Fig. 12.1
Rectocele. Rectal prolapse can also seen at the verge that was easily visualized with straining (Courtesy of M. Shane McNevin, MD)
A careful urogynecologic examination is also important, particularly in any patient with a suggestion of rectal or other pelvic organ prolapse. If prolapse is visible at the vaginal introitus or a bulge is noted during the Valsalva maneuver, a systematic examination should be performed. With the patient in a supine position and the head of the examination table elevated to 45°, an appropriately sized vaginal speculum is placed in the vagina to view the cervix or vaginal cuff. While the patient is performing the Valsalva maneuver, the speculum is slowly removed. The extent to which the cervix or the vaginal vault follows the speculum through and out of the vagina is noted. The speculum is disassembled and the posterior or fixed blade is used for examination. To examine the anterior vaginal wall, the posterior vaginal wall is retracted with the fixed blade and the extent of any anterior vaginal prolapse during the Valsalva maneuver is noted. To examine the posterior vaginal wall, the fixed blade is inverted, the anterior vaginal wall is retracted, and the patient is instructed to repeat the Valsalva maneuver. Any resulting prolapse or bulge is noted. Bimanual and rectovaginal examinations help identify any coexisting pelvic abnormalities, including those of the perineal body. If pelvic organ prolapse is not evident, especially in a woman feeling a bulge, the patient should be examined in the standing position while she performs the Valsalva maneuver [2].
Endoscopy
The next step in evaluation of these patients is anoscopy and flexible sigmoidoscopy or colonoscopy. Anoscopy may identify circumferential folds in the rectal wall descending from above indicating a possible prolapse or intussusception. While the choice of endoscopic procedure is determined by the patient’s age and interval since last screening examination, it is necessary to exclude polyps or neoplasm as the cause of the obstructive symptoms. The examination may also identify a solitary rectal ulcer, colitis cystica profunda, or any suspicious lesion that should be biopsied to exclude a neoplastic process and confirm the diagnosis. If the endoscopy is negative, the next step depends upon your clinical suspicion for another diagnosis and the patient’s response to conservative therapy
Adjunctive Tests
Key Concept: Additional testing is useful if the patient has mixed symptoms of infrequent urge and obstructed defecation. The testing is also performed to confirm findings on physical examination as the source of the symptoms or to identify the cause if there is uncertainty on the physical examination.
There is no single test that is able to definitively diagnose obstructive defecation or the underlying etiology. Several adjunctive studies may be performed to help arrive at a diagnosis, including transit studies, anorectal manometry, balloon expulsion, EMG, fluoroscopic (conventional) defecography, MR defecography, and perineal ultrasound [3, 4]. If the patient complains of an infrequent urge to defecate, as well as difficult evacuation, colonic transit time and balloon expulsion tests may be useful to determine the primary problem.
Colonic Transit Study
Colonic transit can be evaluated with a colonic marker study (Fig. 12.2a, b) [3]. This test involves asking the patient to swallow a capsule containing radiopaque rings followed by abdominal x-ray typically on day 3 and day 5 to determine the number of markers left and their locations [4]. If more than 5 of the 24 markers are present on day 5, the study suggests slow-transit constipation. The distribution of the markers may also suggest an etiology, with a diffuse pattern suggesting colonic inertia, and markers predominantly present in the distal colon and rectum suggesting that pelvic outlet obstruction is the primary problem, rather than abnormal transit through the colon [5].
