Fig. 30.1
Draping technique to facilitate exposure to the perineum
Initial Survey
A survey of the available instrumentation, possible endoscopic or radiologic needs, and additional available staff should be addressed with the operating room personnel. In years past, when I traveled to multiple hospitals, I used to carry a set of “special” instruments in my car. The basic operating instruments are now available in most hospitals. In the cases of endometriosis that cannot be differentiated from rectal cancer or other situations requiring identification or localization of lesions, rigid proctosigmoidoscopy or flexible sigmoidoscopy can be very helpful. To gain a better understanding of the anatomy and allow planning your approach, you may even consider doing these procedures prior to scrubbing. Rarely is radiology necessary beyond confirming instrument and sponge counts. You may find the need for ureteral stents to be placed. The lithotomy position facilitates stent placement. If you are not in a hospital where you work frequently, asking the consulting surgeon for their choice of urologist may be wise.
Examination
Key concept: Don’t just focus on the problem at hand, but the entire situation. Establish roles early.
Once at the patient’s side, look first at the problem for which you are consulted. Next, evaluate the rest of the abdominal viscera including the extent of adhesions and the magnitude of any injury present. The length of the remaining small bowel and its accessibility are important. After appraising the situation and the interventions required, you must determine whether you will assume control of the case or “assist” the requesting physician. If the consulting surgeon needs assistance with adhesiolysis for improved exposure of their operative field, I normally act only as a consultant and return the operation to the primary surgeon for completion. If, on the other hand, there is a significant bowel injury or a need for bowel resection, then I would assume primary control of the operation and often the postoperative care. Again, this has a lot to do with my prior relationships with the primary surgeon.
Exposure/Operative Procedure
Key concept: You should be familiar with several methods to extend your incision and provide adequate exposure.
After deciding what needs to be done, you must determine if the exposure is adequate to perform the indicated procedures. The beauty of a midline incision is that it can be extended as needed. If you have a Pfannenstiel incision and need greater exposure, it can be enlarged by conversion to a Cherney incision. This involves extending the fascial incision to the pubic tubercles laterally and dividing the tendonous attachments of the rectus abdominis muscles to the pubic crest [2]. Additionally, the Maylard incision divides the rectus muscle to facilitate improved exposure, but is limited to the lower part of the peritoneal cavity (Fig. 30.2a–d). If the upper abdomen requires significant attention that cannot be reached through the lower incision, it may be necessary to convert to a midline incision extending up as high as necessary. This is referred to as an “inverted T” incision and may provide good exposure for the entire abdomen [3]. Another approach is the “hockey-stick” incision. This is created by a vertical incision at the lateral aspect of the lower transverse incision. It is made lateral to the rectus muscle, which is usually divided. The incision can be extended upward to the costal margin, if needed, for further exposure [4].
