Fig. 18.1
The contribution of anastomotic collagen synthesis and lysis to overall anastomotic strength (With permission from Munireddy et al. [2])
Remodeling
The provisional matrix previously formed is remodeled into a stronger thinner area with fibroblast proliferation and transition in collagen formation from type III to type I [2]. This is the time period in which fibroblast-mediated wound contraction occurs. It has been suggested that fibrosis occurs because of reorganization of granulation tissue into scar that is likely more pronounced in more ischemic tissue. An increase in ischemic tissue is one possible explanation for the higher incidence of stenosis seen with stapled anastomosis compared to hand sutured [2, 12].
Failed Anastomotic Healing
The healing of the gastrointestinal anastomosis is a timely and orderly process which occurs successfully the majority of the time. Failure of this process is caused by local or systemic factors that interrupt the “timely recovery of the injured tissue’s mechanical integrity [13].” Tissue perfusion is a major factor that affects healing locally.
Tissue Perfusion
Key Concept: Macro– and microvascular blood flow provide the necessary factors to enable anastomotic healing. Avoid using the sigmoid colon, when possible, and fully mobilize the splenic flexure to provide a tension–free low colorectal anastomosis.
For the normal healing process of an anastomosis to take place, it must have ample tissue perfusion to deliver the influx of inflammatory cells, growth factors, and oxygen. Ample tissue perfusion of a healing anastomosis is determined by the macrovascular and microvascular anatomy as well as the arterial tissue oxygen saturation [5].
Macrovascular Anatomy
The mucosa, which receives two-thirds of the blood supply of the colon [14], is extremely sensitive to reducing blood flow. This leads to ischemia that can rapidly become transmural and irreversible. In addition, reperfusion of ischemic bowel can cause further tissue damage that extends beyond the boundaries of the previous injury. The vasculature of the colon and rectum, along with the multiple variations that exist, is well known to the surgeon. This knowledge is a necessity in order to perform a safe and successful oncologic resection, but for the purpose of the intestinal anastomosis and why it fails, it is far more instructive to focus on the specific areas of relative vascular insufficiency. These areas of vascular insufficiency can be congenital or specifically result from surgical resection. Below are the notable areas of concern.
Griffiths’ Point
J. D. Griffiths described a “critical point” that exists at the splenic flexure where the marginal artery is often diminished. The marginal artery in this area is dependent on the left branch of the middle colic and branches of the ascending left colic artery to provide blood flow [15]. Indeed Griffiths’ point is one of the “water shed” areas that develops poor perfusion during systemic hypotension. This area of the splenic flexure, as well as the proximal and mid-descending colon, has also been shown to contain more widely spaced and infrequent vasa recta compared to more frequent and one centimeter apart spacing seen in other areas of the colon [15] (Fig. 18.2). Griffiths, along with other surgeons, has recommended that the branches of the left colic artery be preserved when ligating the inferior mesenteric artery (IMA) during a sigmoid or rectal resection [16]. The actual significance of left colic preservation remains to be proven at this time. It is clear that a decrease in flow of up to 50 % can be seen in the marginal artery after IMA ligation. It should be noted that the area with the poorest perfusion following IMA ligation will not be the splenic flexure but the area involving the sigmoid colon. The sigmoid colon has a relative deficiency of the marginal artery and is the least perfused segment when the IMA is proximally ligated. Therefore, as long as the sigmoid is resected, ligation of the IMA proximal to the left colonic branch (high ligation) should not result in colonic ischemia [15, 17].
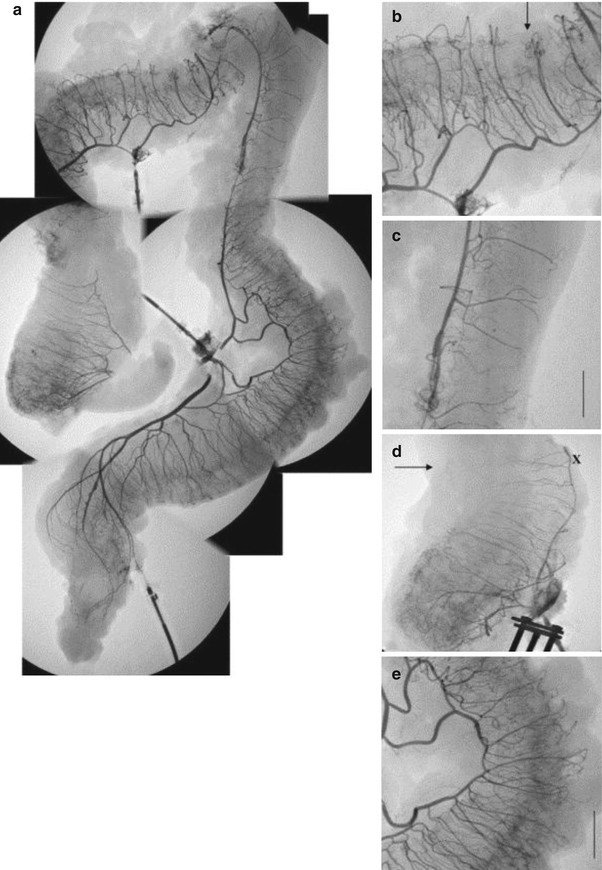

Fig. 18.2
Images (a–e) are angiographs taken of the entire colon and rectum. (a) Combined angiographic images of the colon and rectum. (b) Transverse colon. (c) Descending colon. In this portion of the descending colon, there is wide interspacing between vasa recta with relative absence of collaterals at the antimesenteric border. This is in comparison to the transverse and right colon (With permission from Allison et al. [15]). Arrow indicates point of ischemia in (b, d)
Additionally, the marginal artery of Drummond may not exist or be patent in a significant number of patients. The lack of blood flow to the left colon, via the marginal artery from the left middle colic artery, results in ischemia of the entire left colon after high ligation of the IMA at the aorta. This will be immediately apparent and should result in a change in plan to use more proximal colon for a colorectal anastomosis. It is the author’s opinion that during a low anterior resection, the descending colon should be used as the proximal end of the anastomosis to the rectum and the splenic flexure should be routinely mobilized. In general, high ligation seems safe and potentially provides an oncological benefit, though one exception should be noted. Elderly males were shown in one study to have a much more reduced blood flow within the descending colon following high ligation than females. This is thought to be due to atherosclerotic changes. Men are known to have earlier development and more severe atherosclerotic lesions than do women [17]. The average age of a man with newly diagnosed colon and rectal cancer is 69, and therefore, most men with colon and rectal cancer are at risk of atherosclerotic lesions. This could explain why the male gender has been previously shown to be a risk factor for anastomotic leaks in low colorectal anastomosis. Elderly males undergoing a low anterior resection who have evidence of significant atherosclerosis could potentially benefit from a more distal ligation of the IMA in order to preserve the LCA and adequate distal perfusion. Intraoperative evaluation of perfusion could be of use in this subset of patients.