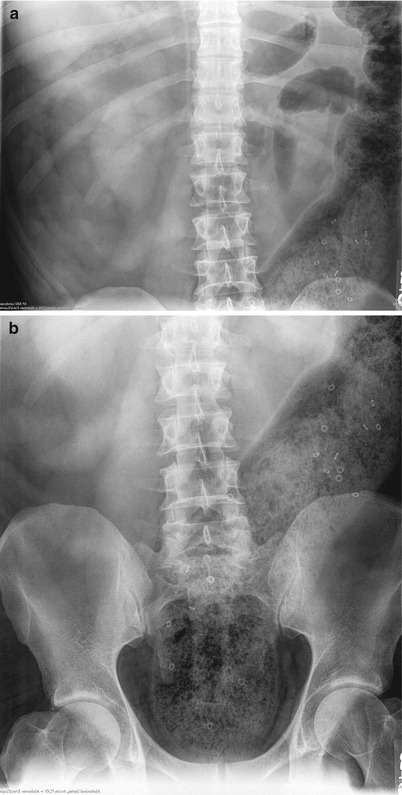

Fig. 12.2
(a, b) Sitzmark study demonstrating large stool burden in the rectum and left colon and retained sitzmarkers
Balloon Expulsion
A balloon expulsion test involves inflation of a rubber balloon inserted into the rectum; the patient is then asked to expel the balloon. Normal subjects can expel the balloon in 1 min. While a positive test (i.e., inability to expel) indicates obstructive defecation, some patients with obstructive defecation may still be able to expel the balloon. Although a simple test, the methods for performing the balloon expulsion test are not standardized in regard to the filling volume of the balloon, position of the patient, and expulsion time [6]. It also does not define the mechanism of obstructed defecation, but simply helps confirm it is the cause of the patient’s symptoms. Therefore, it is best used as a screening test for a functional defecation disorder [7].
Key Concept: The primary purpose of some tests is to identify pelvic floor dyssynergia. One of the tests, manometry, also excludes Hirschsprung’s disease.
If the patient complains only of obstructive defecation symptoms, the goal of additional testing is to identify the underlying etiology. More than one contributing factor may be seen on the testing. The following tests may be useful:
Anorectal Manometry
Anorectal manometry testing will tell you the resting anal pressure, squeeze pressure, rectoanal inhibitory reflex, rectal sensations (first and maximum tolerable), rectal compliance, and rectal and anal pressure during attempted defecation [6, 7]. Abnormalities in these parameters may direct the clinician towards potential pathophysiology causing obstructive defecation. The main abnormality in obstructive defecation is absent or inadequate relaxation of the anal sphincter, sometimes associated with contraction during straining [6]. An absent rectoanal inhibitory reflex (RAIR) is an indication of Hirschsprung’s disease, which is usually diagnosed in childhood, rather than obstructive defecation. Elevated sensory thresholds, increased compliance, and rectal motor dysfunction may be seen with obstructive defecation and can be treated with biofeedback [6, 8]. You should be aware that manometry may overdiagnose dyssynergia. Paradoxical sphincter contraction has been shown in 22 % of asymptomatic controls, and the rate was not statistically different in constipated patients [7, 9]. The finding may be due to the horizontal patient position and simulated environment.
Electromyography (EMG)
EMG is a direct and specific test for the examination of somatic muscular activity of the external anal sphincter, puborectalis muscle, and pubococcygeus muscle during attempted defecation [10]. EMG may be performed with a needle study, but more frequently EMG recruitment testing is done with a sponge for the patient’s comfort. With either method, the patient is asked to tighten the sphincter muscle and then to bear down. Myoelectrical activity at rest, during squeeze, and push are recorded. Non-relaxation, or even increased activity, in these muscles during attempted defecation is considered abnormal and may indicate that the patient’s symptoms are secondary to dysfunctional muscle. However, paradoxical activation of the puborectalis and external sphincter has been observed in disorders other than obstructive defecation and in normal subjects [11, 12]. Also, some patients with symptoms of obstructive defecation demonstrate normal inhibition of external sphincter and puborectalis activity [11, 13, 14], suggesting another etiology.
Imaging
Key Concept: Imaging is used when rectal prolapse, rectocele, solitary rectal ulcer syndrome, or findings suggestive of pelvic organ prolapse are seen on examination. Imaging may also confirm or refute the presence of non–relaxation of the puborectalis muscle.
Defecography
Defecography can identify structural abnormalities and also assess functional parameters [15]. Defecography may allow diagnosis of several problems that may be contributing to the patient’s symptoms such as internal intussusception, external rectal prolapse, rectocele, sigmoidocele, enterocele, and paradoxical contraction of the puborectalis muscle (Figs. 12.3, 12.4 and 12.5) [6, 7]. However, other than rectal prolapse, many of these findings are present in asymptomatic controls, so the defecography findings ought not to be the sole indication for surgery [6, 16, 17]. Anorectal angle measurements among observers are greatly variable, as there is no consensus on whether the rectal axis should be drawn through the anterior, central, or posterior wall [7, 18].