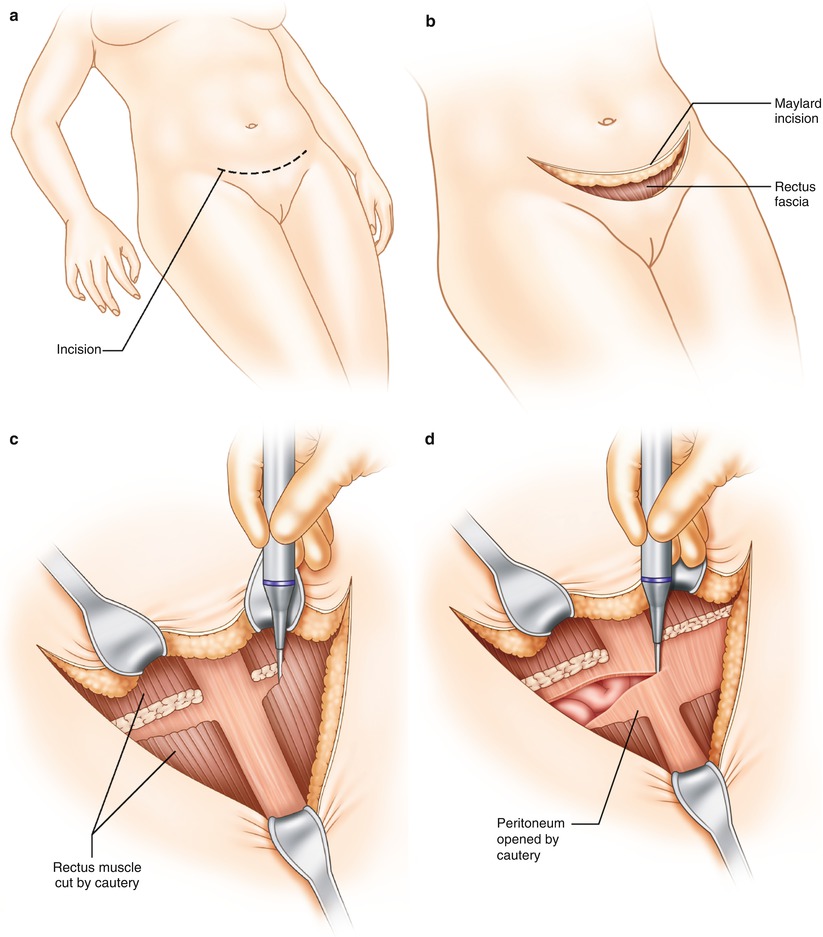

Fig. 30.2
(a–d) Maylard incision technique
Common Intraoperative Consults
Extensive Adhesions
Key concept: Meticulous dissection and knowing when to divert avoid additional problems.
One situation that you may encounter is that of dense adhesions that prevent access to a surgical site. What seems like a difficult situation to the surgeon who does not deal with bowel on a daily basis may be relatively straightforward to the experienced GI surgeon. In the face of difficult and tedious adhesions, careful and patient adhesiolysis will often be rewarded by a good result regardless of etiology. In performing a difficult adhesiolysis, it is usually better to leave a bit of peritoneum or fascia on the bowel rather than risk an enterotomy. Another technique used by those facile with knife dissection is to use a blade rather than scissors to divide adhesions.
A patient who has had very recent abdominal surgery may have fusion of tissue planes between the small bowel and surrounding structures. In this circumstance, if it is not possible to safely separate the loops with careful dissection, it may be best to perform some type of intestinal diversion and a gastrostomy with plans for intravenous nutrition and return in 6–12 weeks for a repeat operation.
Injury to Large or Small Bowel
Key concept: The extent of injury will help determine the degree of repair or resection required.
The calls for injury to the bowel that cannot be repaired by the primary surgeon typically come from the gynecologist or urologist due to their lack of comfort with bowel surgery. Most small bowel injuries can be resected or repaired primarily. You must properly examine each injury by fully mobilizing the area of involved bowel. It is difficult to establish “rules” as to when to repair serosal or seromuscular injuries. Typically, I do not repair simple serosal injuries. This is due to the fact that the major strength of the bowel wall comes from the submucosa, with only minimal contributions from the serosa and muscular layers [5]. If the muscularis has also been stripped from the submucosa and there is bulging of the mucosa, I will often repair. Milking the bowel content past the area of injury will often help identify an area of full-thickness injury as well as the adequacy of the repair. To avoid narrowing the bowel, I try to close the defects transversely with interrupted Lembert sutures. I then return at the end of the procedure and carefully inspect the repair for evidence of ischemia. If it looks questionable or ischemic, I would favor segmental resection. With electrosurgical injuries, it can be difficult to evaluate the depth or extent of the thermal damage and the potential exists for delayed perforation. The simplest and safest approach is imbrication of the site of potential injury with a series of Lembert sutures as described above [6]. In situations where there is concern for breakdown of repair, malnutrition, and steroid use, a drain left near the repair can potentially establish a controlled fistula. Once the entirety of the abdominal viscera has been evaluated, it becomes important to assess the remaining length, particularly of the small bowel. Those who have had a significant amount of their small bowel resected may be at risk for short bowel syndrome [7].