Sudeck’s Point
This area is described as the point between the last sigmoidal branch and the left branch of the superior rectal artery [17]. Its main relevance has been seen in episodes of intestinal ischemia, commonly after abdominal aortic aneurysm repair and IMA ligation. However, this area may also be of significance if the majority of the sigmoid remains and is used in the anastomosis following rectal resections. It is therefore important to avoid using the sigmoid for the anastomosis for multiple reasons.
Rectal Stump
The rectum has been traditionally viewed as having a robust blood supply with a rich network of collaterals. This is based on the clinical finding that the rectum, as opposed to the colon, is very rarely involved in clinical episodes of intestinal ischemia. In reality, the distal rectum does not seem to have this robust blood supply nor the same degree of resistance to ischemia, following a low anterior resection (LAR). This observation was first described by Goligher in 1949. More recently Allison et al. [15] performed angiography of resected specimens’ specific reasons for this phenomenon (Fig. 18.3). They observed that the upper rectum had an adequate network of collateral vessels based upon the superior rectal artery. In contrast, the lower rectum had a much poorer collateral network that mainly consisted of intramural vessels. Prior to the LAR, the blood flow from the rectum would preferentially travel down the posterior left and right branches of the superior rectal artery to end in the mesentery or rectal wall. The anterior left and right branches were the only vessels seen to give direct collaterals to the middle and inferior rectal arteries. Following LAR, the rectal stump is dependent upon flow from the middle and inferior rectal arteries. Angiography performed on the rectal stump using the middle rectal artery (Fig. 18.3) retrograde showed blood flow only through the anterior branch of the superior rectal artery. The posterior rectum was shown to be dependent upon a variable amount of intramural collaterals between the anterior and posterior branches. This results in a poorly perfused posterior-inferior rectal stump, and is likely why it is not too uncommon for leaks to occur at the posterior aspect of the anastomosis [15]. Another report did not show decreased perfusion specifically in the posterior-inferior rectum, but the rectal stump had a greater reduction in blood flow as compared to the proximal end [18]. In addition they found significantly more leaks in those patients where there was a blood flow reduction of 16 % or greater [18].
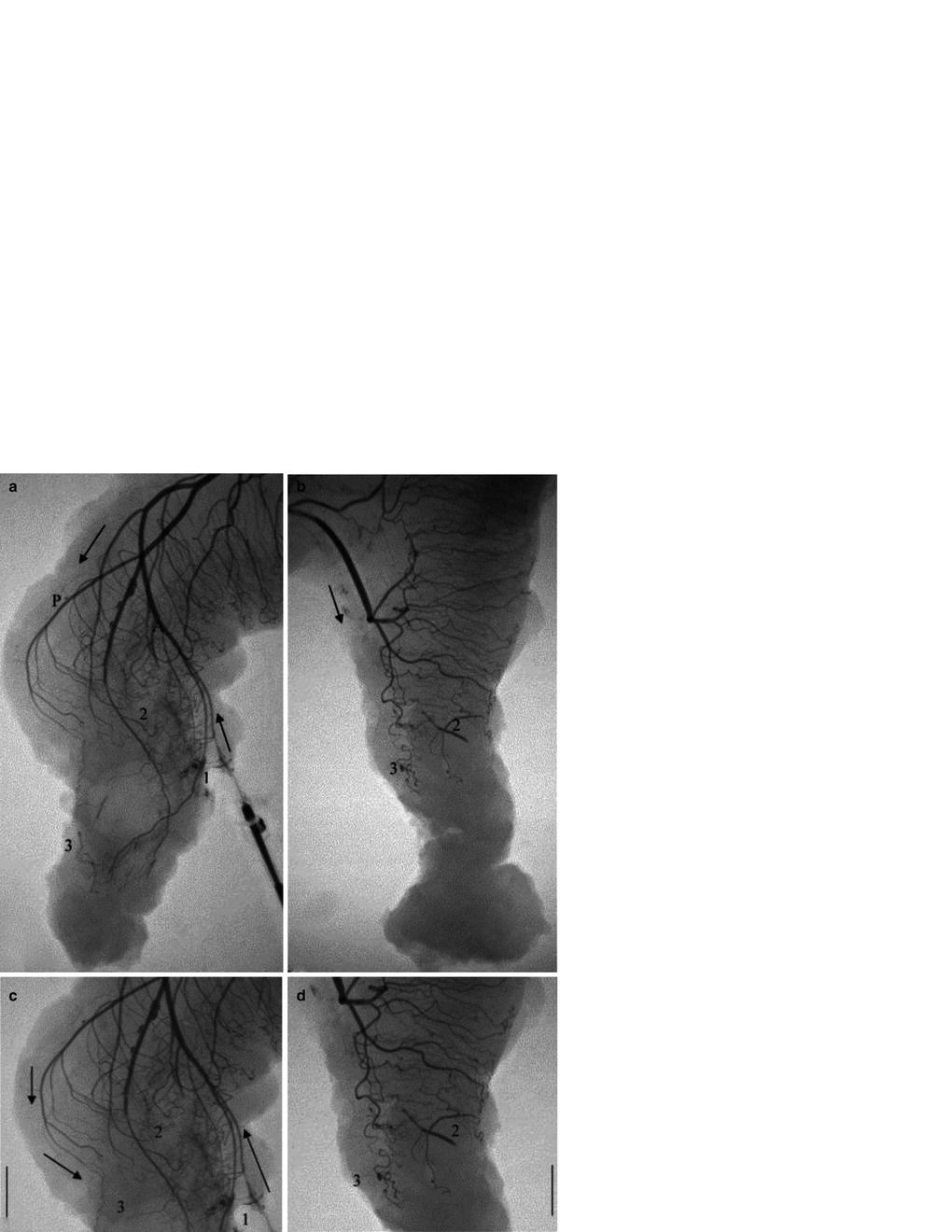

Fig. 18.3
The superior rectal artery (SRA) divides into a right and left branch in the upper mesorectum. Both the right and left branch give off smaller anterior and posterior arteries that supply the rectum. Only the anterior branches communicate directly with the middle rectal artery (With permission from Allison et al. [15]). (a) Injection through middle rectal artery. After rectal resection and sacrifice of the SRA, blood preferentially flows down the anterior branches (a) to the bifurcation point in the upper mesorectum. Blood then travels antegrade down the posterior branches (P) to the posterior portion of the rectum. (b–d) Rectal resections distal to the SRA bifurcation prevent direct flow from the anterior branches (1) to the posterior branches. Instead, the posterior rectum must rely on small vessel intramural collaterals (2) and inferior collaterals of the posterior branches (3) Arrows indicates blood flow
Microvascular Anatomy
Small vessel collaterals can be of significance at specific locations of the colon and rectum. Just as a decrease in the number of these collaterals can affect local tissue perfusion, local vasomotor control over these collaterals can also have a profound effect. This is most profound when splanchnic vasoconstriction occurs in the setting of blood loss and hypotension, as well as increased sympathetic activity, and can dramatically impact the healing anastomosis.
Arterial Oxygen Tension