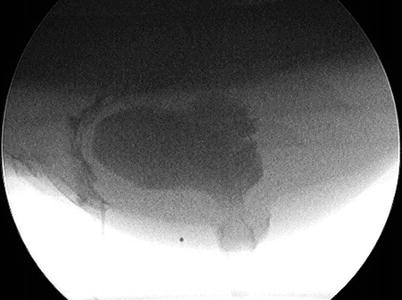
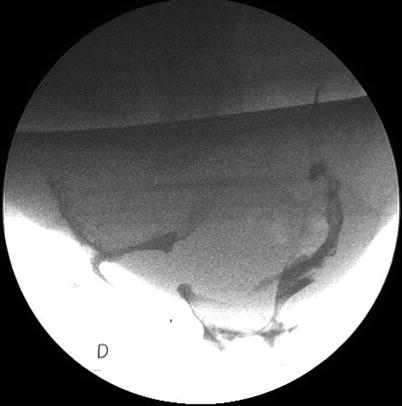
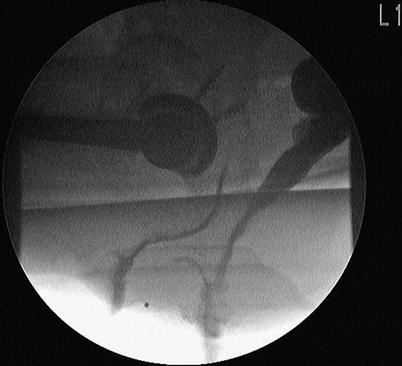

Fig. 12.3
Defecography of rectocele

Fig. 12.4
Defecography of rectal prolapse with enterocele

Fig. 12.5
Defecography of deep internal intussusception
MR defecography is a newer modality that allows anatomic and dynamic pelvic floor evaluation in real time without radiation exposure (Figs. 12.6 and 12.7) [7, 19]. Several studies have compared conventional defecography to MR defecography with differing results. MR defecography, unless open, requires a horizontal positioning of the patient, which is not a physiologic defecatory position but, however, has shown more reproducible results, compared to conventional fluoroscopic defecography [6, 20]. In a small study, seated open MR defecography compared to closed supine MR defecography shows similar detection of most clinically relevant findings. Rectal intussusception was seen only on seated MR [21]. Studies comparing conventional defecography and supine MR with evacuation phase showed no significant differences in sphincter hypotonia, dyssynergia, rectocele, or rectal prolapse [22, 23]. In contrast, a small study comparing dynamic pelvic MRI and videoproctography showed videoproctography to be more sensitive in detecting anterior and posterior rectoceles, rectoanal intussusceptions, sigmoidoceles, and perineal descent [24]. A different small study showed dynamic cystocolpoproctography and dynamic pelvic MR to be concordant for rectocele, enterocele, cystocele, and perineal descent; dynamic MR was the only modality that identified levator ani hernias; dynamic cystocolpoproctography identified sigmoidoceles and internal rectal prolapse more often than dynamic MR [25]. Likely, your local expertise will dictate the appropriate imaging modality at your institution.
