- •
Obstructing ureterocele with systemic infection
- •
Obstructing ureterocele with severe nonrefluxing hydroureteronephrosis
- •
Intravesical ureterocele within a single nonrefluxing system
- •
Ectopic ureterocele
- •
Duplex system
- •
High-grade preoperative VUR
- •
Postnatal diagnosis
This article next addresses (1) the general consensus that endoscopic puncture is an appropriate initial intervention for single-system intravesical ureteroceles. This topic is followed by a review of more controversial applications of endoscopic intervention, including puncture as an initial intervention for children with (2) ectopic ureteroceles, (3) high-grade ipsilateral VUR, and (4) poorly functioning or nonfunctioning ureterocele-associated moieties.
Endoscopic Puncture for Single-system Intravesical Ureteroceles
Endoscopic puncture offers the greatest potential as a definitive treatment of patients with intravesical single-system ureteroceles. Successful decompression without reflux may be achieved in 70% to 80% of such cases.
In a series of meta analyses, Byun and Merguerian found a higher relative risk of reoperation after endoscopic management of ureteroceles that were (1) ectopic versus intravesical, (2) associated with duplex systems, and (3) associated with VUR preoperatively. The presence of multiple factors did not result in an additive effect on risk for reoperation. The investigators concluded that factors 1 to 3 represent proxies for trigonal anatomic distortion rather than independent risk factors for reoperation. This finding was subsequently corroborated by Di Renzo and colleagues in a series of 45 patients undergoing transurethral puncture. Initial therapy was definitive in 24 of 45 patients (53%), whereas 21 (47%) required further surgery. Secondary surgery was more likely in the setting of duplex (58%) versus single system (18%), ectopic (61%) versus intravesical position (30%), and in the presence (61%) versus absence (37%) of preoperative VUR.
Endoscopic Puncture for Ectopic and Duplex System Ureteroceles
Ectopic position is associated with high reoperation rates after endoscopic incision. Husmann and colleagues found that, in 28 patients with ectopic ureterocele undergoing endoscopic decompression, 18 (64%) required additional surgical treatment usually because of ipsilateral reflux.
Nonetheless, some investigators have begun to broaden their use of endoscopic puncture to include ectopic ureteroceles, whereas others have combined endoscopic puncture with ureteral bulking agent injection in the setting of associated high-grade reflux ( Table 2 ).
| Preoperative Ipsilateral VUR (%) | Resolved or Downgraded VUR (%) | De-novo VUR (%) | Secondary Surgery (%) | |
|---|---|---|---|---|
| Castagnetti & El-Ghoneimi, 2009, (N = 41) | 32 | 18 | 32 | 51 |
| Calisti et al, 2011, (N = 46) | 70 a | 63 a | 39 | 41 |
a Grade III or higher; patients received simultaneous puncture and bulking agent injection.
Castagnetti and El-Ghoneimi examined 41 neonates who underwent transurethral incision of duplex system ureteroceles within the first month of life. Twenty-four of 41 (58%) ureteroceles were ectopic. There was associated ipsilateral VUR in 13 (32%) and contralateral VUR in 7 (17%) patients. Endoscopic incision was an effective means of decompression in 40 of 41 patients. Ipsilateral VUR ceased in 6 of 13 ipsilateral IMs and 2 of 7 contralateral renal units. De-novo VUR in the punctured moiety developed in 13 of 41 cases (32%). Although 21 patients (51%) required secondary surgery (additional endoscopic procedure, reimplantation, or heminephrectomy) only 2 (5%) required surgery for de-novo reflux. There was no significant difference between intravesical and ectopic ureteroceles in the occurrence of VUR in the punctured moiety, rate of nonfunctioning SMs, or need for secondary surgery. The need for secondary surgery was significantly greater in patients with reflux before endoscopic incision.
Calisti and colleagues applied an all-endoscopic approach in 46 patients with duplex systems associated with ureterocele and/or vesicoureteral reflux and examined the need for subsequent open surgery. After full diagnostic evaluation, patients underwent ureterocele puncture, bulking agent injection, or simultaneous puncture and injection. Indications to perform puncture included dilated upper system, recurrent UTI, or obstructive renographic pattern in a still functional renal moiety. Indications for ureteral bulking agent injection included patients with persistent grade 3 or greater VUR and recurrent UTI after antibiotic prophylaxis. Twenty extravesical ureteroceles and 13 intravesical ureteroceles were punctured. Twelve of 20 extravesical ureteroceles were associated with grade 3 or greater IM VUR, as were 11 of 13 extravesical ureteroceles. These patients underwent simultaneous ureterocele incision and bulking agent injection. Bulking agent injections alone were administered in 23 refluxing duplex renal units without associated ureterocele.