As humans we are obligate aerobes. In addition to aerobic metabolism, oxygen is needed in collagen synthesis; however, when the oxygen tension drops below 40 mmHg, collagen synthesis ceases [5]. The amount of oxygen that is delivered to the tissues is dependent upon a multitude of factors that includes cardiac output, local vascular resistance, and hemoglobin content. Both cardiac output and local vascular resistance have a more profound impact on tissue oxygenation than hemoglobin as only a mere half of all oxygen-carrying hemoglobin is needed at any point in time for aerobic metabolism [19]. As such, anemia in and of itself is less likely a major contributing risk factor of anastomotic leaks.
Summary Pearl
Currently the list of identified risk factors for anastomotic leaks is extensive, and their exact relationship to, and significance in, anastomotic leaks is hard to define. You are therefore faced with a complex array of risk factors, all with varying degrees of importance, affecting to some degree one or more components of anastomotic healing, and you must decide which one is at play in a particular patient.
Risk Factors
Key Concept: Risk factors for anastomotic leak fall into the three broad categories: patient–related, location–related, and intraoperative factors. Knowledge of such risk factors should ultimately provide the basis of future preventive techniques while currently highlighting those patients in whom proximal diversion may be warranted.
Patient-Related
Key Concept: Certain inherent risk factors are present with every operation, though identifying and targeting modifiable risk factors, when possible, may mitigate the development of leaks.
Weakly associated patient-related risk factors include age, tobacco and alcohol use, and obesity [20]. Obesity is not felt to be a risk factor at all in right-sided resections; however, there tends to be a more significant association with rectal resections [21]. A combination of risk factors may yield even more significance. This is likely why a higher American Society of Anesthesia (ASA) classification (usually scores of 3 or more) is one of the more consistently found risk factors for leak [20]. Likewise, patients with Charleston Comorbidity Index scores of 3 or more have also been found to have significantly higher anastomotic leaks [17]. These and other types of scoring systems might be more useful indicators of a patient’s risk of anastomotic leak.
Poor Nutritional Status
Key Concept: Nutrition is a potentially modifiable risk factor that can often be improved prior to surgery. Its impact on anastomotic leak is somewhat variable, but poor nutrition is commonly associated with higher rates of leak, specifically for right–sided resections.
Certain preventive measures can be taken even before the operation is begun to reduce the risk for leak, primarily in the elective setting. Wexner suggests that these preventive measures are more commonly encountered preoperatively in a right colectomy and intraoperatively in a left colectomy [22]. This statement has been further validated by other studies, including one multicenter prospective trial [23]. Identifying those patients who are of poor nutritional status and treating them preoperatively may reduce the risk for and the morbidity and mortality from anastomotic leaks [23]. Generally, poor nutritional status has been defined in the literature as weight loss ≥10 %, serum albumin <3.5, and serum proteins <5.5 g/dL [24–27]. It is important to define what level of severity of malnutrition requires preoperative nutritional support. Most studies have used a weight loss of ≥10 % to define malnutrition [28]. However, according to Jie et al., this only represents the cutoff value for which complications are seen to increase [28]. The nutritional risk score (NRS) may be a better indicator of severe malnutrition for which preoperative nutrition is indicated. Parameters which are used in the NRS include weight loss in the last 3 months, decrease in food intake, body mass index, severity of disease, and age. Thus, a 65-year-old patient with colon cancer who has greater than 15 % weight loss in the last 3 months and needs a right colectomy would have a score of 5. With this scoring system, Jie found that in those patients with a NRS ≥5 and a lower GI resection, preoperative nutrition decreased the complication rate from 45 % in the control group to 27 % in those patients with preoperative nutrition [28]. An in-depth discussion regarding the impact of nutritional support can be found in Chap. 3 by Dr. Maykel.