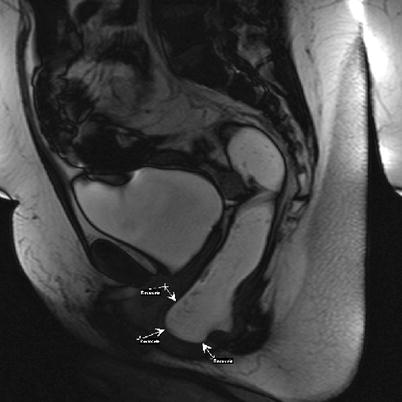
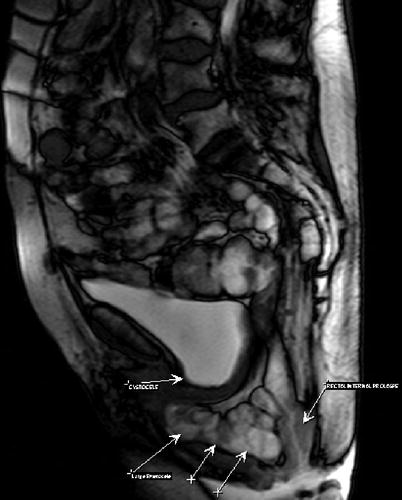

Fig. 12.6
MRI defecography of rectocele

Fig. 12.7
MRI defecography of enterocele, cystocele, and rectal invagination
Perineal Ultrasound
Dynamic perineal ultrasound is performed by placing a probe on the perineum, between the anus and the introitus. Compared to healthy controls, patients with symptoms of obstructive defecation demonstrate significantly greater absence of relaxation of the puborectalis muscle on straining and significantly higher incidence of rectal internal mucosal prolapse on dynamic perineal ultrasound [26]. There is good concordance between dynamic transperineal ultrasound and defecography for identifying rectocele, rectoanal intussusception, anorectal angle, and dyssynergic contraction of the puborectalis [27, 28]. Like many studies of this nature, the performance and interpretation are reliant on the experience and expertise of the user.
Our Recommendations
Key Concept: The choice of adjunctive tests depends upon the patient’s symptoms and the findings on physical examination.
In our practice, after thorough history, physical examination, and endoscopy, we start with conservative management, including stool bulking and osmotic laxatives. If the patient has combined symptoms, we perform transit studies to help qualify the type of constipation the patient may be experiencing: slow transit vs. pelvic outlet obstruction vs. combination. Currently defecography is done for patients with demonstrated or suspected rectal prolapse or solitary rectal ulcer syndrome, as well as patients with symptomatic rectocele or enterocele. The goals are better understanding of the anatomy and exclusion of concomitant abnormalities. Admittedly, defecography is readily available at our site; however, when it is not, dynamic MRI is an option, but it is important to have a radiologist with the interest and training for appropriate interpretation. It is possible that in the future perineal ultrasound will replace both tests for radiation exposure and cost reasons, but at the present, there is only limited experience. EMG recruitment is done on patients with obstructive defecation unless full-thickness rectal prolapse is present. In all other patients, non-relaxation would be addressed first even if an indication for surgery was present. In addition, EMG recruitment helps to confirm or refute the diagnosis of anismus. We perform manometry on these patients but largely for research purposes rather than as aid for clinical decision-making. Balloon expulsion testing is rarely done. It is helpful however when the diagnosis is uncertain or if the patient is diverted.
Etiology and Treatment Options
Key Concept: Treatment decisions depend upon the etiology of obstructive defecation.
If a full-thickness rectal prolapse is diagnosed, surgical correction is performed; any persistent evacuation difficulties are addressed postoperatively. Please see Chap. 11 where Drs. Wexner and Hayden address the options for repair. If non-relaxation of the puborectalis muscle is identified on testing, particularly if seen on two tests, that issue is addressed first even if a rectocele, internal intussusception, or enterocele is also identified. If a rectocele is the only abnormality identified, it is addressed; the same is true for enterocele and sigmoidocele in some circumstances.
Non-relaxing Puborectalis
Key Concept: Non–relaxation of the puborectalis muscle may be an isolated finding or seen in combination with other abnormalities. Initial treatment is medical management including dietary recommendations and possible recommendation of fiber supplements or osmotic laxatives combined with biofeedback therapy.
Non-relaxation of the puborectalis may be diagnosed on EMG recruitment, defecography, balloon expulsion tests, and ultrasound. There is controversy about the diagnostic criteria and even the existence of this finding [9, 12]. The false-positive rate is poorly documented but false positives do occur, likely secondary to a poor understanding of instructions or embarrassment [9]. In our institution, 15 % of patients were able to demonstrate appropriate puborectalis function during their first biofeedback appointment, suggesting a false-positive diagnosis (unpublished data).