For colonic injuries, the same decisions must be made, to resect or repair. These situations should be handled similar to a trauma setting. There are several studies that support the primary repair of injuries that involve <50 % of the bowel wall and have no evidence of devascularization. If there are perforations or injuries that involve >50 % of the bowel wall, result in complete transection, and have significant tissue loss or evidence of vascular compromise, resection should be undertaken. The experience from the University of Tennessee has shown that in these patients a management algorithm based on patient comorbidities and transfusion of greater than six units of blood can help determine the operative approach. They suggest that patients with chronic renal failure, congestive heart failure, HIV, and cirrhosis and those on chronic steroids or who have been transfused more than six units of blood should be diverted. Otherwise, resection and primary anastomosis is typically safe [8].
Special circumstances include the presence of multiple colonic injuries and injuries that are proximal to an anastomosis. You may perform a resection of one colonic injury, but may not be able to include them all, leaving questionable bowel to be addressed. The use of omental patches may be useful to cover or reinforce these types of injuries. The use of serosal patches (suturing another segment of healthy bowel over an injury) has been described, but I have used this technique very rarely. Fibrin glue has also been employed to reinforce bowel repairs with variable results [6]. Another acceptable approach is repair and proximal diversion. An adjunctive maneuver when dealing with colonic repairs in an unprepared colon is the milking of solid stool into a segment of colon that is to be resected. This will decrease the amount of stool remaining in the bowel, particularly proximal to an anastomosis.
Injury to Rectum
Key concept: While diversion plays a larger role with rectal injuries, factors such as the location of the injury, patient’s clinical status, tissue health, and degree of contamination are extremely important in decision-making.
Rectal injuries are among the more common reasons for intraoperative consultation. Whether or not the patient has had pelvic radiation, dates of administration and the dose given are important details for safe operative decision-making. The timing of the radiation is at least as important as the dosage. Beyond 8–12 weeks, intimal fibrosis and thickening may lead to a decrease in blood flow to and impaired healing of the radiated segment of bowel. Performing a proximal diversion or bringing in well-vascularized tissue to buttress the repair of radiated bowel should be considered. Possibilities for such reinforcement include an omental pedicle or muscle flaps such as gracilis or rectus abdominis. These can also be valuable techniques to use in patients who have an injury to the rectum during a hysterectomy. Interposing tissue such as the omentum between the rectal repair and fresh vaginal cuff may reduce the incidence of postoperative rectovaginal fistula formation.
As in colon injuries, much of the decision-making in rectal injuries is similar to that in the treatment of trauma. The mechanism of injury is of importance whether it be sharp, avulsion, or thermal (Fig. 30.3). The amount of contamination, the stability of the patient, and whether the injury is intraperitoneal or extraperitoneal are also of consideration. If a bowel injury is nondestructive and in the upper one-third of the rectum (intraperitoneal), these can safely be managed with primary repair and selective diversion depending on the circumstances of the consult. In the lower two-thirds of the rectum (extraperitoneal), injuries should be debrided to healthy tissue and repaired; if possible, a presacral drain (brought out through the abdominal wall) and diversion should be considered [9]. If the patient is unstable, has received several transfusions, or is medically unfit, diversion should be very strongly considered. Additionally, I may have a lower threshold to divert in a consulting situation than I would in an operation where I am the primary surgeon. This holds true because you may or may not know every clinical aspect of the case and you want to do the safest thing possible. The patient may have issues with incontinence that no one has addressed, and creating a low colorectal anastomosis may exacerbate this.
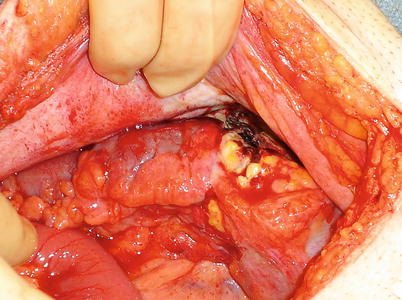

Fig. 30.3
Rectosigmoid perforation from a prior colonic stent (Courtesy of Philip Y. Pearson, MD)
Mass
Key concept: In the absence of obstruction, obtaining issue and returning at a future time with more information and discussion with the patient are often beneficial.