Immunosuppression
Key Concept: Patients with malignancy and inflammatory bowel disease often require surgical resection and are commonly immunosuppressed or taking immunosuppression agents preoperatively. You need to have a thorough understanding of how this can impact postoperative morbidity including anastomotic leaks.
Steroids
Corticosteroids decrease the activation and infiltration of inflammatory cells into wounds along with inhibiting certain growth factors that are necessary for collagen synthesis [29]. Intuitively it would seem that corticosteroids impair anastomotic healing; however, their exact effect on anastomotic leaks is less clear. Multiple studies have shown no increased risk with preoperative steroid use [30–34]. These studies are mainly retrospective and include patients with variable dose and duration of steroid usage and type of operation performed. A more recent prospective study found that both long-term and perioperative usage are associated with a higher risk of anastomotic leakage [35]. Steroids have also been shown to increase the rates of wound infections and septic complications [36, 37]. If possible steroids should be weaned preoperatively, and you should more readily consider proximal diversion (especially in the case of a rectal anastomosis).
Infliximab, AZA, 6-MP
There does not seem to be an association with anastomotic leaks or other postoperative complications with the use of infliximab, though this is controversial. This holds even when infliximab is used in combination with azathioprine or 6-mercaptopurine [32, 36]. There does not seem to be any benefit in stopping these medications preoperatively or delaying surgery in Crohn’s patients. However, infliximab has been shown to negatively impact outcomes after operations for ulcerative colitis [38].
Crohn’s Disease
Key Concept: In addition to the pathophysiology of the disease itself, the Crohn’s patient is likely to present with other risk factors for anastomotic leaks including preoperative malnutrition and immunosuppressive medication use.
Multiple studies have shown a high risk of intra-abdominal septic complications in patients with Crohn’s disease [39, 40]. Other risk factors which are much more specific to Crohn’s disease include a hand-sewn anastomosis, end-to-end anastomoses, histologic positive margins, penetrating-type disease, and the need for sigmoid resection [20, 30, 34, 41]. We recommend stapled side-to-side anastomoses when performing an ileocolostomy and to highly consider performing proximal diversion in cases where both small bowel and sigmoid resections are needed. Grossly histologic negative margins are almost impossible to achieve in a patient with Crohn’s disease without causing unnecessary bowel resection. A grossly negative margin, as indicated by the soft, thin mesentery at the point of resection, is adequate.
Radiation
Key Concept: Radiation alone may not increase the risk of leak, and does not mandate the need for diversion, typically, unless when combined with chemotherapy.
Neoadjuvant radiotherapy in the pelvis may result in increased risk of anastomotic leak; however, this belief has not been definitively established in the literature [42]. A study that looked at 1,338 patients with rectal cancer over a 30-year period was unable to find any significant association between neoadjuvant radiotherapy and anastomotic leaks. Other factors such as location and size of tumor were found to be of more significance. This has been confirmed in other reports, including a fairly large randomized trial [43]. There is no need for routine diversion in patients who have received neoadjuvant radiotherapy alone. If chemotherapy is added to the radiation, a higher risk is likely, and a diverting stoma is recommended. As will be further discussed later, proximal diversion does not prevent a leak but reduces its impact.
Diverticulitis and Emergency Surgery
Key Concept: Although emergent surgery is a well–known risk factor for complications, diverticulitis may be an independent risk factor for anastomotic leak.
Diverticulitis and emergency operations have previously been identified as risk factors for anastomotic leaks [44–47]. Emergency operations are at an increased risk of postoperative complications in general, which include wound infections, intra-abdominal abscesses, anastomotic leak, wound dehiscence, and mortality [44]. In addition, other risk factors such as the disease process itself, location of the anastomosis to be performed, and condition of the patient play a role in the development of a leak. Diverticulitis commonly involves some of these same factors but may be an independent risk factor for leak, even in nonemergent conditions. This increased risk may be due to persistent inflammation or unresolved abscesses at the time of the operation and decreased anastomotic strength due to an increase in wall thickness secondary to muscular hypertrophy and an inappropriate selection of staple height [23]. One preventable risk factor for leak is avoiding any retained sigmoid on the rectal stump. An anastomosis between the left colon and soft rectum is essential to cure diverticulitis and prevent an anastomotic leak.
Peritonitis
It is a commonly held belief that an anastomosis created in the setting of peritonitis will be at increased risk of anastomotic dehiscence [48, 49]. However, previous animal studies and other clinical studies have failed to show any increase in risk of leaks in the setting of peritonitis [49, 50]. There may be a difference between purulent versus fecal peritonitis (e.g., Hinchey III vs. IV) and the risk of leak, as previously reported by Biondo and colleagues [50]. In a follow-up study, they were able to perform a primary resection and anastomosis with a respectable 5.7 % leak rate without the use of proximal diversion in patients with purulent peritonitis. They excluded those with fecal peritonitis, as well as ASA IV, and unstable or immunocompromised patients. Of the remaining 208 patients, 50 % of the patients had peritonitis, of which half of these had diffuse peritonitis. Peritonitis was not found to be an independently associated risk factor for anastomotic leaks [47]. At present it does not appear that purulent peritonitis alone is a risk factor for anastomotic leak [47, 50–52].