Our preferred initial treatment of non-relaxation of the puborectalis is biofeedback therapy. Regardless of the technique utilized, the goals of biofeedback therapy are to correct the lack of appropriate coordination of the abdominal muscles and sphincter mechanism and to enhance rectal sensory perception. Published methods include manometry-based biofeedback, EMG biofeedback, balloon defecation, and home device training. Surface EMG electrodes may be used on the abdominal and gluteal muscles. Protocols vary but typically include four to six training sessions. Improvement of symptoms varies between 44 and 100 % in several uncontrolled trials [29]. The wide range is likely due to the vague definition of endpoints, variable duration of follow-up, and inconsistent patient selection. What is clear is that results are better in patients who complete the full-prescribed course of treatment [30]. There have been several randomized controlled trials [31–36] where biofeedback was compared to medical management, polyethylene glycol, diazepam/placebo, balloon defecation treatment, and sham feedback therapy. In all of these studies, biofeedback was found to be superior to the other treatment options. An additional 1-year long-term follow-up study reported that biofeedback was superior to medical management [34]. Another recent study showed benefit in patients with anismus with and without IBS [37]. Overall, in addition to the positive outcomes, biofeedback is inexpensive and safe without reported adverse events.
Failure of Initial Management/Surgical Options
Key Concept: If symptoms do not resolve with biofeedback therapy, reported alternative treatments include botulinum neurotoxin injection and surgical division of the puborectalis muscle. Botulinum neurotoxin appears to be safe, while surgical division of the puborectalis muscle is no longer recommended because of reported complications.
If obstructive defecation symptoms persist in patients whose only abnormality is non-relaxation of the puborectalis muscle, then botulinum neurotoxin injection may be considered. In reported studies two bilateral injections of botulinum neurotoxin into the puborectalis and external sphincter muscles are performed with total amounts varying from 60 to 100 units [38–44]. The injections are done under either digital or ultrasound guidance and with either local or general anesthesia. Improvement in symptoms varies from 33 to 79 % [38, 40–45]. In the studies with postoperative evaluation, the sphincter pressures and anorectal angle decreased. The improvement typically lasts only 3 months, so repeat injections are often required. Careful selection of patients is important. In one study, examination under anesthesia of nonresponders revealed significant abnormalities (rectal prolapse, fissure, internal anal sphincter myopathy) in 97 % of patients [38]. When those patients were excluded, the response to botulinum toxin was 96 %. In that same study, gender and presenting symptoms were the only factors predictive of success, with men and patients presenting with obstructive defecation symptoms alone more likely to respond. A randomized study compared biofeedback to botulinum neurotoxin injection in treatment of anismus [39]. Initial improvement was significantly better in the injection group, but there was no difference in long-term success or patient satisfaction. None of the studies reported serious complications, though temporary incontinence of flatus is reported in several studies. Our feelings are that while the primary disadvantage of botulinum neurotoxin injection is the potential need for repeat treatment, it appears to be safe and a reasonable option in the treatment of these patients.
On the other hand, surgical division of the puborectalis muscle, a previously reported option has been largely abandoned because of the high rate of incontinence [46–48]. However, one recent study compared biofeedback, botulinum neurotoxin injection, and surgical division of the puborectalis muscle [49]. Patients undergoing surgical treatment had the best long-term improvement, with 70 % improvement at 1 year vs. biofeedback (30 %) or botulinum toxin injection (35 %). Incontinence was reported in 13 %. Outpatient anal dilatation is described as another option. Thirteen patients performed daily insertion of anal dilatators for 30 min for 3 months [50]. At 6 months from the end of treatment, all patients reported improvement. For patients who prefer or do not respond to other treatments, the remaining surgical option is diversion.
Our Recommendations
In our practice, patients with non-relaxation of the puborectalis muscle, either as an isolated or combined abnormality, are referred for biofeedback. For those patients with isolated non-relaxation that persists after biofeedback, botulinum neurotoxin injection is offered. We do not recommend division of the puborectalis muscle because of the risk of irreversible incontinence. Diversion is rarely indicated but may be offered when the impact on the symptoms severely impair patient’s quality of life.