When the investigators applied puncture alone to duplex system ureteroceles, they observed successful ureterocele collapse in 9 of 10 (90%), secondary VUR in 5 of 10, and need for secondary surgery in 5 of 10 (50%) renal units.
When the investigators applied simultaneous puncture and ureteral bulking agent injection to duplex system ureteroceles with grade III VUR, they observed successful ureterocele collapse in 23 of 23 (100%), resolved or downgraded VUR in 11 of 23 (48%), secondary VUR in 8 of 23 (35%), and need for secondary surgery in 13 of 23 renal units (57%).
Overall, the all-endoscopic approach was successful and resolutive in 23 of 46 patients (50%) and significantly influenced by the grade of reflux. Seventeen of 46 patients (37%) subsequently required open ureteral reimplantation for persistent reflux associated with febrile UTI and 6 of 43 patients (13%) subsequently required partial or complete nephrectomy for severe renal dysplastic changes.
In the setting of ectopic ureterocele, transurethral puncture represents an effective short-term correction of upper pole obstruction but may not represent definitive therapy in most cases. Many children require repeat puncture for adequate decompression or, more commonly, subsequent reconstructive surgery for persistent obstruction, recurrent infection, or persistent or de-novo reflux. Furthermore, incision or puncture may increase the likelihood of future surgery in patients with no preoperative reflux, perhaps because of procedure-related de-novo reflux, although this remains unclear. In light of these concerns, an upper tract approach has traditionally been used for ectopic ureteroceles.
Endoscopic Puncture in the Setting of Preoperative Vesicoureteral Reflux
There is some evidence to suggest that endoscopic puncture may be used irrespective of the presence of reflux, and that minimally invasive techniques may be used to treat children with VUR either inherent to a duplex system or resulting from previous endoscopic puncture.
Adorisio and colleagues applied endoscopic ureterocele puncture in 46 consecutive cases irrespective of the presence of reflux. Among 14 patients who had prepuncture VUR, 10 had spontaneous resolution in follow-up and the remaining 4 were corrected with endoscopic injection. Five of 46 patients developed de-novo reflux into the ipsilateral upper pole moiety. Two of these experienced spontaneous resolution, whereas 2 underwent endoscopic correction.
Calisti and colleagues used bulking agent injection to refluxing duplex system ureters without ureterocele; they observed resolution or improvement of VUR in 18 of 23 (78%) and need for secondary surgery in 5 of 23 renal units (22%). Perez-Brayfield and colleagues similarly reported successful outcomes after endoscopic correction of complex ureterovesical anatomy in 72 patients, including 5 with VUR following ureterocele puncture or incision.
Endoscopic Puncture in the Setting of Poor or Absent Ureterocele Moiety Function
The risk of subsequent morbidity (eg, UTI, urolithiasis, hypertension, malignancy) conferred by leaving a nonfunctioning ureterocele-associated moiety in vivo is not well understood. Chertin and colleagues examined the long-term morbidity associated with a nonfunctioning or poorly functioning moiety after endoscopic puncture in children with prenatal versus postnatal diagnosis. The groups had comparable percentages of intravesical and ectopic ureteroceles and nonfunctioning ureterocele-associated moieties. VUR was present in 23 of 35 of the prenatally diagnosed children (66%) and 12 of 13 (92%) of the postnatally diagnosed children. None of the prenatally diagnosed children had a postpuncture UTI, and they were less likely to require a secondary puncture or a secondary reflux procedure. Only 1 prenatally diagnosed child required subsequent heminephrectomy for high-grade reflux. These data suggest that nonfunctioning or poorly functioning renal moieties left in situ after endoscopic ureterocele decompression may not contribute to additional morbidity or frequently necessitate subsequent heminephrectomy. This possibility seems to be especially true in prenatally diagnosed children ( Table 3 ).