“Loaded Colon”
One of the reasons why emergency surgery is felt to be associated with higher leaks is that these operations are performed on the unprepped colon. The “loaded colon” has been reported to have up to a threefold increase in anastomotic leaks [53]. This is contradictory to the most recent studies on mechanical bowel preparation that have concluded that it can be safely omitted. Methods such as intraoperative colonic lavage have been shown to have a positive effect on anastomotic integrity and collagen metabolism and can allow for a primary anastomosis to be performed without diversion in emergency operations for colonic obstruction [49, 54]. Until additional evidence to the contrary emerges, it is recommended that colonic lavage be performed when distal colon and rectal anastomoses are created in the “loaded colon.” In contrast, elective operations to remove the left colon or rectum can be safely performed with only enemas (without a complete bowel preparation) to empty the stool.
Hemodynamic Instability
Since the healing anastomosis is extremely dependent upon adequate perfusion, episodes of hypotension, and especially those requiring vasopressors, should be an absolute indication for preventive measures. In fact, shock was one of the only two risk factors for which proximal diversion would be needed, according to an AAST multicenter trial, in the cases of traumatic injuries to the colon [55].
In the majority of emergency or diverticular operations—in the absence of either fecal peritonitis or shock—a primary resection and anastomosis can be performed safely. Lower rates of anastomotic leak, re-interventions, and other wound infections are seen with proximal diversion when other risk factors are present. As previously stated, intraoperative lavage is recommended for emergency resections involving the impacted left colon to allow primary anastomosis [20].
Location
The site of the anastomosis has been the most consistent and significant risk factor for anastomotic leak [56]. The further distal an anastomosis is created, the higher the risk of leak. An ileocolic anastomosis has a leak rate of 2–3 % compared to a 10–17 % leak rate in coloanal anastomosis. Even in rectal anastomoses, a significant difference can be seen the closer the staple line gets to the anal verge [56]. The highest risk of anastomotic leak can be seen for anastomoses at and below 5–8 cm from the anal verge [23].
There are several proposed reasons for the difference in leak rates between proximal and distal locations including:
4.
Increased intraluminal pressure of rectum during a closed anal sphincter and defecation
Obesity and Male Gender
Key Concept: These two risk factors for leak are primarily seen in association with a low rectal anastomosis.
Obesity is known to be a risk factor for postoperative wound infections, prolonged open operations for rectal resection, and conversion from laparoscopic to open [57]. While obesity is variably associated with anastomotic leakage, there is considerable evidence that obesity affects the leak rate for low rectal anastomoses. In several studies, obesity has been shown to be the strongest risk factor for the development of a leak [21, 58, 59]. Additionally, the male gender is mainly found as a risk factor for problems with low rectal anastomosis and usually is not found to be significant for more proximal anastomoses [23, 60–62]. Both obesity and male gender, and more specifically the deep narrow male pelvis, can make low rectal operations significantly more difficult. Men also may be at increased risk of poor anastomotic perfusion. It is known that men can have altered intestinal microcirculation in response to hormones and are at an increased risk of advanced atherosclerosis compared to women [63].
Operative Risk Factors
Key Concept: There is no method of intestinal anastomosis that is leak free. Although you have a somewhat limited ability to prevent anastomotic leaks, your performance at the time of the operation can have a major influence.
Blood Loss, Transfusions, and Operative Time
Key Concept: One way you can reduce the risk of anastomotic failure during the operation is by limiting intraoperative blood loss and the time it takes to perform the operation.
While both of these parameters have been confirmed to decrease leak rate on multivariate analysis [64, 65], the actual significance is not completely clear, as this has not been uniform across the literature [66, 67]. Furthermore, the actual amount of blood loss or duration of operation that matters is much less clear. Operative times found to be of significance ranged from 120 to 270 min [17, 24, 66, 67], while meaningful operative blood loss has been defined as that which requires blood transfusion—a highly variable definition [17]. What seems clearer is that increased operative time leads to more exposure of the patient to tissue trauma and bacteria [25] and correlates with hypothermia in most patients. Primary hypothermia correlates with increased infectious complications and hospital length of stay [68]. Even more, increased operative times and greater amounts of blood loss are surrogate markers for the degree of difficulty of the surgery. You must be mindful of these objective indicators of a more difficult operation in order to accurately decide whether or not to perform proximal diversion. While occasionally viewed as a “failure,” it is never wrong to err on the side of diversion.
Intraoperative Complications
Key Concept: Adverse events during the operation, even if not directly involving the anastomosis, can increase the risk of anastomotic leak.
Trencheva et al. defined an intraoperative complication as injury to the bowel, other organs, or blood vessels. In addition, stapling device malfunction, hypotension, oxygen saturation less than 90 % for more than 5 min, pH less than 7.3, and even blood loss requiring intraoperative blood transfusion were also classified as an intraoperative complication. In their series, any patient with an intraoperative complication was four times as likely to have an anastomotic leak [17]. On one hand, this may again be a surrogate for a more difficult operation. More appropriately, this highlights the degree of interconnectivity among all aspects of an operation and the impact one problem can have on another.
Total Mesorectal Excision (TME)
Key Concept: Although oncologically sound, TME results in a lower anastomosis and the potential for loss of blood supply that may increase the leak rate.
During the widespread adoption of total mesorectal excision, there was a substantial rise in leak rates from the previously reported 9 to ~23 % [69]. Over the next 4 years following this initial study, the leak rate eventually did return to the level seen before TME [69]. A study looking at laparoscopic resections showed that the addition of TME more than doubled the leak rate for upper rectal cancer [67]. TME for high rectal cancer may result in insufficient blood supply to the posterior portion of the proximal rectum, which is further evidenced by the fact that tumor-specific mesorectal excisions have lower rates of anastomotic leaks [15, 70].