Rectoceles
Key Concept: Rectoceles are common findings in parous women; caution must be exercised in attributing symptoms to them.
The prevalence of rectoceles is poorly documented, as many women are asymptomatic, with reported rates varying from 18 to 40 % in limited studies (Fig. 12.8) [17, 51]. Confounding this, rectoceles are seen on defecography in 81 % of asymptomatic women [17]. Because rectoceles are quite common, you should be cautious about attributing symptoms to that finding alone. In addition, a recent review demonstrated variable association of the degree of posterior compartment prolapse and symptoms of obstructive defecation [52].
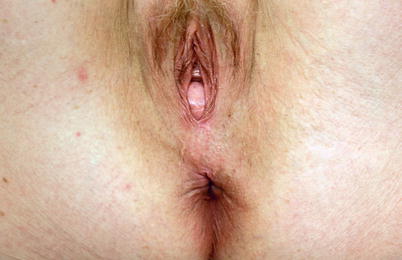

Fig. 12.8
Rectocele (Courtesy of M. Shane McNevin, MD)
Surgical Indications
Key Concept: Surgery is indicated for persistent obstructive defecation symptoms and/or a symptomatic vaginal bulge.
Women with obstructive defecation secondary to a rectocele typically complain of a sense of incomplete evacuation with a sensation of stool trapped in a visible vaginal bulge or rectal pocket. Perineal or posterior vaginal wall pressure facilitates evacuation and may serve as an indication that rectocele repair will alleviate the symptoms. While some surgeons use rectocele size over 3 cm as a criterion for surgery [53], others have found that this does not correlate well with the extent of symptoms [54]. Some authors believe that retention of dye in the rectocele is an indication of the clinical significance of the rectocele, while others maintain that this finding does not relate to the relief of symptoms postoperatively [55–57].
Key Concept: A number of surgical options are available for rectocele repair; there is no clear evidence of superiority of one approach.
Rectoceles occur because of disruption or diffuse thinning of the fascial tissue between the rectum and the vagina. They are essentially a hernia. In other areas of the body, mesh is frequently utilized now to reduce recurrence rates. Similarly, because of recurrence rates with traditional native tissue repairs of rectoceles, repairs utilizing mesh were developed. Some of those repairs, with the use of synthetic mesh, for example, have been complicated by persistent pain, mesh erosion, and infection. Another approach, stapled transanal resection of the rectum (STARR procedure) involves resection of redundant tissue in the rectum without repair of the fascia using linear staplers. While certain patients benefit, the procedure does not resolve symptoms in all women with rectoceles, and there is a generalized paucity of data describing the results in patients with rectoceles alone.
Ventral rectopexy with mesh anchored to the perineal body is also currently being evaluated with the goal of repairing the fascial defect with reduced complication rates because of the abdominal approach. Unfortunately, no single approach has yet been shown to be superior to the others. An issue with the literature regarding rectocele is that the definition of anatomically successful repair is not standardized. Furthermore, in most cases, the different approaches vary in success rates with regard to the outcome measured (i.e., recurrence, function, complications).
Transvaginal surgical choices include posterior colporrhaphy, site-specific rectocele repair, and mesh implantation. Posterior colporrhaphy involves midline plication of tissue in the rectovaginal septum often with perineoplasty. After dissection of the vaginal epithelium from the rectovaginal septum, the fascia, and in some hands the levator ani muscles, is plicated in the midline. The fibromuscular tissue adjacent to the perineal skin is also plicated to complete the perineoplasty. Generally, the anatomic abnormality is successfully repaired, but dyspareunia is common and the functional results variable [58–64]. Recurrence of symptoms appears to increase with the length of follow-up [65].
Site-specific repairs consist of identification and repair of the fascial defect in the rectovaginal septum [66–71]. A further modification added a perineal repair [72]. These repairs also result in acceptable anatomic correction with less sexual consequences, but the recurrence rates appear to be higher. A randomized trial of posterior colporrhaphy compared to site-specific repair with and without graft augmentation demonstrated slightly better anatomic success in the posterior colporrhaphy patients with no difference in functional outcome or dyspareunia [58]. Another comparative study confirmed those findings [73]. Overall, relief of defecation symptoms varies from 46 to 72 % [72–74].