The operating surgeon may encounter a mass either by palpation of the colon during routine exploration or in an extraluminal location with involvement of the bowel. In the initial evaluation of the mass, the degree of obstruction that is present must be determined. If obstruction is not an issue or malignancy can be ruled out (as in endometriosis), it may be best to return at another time after proper bowel evaluation and after obtaining informed consent from the patient. If obstruction is present in the colon and it has not been prepped, the risk of contamination and anastomotic problems should be considered. At this time, my preference remains in favor of bowel preparation for elective cases. In the consulting situation, if the patient has not been prepped, I am willing to do anastomoses without bowel preparation in light of the data that have shown no increased complications. A Cochrane Review performed in 2011 showed no statistically significant evidence that patients benefit from mechanical bowel preparation nor the use of rectal enemas [10]. In addition, one study showed that the liquid stool present in patients that had bowel preps caused a significantly higher rate of spillage and therefore could lead to a higher rate of infection [11]. As mentioned previously, solid stool can be maneuvered into the portion to be removed to decrease the amount of stool burden proximal to your anastomosis. It must be remembered that the data regarding colonic resection and bowel preparation do not apply to situations where the bowel is obstructed. The intestine proximal to an obstruction is typically dilated and congested, and I feel that anastomosis to such a segment of bowel is dangerous. If the bowel is resected back to healthy small bowel, an anastomosis can still be considered. A bypass may also be considered if the mass is unable to be removed.
If the mass is extraluminal and not causing obstruction, biopsy should be performed to determine the nature of the problem as well as to determine the presence of possible malignancy. Removal can be considered if the risks do not seem excessive. In addition to adenocarcinoma, other less frequent findings on biopsy include endometriosis (which will be discussed later), carcinoid, desmoid, lymphoma, and necrotic tissue (such as a lymph node). Carcinoid frequently involves the appendix or terminal ileum. Resection is frequently safe and advisable for carcinoid tumors. Remember that ileal carcinoids are often multiple, so careful examination of the adjacent bowel is advisable. Desmoid tumors are seen most frequently in patients with familial adenomatous polyposis and can involve the mesentery or the abdominal wall. My general approach to desmoids would be not to resect. Smaller lesions on the abdominal wall may be safely removed, but with nonobstructing smaller lesions involving the mesentery, the first line of therapy is often medical with sulindac and anti-estrogens. Larger desmoids may be difficult to remove safely due to the involvement of mesenteric vessels and often result in significant portions of bowel being resected. These are best treated with chemotherapy [12].
Isolated gastrointestinal lymphomas rarely cause obstruction or perforation due to their pliable nature and most often present with acute abdominal pain. They should be resected for potential cure, staging information, and avoidance of complications. A recent review of primary small bowel and colonic lymphoma showed that small bowel lymphoma did benefit from postoperative chemotherapy. Colorectal lymphoma on the other hand did not show a significant difference with chemotherapy or surgery alone as most required surgery for complications or diagnosis [13]. Necrotic lymph node tissue may need to be debrided, but does not need to be resected to clear margins. This may be an indication for a drain.
Colonic Inflammation
Key concept: Many inflammatory conditions can be treated medically, while perforations should be repaired at the time.
These consultations usually arise in the setting of pelvic surgery, typically gynecological or urologic procedures. They may also arise in the setting of right lower quadrant pain and immunosuppression. The sigmoid or descending colon may be noted to have inflammation or be adhered significantly to structures of interest to the requesting surgeon. It is important to note that inflammation of the colon in the face of diverticulosis may be related to a variety of colitides that may best be treated medically. Therefore, inflammation of the colon, by itself, is not an indication for resection. Purulence is often encountered. Most of these patients have been asymptomatic as far as episodes of acute diverticulitis are concerned. A detailed history, if available, is helpful in these situations. This is often not readily available, and discussions with family members may or may not yield any additional clinical information.