Tension and Splenic Flexure Mobilization
Key Concept: Avoiding tension has been classically viewed as one of the fundamental principles of a healing anastomosis and remains a significant preventative measure in reducing anastomotic leak.
The colon seems to be especially effected by applied tension, even more so than the small intestine. Blood flow did not return to preoperative levels until the seventh postoperative day following experimentally applied tension in animal studies [71]. Mobilization of the splenic flexure has commonly been used to decrease tension in the rectal anastomosis. Karanja et al. found a 9 % leak rate with splenic flexure mobilization compared to 22 % without mobilization [72]. Additional evidence supporting its actual significance is limited. The anastomosis is subjected to other sources of tension during peristalsis and defecation that result in radial tension for which splenic flexure mobilization would seem to provide less benefit. The major importance in splenic flexure mobilization may ultimately be improved blood supply of the descending colon (rather than the sigmoid) when used for the colorectal anastomosis.
Drains
Key Concept: Drains may be useful in low extraperitoneal anastomosis, but are not typically indicated when the anastomosis is intraperitoneal.
The use of drains has been extensively debated over the last decade, largely because they offer both real and theoretical benefits and risks, yet are only one of many factors that play a role in a proper anastomosis. Furthermore, they are widely variable in their use, type, and rationale for placement. There is extensive evidence that draining an intraperitoneal anastomosis is of no benefit [69]. In the case of gross contamination or abscess at the time of resection, the intraperitoneal anastomosis should be created in a less hostile location and after extensive contamination control. Draining the pelvic anastomosis may be of some benefit. The pelvis does seem to be unique as compared to the abdominal cavity in that fluid is much more likely to accumulate in the most dependent area of the pelvis around the anastomosis and the non-peritonealized pelvic floor fails to absorb fluid efficiently [47, 73]. However, it is unclear how effectively our drains remove this fluid or what impact this has on the healing anastomosis [17, 47, 74]. The other proposed benefit of draining the pelvic anastomosis is detection of an anastomotic leak. On one hand, studies have shown that drains have very poor detection rates and that other clinical signs are more likely to appear before any change in drain effluent [61]. Others have shown some benefit in leak detection where drains detected a leak in 80 % of patients and in 40 % of cases this preceded any other clinical signs [75]. In the most recent meta-analysis, there was not enough sufficient evidence to indicate that drains are able to prevent anastomotic leaks or other anastomotic complications; however, the authors acknowledged the need for more randomized control trials specifically looking at lower rectal anastomosis [66]. It is our practice to use drains selectively in cases where build up of fluid in the pelvis begins during the case despite good hemostasis or after a very bloody operation where every vessel could not have been controlled. In general, we remove the drains once the effluent is less than 15 cc/day or if clear.
Laparoscopy
Key Concept: Laparoscopic approaches with experienced surgeons may result in a decrease in leak rates.
A study using the Nationwide Inpatient Sample population database found a decrease in the rate of anastomotic leak along with a corresponding decrease in wound infection following laparoscopic surgery, despite an increased leak rate with conversion to open surgery [76]. The recently published Danish nationwide cohort study demonstrated an increase risk of leak with minimally invasive approaches; however, this study was performed during the period when the laparoscopic method was first being used [77]. While no definite conclusion can be drawn, this latter study does highlight the potential impact that inexperience can play in leak rates. Interestingly anastomotic leaks have been diagnosed earlier following laparoscopic surgery, and as a result of the primary operation being laparoscopic, laparoscopic management of the leak was possible [67]. A possible cause of low rectal anastomotic leaks in totally laparoscopic cases may be the use of multiple firings of the endoscopic stapler to transect the rectum. Crossing staple lines and poor perfusion always put an anastomosis at risk, regardless of the surgical approach.
Omental Wrapping
Key Concept: Wrapping the anastomosis with a well-vascularized pedicle of omentum has been associated with decreased leaks in small studies.
Animal studies have proven the unique ability of the omentum to adhere to and effectively bridge the anastomosis [78, 79] and allow for absorption of fluid [80]. Some have cautioned its use secondary to the likely negative impact in cases where the omental pedicle is devascularized [80]. The most recent meta-analysis found a significant reduction in clinical anastomotic leak only. Issues involving blinding and a small number of patients within each study limited the strength of the conclusion. Omentoplasty should be left up to the surgeon’s personal experience.
Simultaneous Liver Resection
Key Concept: Staged resections may decrease the overall morbidity and leak rate for extensive disease, while simultaneous resection is generally safe in carefully select patients.
Synchronous liver metastases are present in 23–51 % of newly diagnosed patients [81], and liver resection remains the best option for those patients with resectable disease [82–84]. Staged procedures have been the traditional approach [83]. Most studies evaluating safety and efficacy are retrospective and therefore suffer from selection bias resulting in more extensive liver resections in the group of patients with a staged approach to extensive liver resections [83]. These studies do show that limited liver resections can be performed at the same time as the colon resections with equivalent morbidity and mortality [82–84]. With more extensive liver resections and in those 70 years or older, the morbidity and mortality significantly increase, favoring a staged approach [82]. Age and extensive liver resections seem to be the main factors to consider when deciding upon a staged versus simultaneous resection. Leak is just one of the many causes of postoperative morbidity and mortality. The only study focused on anastomotic leak after simultaneous liver resection showed an operative time greater than 8 h was the most significant risk factor for anastomotic leak regardless of the extent of the liver resection. The majority of leaks occurred with rectal resections (36 % leak rate). Patients with colonic resections were observed to have a 13 % leak rate, but this was still higher than those patients who only underwent a colonic resection [82].