Disappointing recurrence rates motivated the search for alternative repairs [65, 75, 76]. The third vaginal approach option is mesh implantation, which can occur either through use of a mesh-kit technique or as a supplement to any of the other procedures. Synthetic permanent, synthetic absorbable, and biologic meshes have been used. A number of comparative studies of synthetic mesh demonstrated comparative anatomic results to native tissue repair [77–81]. Four randomized controlled trials comparing transvaginal permanent synthetic mesh to traditional native tissue repair have been published; two of the studies involved the use of mesh kits [82–85]. One reported fewer recurrences with the use of mesh, while the other three reported no differences. The rate of mesh extrusion ranged from 5.6 to 16.9 %. Interestingly, the study with the positive results also revealed a much higher rate of new prolapse in an untreated compartment than with traditional repair [86]. Complications related to the mesh including mesh extraction, mesh retraction, pelvic pain, and sexual dysfunction were reported. Recent concerns about vaginal erosions of the mesh and mesh contractions resulting in chronic pain and vaginal shortening resulted in an update warning from the FDA in 2011 counseling surgeons about the risks and need for informed consent [87]. Your patients should be informed that surgical intervention may be necessary to correct the mesh extrusion and pain secondary to the mesh, should it occur. At the present there appears to be additional risk and insufficient benefit for recommending the use of synthetic mesh [88].
Those findings and recommendations led to use of other materials. One randomized controlled trial comparing synthetic absorbable mesh to native tissue found no difference in recurrence rates [89]. Unfortunately, the authors did not report bowel or sexual functional outcomes. There is some evidence that the complication rate is lower with biologic mesh than with other mesh. However, two studies comparing its use to native tissue found either no benefit [85] or a higher recurrence rate [58].
Transperineal repairs of rectoceles involve a perineal incision with subsequent dissection of the rectovaginal septum separating the posterior vaginal wall from the external sphincter distally and rectal wall proximally. The dissection proceeds cephalad to the posterior fornix. Imbrication of the fascial tissue, along with a site-specific repair or insertion of mesh, completes the repair [90–93]. A levatorplasty may also be added. A randomized controlled trial compared transanal repair, transperineal repair alone, and transperineal repair with levatorplasty [94]. Defecography revealed a reduction in the size of the rectocele in all groups, but functional scores improved only in the two transperineal groups. The combination of a transperineal approach with levatorplasty yielded the most improvement in functional outcome.
Transanal repair of rectocele involves the elevation of rectal mucosal flaps for the length of the rectocele [53, 95, 96]. The tissue in the rectovaginal septum is then imbricated; anterior levatorplasty and internal sphincter muscle plication may be added. The mucosal flaps are then re-approximated. Several investigators report high rates of symptomatic improvement after transanal repair [53, 95–101]. Typically, low rates of dyspareunia are demonstrated. However, a randomized controlled trial comparing vaginal to rectal repairs found a higher recurrence rate (7 % vs. 40 %) and more frequent incontinence of flatus after the transanal repair [102]. As noted above, the trial comparing transanal and perineal approaches demonstrated better functional results with the transperineal approach [94]. Yet, a recent Cochrane review found lower recurrence rates with a vaginal approach compared to transanal repairs, highlighting the differences among the various approaches for each of the outcomes assessed [103].
Regardless of the type of repair, most studies demonstrate improvement in obstructive defecation symptoms including splinting [85, 94, 104]. On the downside, those same studies also reveal a persistence of some symptoms in about 50 % of patients. This data, in addition to the frequency of the anatomic finding in asymptomatic women, emphasizes the importance of careful selection and informed consent for these patients. This is especially pertinent when considering that conservative management, including dietary, medication, and lifestyle changes and possibly a pessary, results in improvement in ~50 % of patients [105].