In general, simply following sound surgical principles applies. A perforation associated with diverticular disease can be identified with air insufflation by inserting a rigid proctoscope and examining the air-filled bowel with saline in the pelvis. If no air leak is identified and there is minimal to no contamination, often times the diverticulitis can be observed with appropriate antibiotic coverage. This may be combined with intra-abdominal drains depending on the comfort level of the operating surgeon. In the face of perforation without gross contamination, a resection with primary anastomosis can be performed [14]. A proximal diverting stoma, colostomy, or loop ileostomy may be added depending on the condition of the patient and the bowel.
Right lower quadrant pain can also mimic other disease states of an inflammatory nature. General surgeons operating for appendicitis often encounter these situations. The finding of diverticulitis of the right colon or appendix is rarely diagnosed correctly preoperatively, with most patients being diagnosed with appendicitis. Appendectomy with or without diverticulectomy followed by antibiotics is appropriate for inflammation of a diverticulum. If there is localized abscess, perforation, or concern for cancer, a right hemicolectomy is the procedure of choice [15].
Another difficult situation is the patient with right lower quadrant pain, neutropenia, and, sometimes, even sepsis. Oftentimes in neutropenic enterocolitis, the most difficult decision is when to take the patient to the operating room, and if you are being consulted intraoperatively, this decision has already been made. Most literature is based on retrospective case series, but do show a trend toward improved outcomes with surgery. Most authors recommend a right hemicolectomy as the mucosal injury can be more extensive than the serosal surface reveals. An end ileostomy with mucous fistula would be the safest approach in this situation involving immunosuppression and potential sepsis [16].
Cancer and Polyps
Key concept: Proper lesion location and adherence to oncologic principles such as en bloc resection is imperative. Other situations may be best handled by closing the patient and obtaining appropriate staging information.
Several issues can arise in operations for malignant processes of the colon and rectum. A common intraoperative consult is the call for assistance in locating a mass. In the colon, this can occur if the lesion was not tattooed or the endoscopist experienced looping of the scope resulting in inaccurate localization. The difficulty in identifying distal lesions of the large bowel may be the variation that can occur with measurements obtained by flexible versus rigid instruments. If the operating surgeon did not confirm the location prior to the abdominal operation, the first step is to place the patient into low lithotomy and examine thoroughly with a finger, rigid proctoscope, or even a flexible sigmoidoscope or colonoscope depending on the likely location of the mass. If the operation is being done laparoscopically, it may be prudent to convert to a hand-assisted or open technique. Intraoperative colonoscopy may be difficult in a laparoscopic procedure due to massive distention of the colon. In a hand-assisted operation, it may be possible to pass the scope through the colon with minimal insufflation. Then, short segments of the colon can be insufflated and decompressed with the aid of the intra-abdominal hand. CO2 insufflation has been described, but may be difficult to setup unless you have worked out the details of the technique in advance. Also, the CO2 absorbs more rapidly than room air, but still may keep the bowel distended making resection more difficult. Methodical palpation of the colon can also aid in locating the mass. Once the lesion is identified, you may have finished your consultation. If, on the other hand, the lesion is not identified even after these maneuvers, it may be prudent to close the patient and reevaluate at a later date.
Another circumstance which may arise is the cancer that is located much lower in the rectum than the operating surgeon expected, and he/she is not comfortable performing the resection at that level. This becomes a situation in which (unless you are consulted by your partner or someone in your rounding group) you must become the primary surgeon and decision-maker. Standard principles for resection of rectal cancer should apply, and the need for neoadjuvant therapy should be considered. It may be best to close the abdomen and refer the patient for neoadjuvant therapy if this has not been done. This decision should be made before mobilizing the rectum since a second attempt at mobilization is much more difficult and dangerous. If the staging of the rectal tumor is not clear, intraoperative ultrasound can be performed to assist in determining if the patient would be a candidate for such therapy. This may be a situation where you may wish to discuss the case with the family prior to going forward with any intervention and fully discuss the risks and benefits of the indicated procedure.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