Proximal Diversion
Key Concept: Understand your goal with diversion, where it is a crucial part to minimizing morbidity and where it can be safely avoided. When you feel you need to proximally divert a patient, it is generally a good idea.
The effectiveness of proximal diversion, whether a loop colostomy or loop ileostomy, is highly debated. Most studies have focused on whether proximal diversion can prevent anastomotic leak. Some have suggested that proximal diversion does not prevent, but only minimizes the clinical impact of leaks [85]. In a systematic review by Montedori, proximal diversion was found to be useful in preventing both anastomotic leak and the need for urgent reoperations [86]. Proximal diversion also minimizes the clinical impact of leaks by decreasing the leak rate and the need for laparotomy. Unfortunately, there is also added morbidity with proximal diversion. Problems ranging from dehydration and electrolyte abnormalities to mechanical problems can be as high as 30 % [86], resulting in an 18 % readmission rate [87]. In addition, there is a 15–20 % complication rate with ostomy closure [88, 89]. Because of its associated morbidity, proximal diversion should not be routinely performed. The decision for proximal diversion must be carefully weighed against the negative impact of leak and the morbidity of an ostomy. This decision-making process can be simplified by focusing on three key questions.
1.
What is the risk of leak based upon the location of the anastomosis?
Extraperitoneal anastomoses and those within 5–8 cm from the anal verge are at the highest risk of a leak and should generally be diverted [23]. Leaks at this level can negatively impact future bowel function and increase the risk of a permanent stoma [90]. The decision to divert more proximal anastomoses should be based upon the presence of other additional risk factors.
2.
Can the patient tolerate a leak?
Older patients and those with multiple medical comorbidities should be considered for proximal diversion. These patients typically have very little physiologic reserve to tolerate a leak.
3.
What are the patient wishes?
It is important to include the patient in your decision-making. Some patients are adverse to any stoma, temporary or not. Others may be more concerned with the complications from a leak than with having an ostomy. A fully informed patient will be able to better voice their own concerns and be much more satisfied with the eventual outcome. Knowing what the patient wants can simplify intraoperative decision-making.
Mechanical Bowel Preparation (MBP)
Key Concept: MBP does not significantly impact anastomotic leak rates in colon resection, but may decrease complications with rectal resections, and likely should be continued when possible for elective rectal cases.
Multiple studies have concluded that mechanical bowel preparation (MBP) in elective colon resections does not significantly impact anastomotic leaks [91]. The evidence is overwhelmingly in favor of abandoning the use of MBP in colon resections. There is less evidence for the effectiveness of MBP in rectal resections. Some studies have shown that MBP can be safely omitted in rectal resections [92, 93], but most studies have excluded patients with a rectal anastomosis [91]. A multicenter randomized controlled trial showed a higher risk of infectious complications without MBP [94]. They also showed a nonsignificant increase in clinical anastomotic leaks and pelvic sepsis [94]. For now, it would be wise to continue mechanical bowel preparation, when possible, for planned rectal resections.
The results of these studies also do not address some of the other potential benefits of mechanical bowel preparation. A well-prepped bowel allows for:
Better visualization when intraoperative colonoscopy is needed
Easier creation, visualization, and leak testing of an anastomosis when using an EEA stapler
Easier bowel manipulation during laparoscopic surgery
Prevention
Dr. Abbas has questioned whether surgeons should continue to accept the risk of anastomotic leaks [95]. Surgeons have been faced with similar questions in the past. Early surgery for appendicitis was fraught with major difficulties due to poor diagnostic methods and no available methods of antisepsis. This was a time period when abdominal surgery was performed only as a last resort. In 1881, W.A. Byrd stated “I fail to find any recorded cases in which this procedure (laparotomy) has been attempted with success… medicine is useless in these cases except for the production of euthanasia, and surgery cannot even accomplish this.” Six years after this statement, a successful appendectomy was performed by Thomas Morton [96]. It is important that we not become complacent but continually strive to break new barriers.
Intraoperative Anastomotic Assessment
Key Concept: Several methods are available for investigating the integrity of the anastomosis. Whatever method you choose, it should be, in general, a routine part of your practice for all left–sided anastomosis.
Laser Fluorescence Angiography
Following injection of fluorescent dye, a mounted camera with an infrared filter is used to view the resected ends or newly created anastomosis. Special software can be used to compare two different areas of perfusion. A study showed that using the IC-View system® (Pulsion Medical System, Munich, Germany) changed the site of resection in 16 % of patients [97]. Other available forms of fluorescence angiography include the Spy Elite® (Lifecell, Bridgewater, NJ) for open surgeries and the FireflyTM used in the DaVinci® SiTM Surgical System Robot (Intuitive Surgical, Sunnyvale, VA).
Intraoperative Air Leak Test
Studies assessing the effectiveness of air leak testing have shown mixed results [98–100], though typically demonstrate usefulness and no downside. In a large retrospective cohort [101], untested anastomoses had twice the rate of clinical leak than those that were tested. Patients who underwent suture repair after a positive air leak test had a clinical leak rate of 12.2 % compared to 3.8 % for patients with a negative air leak test. Patients who underwent anastomotic revision or proximal diversions after a positive air leak test had a 0 % clinical leak rate [101]. This study provides significant evidence for the use of intraoperative leak testing. A diverting ostomy should always be a consideration with positive air leaks.
Intraoperative Endoscopic Assessment
Li et al. [102] looked at the selective versus routine use of endoscopic examination in bowel resections. Endoscopic examination with air leak testing was performed on the pre-resected bowel, the rectal stump, and the post-anastomotic bowel. This study showed a nonsignificant increase in leaks (5.1 % vs. 0.9 %) with selective versus routine endoscopic examination. The endoscope compared to the proctoscope provides better visualization of the anastomosis and likely a better assessment of its integrity.