Our Recommendations
Recognizing there is not an optimal repair, our preferred approach is a site-specific repair if the defect can be identified and the surrounding tissue seems adequate. If the defect cannot be identified clearly, then a native tissue imbrication with the possible addition of biologic mesh (per surgeon preference) is utilized. Restoration of the perineal body is included. At the present time, we are avoiding the use of synthetic mesh.
Internal Intussusception
Key Concept: If internal intussusception is the only abnormality identified, medical management is the first–line treatment.
Internal intussusception (i.e., rectoanal intussusception, internal rectal prolapse, occult rectal prolapse) is full-thickness, circumferential prolapse of the rectum during evacuation that does not protrude through the anus [106, 107]. Defecography is the best way to diagnose internal intussusception. Importantly, it is unclear whether internal intussusception is pathologic or is a normal variation, as rectal intussusception is seen in 50 % of asymptomatic patients on defecography during defecation [17]. Interestingly, there is no difference in the speed or effectiveness of evacuation between asymptomatic volunteers with and without rectal intussusception. Likewise, symptomatic patients with intussusception did not have significantly different evacuation parameters compared to either asymptomatic group [108]. Furthermore, some authors believe it is a consequence of obstructed defecation rather than a cause of it [109]. The frequency with which obstructed defecation is associated with internal intussusception is unknown [110].
Rectal intussusception may cause symptoms of obstruction by blocking the rectal ampulla during defecation; this may cause a persistent urge to defecate as well as pain in the anal canal [111].
The treatment of internal intussusception is controversial. Initial management is always conservative. Therefore, we and most authors recommend conservative treatment consisting of fiber supplementation, refraining from straining, and biofeedback [3, 109, 112–114]. Over 50 % of patients with rectal intussusception experience complete or partial resolution of constipation symptoms with biofeedback [110].
Surgical Treatment
Key Concept: Caution must be exercised before the recommendation of surgical intervention as the outcomes after traditional surgical correction were disappointing. While more recent results from two new procedures are more encouraging, the role of surgery for this diagnosis is unclear.
Surgery for internal intussusception should be approached with caution. Adequate repair, as evidenced by elimination of the intussusception on defecography, may not equal symptom relief for the patient, and likewise, despite persistence of an anatomic problem (i.e., surgical failure), patients may feel symptomatic relief [109, 111]. Furthermore, the risk of internal prolapse progressing to total rectal prolapse is low [113, 115].
Various surgical techniques have been employed to treat internal intussusception associated with obstructed defecation including traditional rectopexy, ventral rectopexy, and stapled transanal resection of the rectum (STARR). In a survey of constipated patients who underwent laparoscopic resection rectopexy after biofeedback failed, 53 % reported improvement in bowel frequency [116]. In another small study, the majority of patients who underwent resection rectopexy reported an improvement in constipation symptoms [117]. However, others have found rectopexy to be an ineffective treatment for obstructed defecation that may even worsen constipation or tenesmus [118, 119]. Because the functional outcomes of traditional rectopexy (± resection) are unacceptably poor and patients with internal intussusception are unlikely to see improvements in their obstructive defecation with this procedure, a very frank discussion with the patient must precede surgical intervention [120].
Until recently, we, along with most surgeons, avoided surgery for internal intussusception because of the high likelihood of persistent symptoms. A small percentage of patients underwent stoma formation because their symptoms were so troublesome and prior repairs had been ineffective. Recently, however, outcomes from two procedures have been more encouraging. Laparoscopic ventral rectopexy, performed by dissecting exclusively anterior to the rectum, preserving the lateral stalks, and using polypropylene mesh for rectal fixation, has been used for internal intussusception with good results. At 3 months postoperative, 86 % of patients reported improvement in their obstructed defecation, and there were no mesh-related complications [120]. Others have found similar results [119]. Yet, many surgeons fear placing a permanent mesh directly on the bowel due to the potential for erosion or infection. At 6-month follow-up from laparoscopic ventral rectopexy using biologic mesh (Permacol) in patients with internal rectal prolapse and constipation, 82 % reported cured or improved constipation. There were no mesh-related complications [121]. While still in its infancy, ventral rectopexy has promising initial results.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