Intraoperative Dye Test
Using a 22 French Foley, a mixture of sterile water and blue dye is injected intraluminally, while the bowel proximal to the anastomosis is clamped. It takes a volume of 180–240 mL to adequately distend the anastomosis. A study using this method found that the dye test allowed for the easier detection and localization of leaks compared to air leak testing [103].
Intraluminal Devices
Key Concept: These devices are either early in their experience or have not demonstrated a marked benefit to reducing leaks.
Transanal Decompression Devices
These devices are believed to decrease intraluminal pressure by keeping the anal sphincter open. Rectal tubes, usually a Foley catheter, are placed 15 cm above the anastomosis. They provide for both decompression and antibiotic irrigation [104]. There are no comparative studies evaluating the use of rectal tubes. Transanal stents are 4 cm in length and left in place for 5–7 days following insertion [105]. A prospective randomized study in 2006 was prematurely stopped due to an increase in leaks in the stent group [106].
Intraluminal Barriers
Intraluminal barriers prevent the fecal stream from contacting the healing anastomosis. Animal studies have shown that fecal contact negatively impacts the healing [52]. In animal studies, the Coloshield and the Valtrec-Secured Intracolonic Bypass (VIB) have both been very effective in preventing leaks. Leaks were prevented even when an incomplete anastomosis was intentionally created [105]. Both devices are secured proximal to the anastomosis and are spontaneously expelled. Multiple small studies have shown a 0–8.7 % anastomotic leak rate with the use of the Coloshield. These authors claim the Coloshield is a viable alternative to fecal diversion [105, 107]. The VIB device was shown to have an equal rate of leaks in a head to head comparison with a loop ileostomy [108]. The C-seal® (Polyganics Groningen, the Netherlands) is the newest device and can be attached to the bowel proximal to the anastomosis with an EEA stapler. Clinical trials evaluating the C-seal are currently underway.
Compression Anastomosis
A sutureless anastomosis without the associated foreign body has its theorized advantage. It is not a new concept, as the idea dates as far back as 1826—long before Murphy’s button [109]. In the largest study to date, there was a 3.2 % anastomotic leak rate among 1,180 elective open and laparoscopic colorectal anastomoses [109]. The authors concluded that the ColonRing device (novoGI Inc, Netanya, Israel) is feasible and safe and could be considered as an alternative to stapled end-end colorectal anastomosis. Further prospective studies directly comparing the two techniques are needed.
Extraluminal Devices
Methods used to bolster the staple line with bioabsorbable Seamguard® (W.L. Gore & Associates, Flagstaff, AZ) or meshed AlloDerm® (Lifecell, Bridgewater, NJ) have not improved the anastomotic strength [110, 111]. Clinical data evaluating the use of such tissue-bolstering devices for the colorectal anastomosis is limited. Staple line reinforcement has not had the same success in the colorectal anastomosis as is seen in the gastric bypass or sleeve, where there primary purpose is reduction in bleeding [112].
Managing the Failed Anastomosis
Key Concept: Any successful management strategy for anastomotic leak that results in reduced morbidity and mortality and improves the quality of life emphasizes early diagnosis and infectious source control through the use of methods that do not increase the risk of permanent stoma or negatively impact future bowel function.
Anastomotic Leaks
Anastomotic leaks account for a quarter of all deaths following colorectal surgery [45]. These mortality rates have changed very little over the last three decades despite the continuing improvements in critical care management. The mortality and morbidity from anastomotic leaks are greatly influenced by the duration of time before a diagnosis is made and the source of infection controlled [45]. Unfortunately many patients will be discharged home before a diagnosis is made, and others will be treated with more conservative therapies, both of which delay definitive therapy and extend the duration of infection and sepsis. Aside from morbidity and mortality, patients with anastomotic leaks can have a significant decrease in their quality of life, which is mainly due to the high rates of a permanent stoma (up to 72 % in some studies), especially when end ostomies are performed instead of proximal diversion [113].
Clinical Manifestations
Key Concept: Symptoms range widely from nonspecific cardiopulmonary and GI complaints to fever and septic shock. Watching for patients who begin to deviate from the standard postoperative course will aid in early diagnosis.
The timeframe in which patients present with anastomotic leaks follows a bimodal distribution, with symptomatic leaks occurring between 7 and 12 days and asymptomatic leaks diagnosed months later, usually during the evaluation for ostomy closure [45, 70]. The typical symptoms of an anastomotic leak include pulmonary, cardiac, and gastrointestinal symptoms that unfortunately are not too different from postoperative symptoms in patients without leaks. Indeed since these symptoms are not specific for an anastomotic leak, patients sometimes are treated by the surgeon for days to weeks before the anastomotic leak is finally diagnosed [114]. While cardiac, pulmonary, or gastrointestinal symptoms (individually) may be fairly nonspecific, there is evidence that the likelihood of an anastomotic leak significantly increases as a patient develops additional symptoms [115]. The return of bowel function has always been an important component in the postoperative management of the colorectal patient. Lack of bowel function beyond 6th postoperative day is highly predictive of an anastomotic leak, but the presence of bowel function alone is a poor negative predictor [115]. Fever and leukocytosis are fairly insensitive during the initial postoperative stay and are unlikely to reach predictive values while the patient is hospitalized [115]. Operatively placed drains can provide clues to the occurrence of a leak, but surgeons must not be completely dependent on them, as even patients with benign appearing drainage can have anastomotic leaks. Peritonitis is an obvious clinical sign but may not be present in diverted patients or those with an extraperitoneal anastomosis. Purulent anal discharge is fairly specific, but can easily go unnoticed by the surgeon or patient. Sometimes patients may not display any one sign or symptom, but simply fail to follow the standard postoperative course or meet discharge requirements. These patients which are “failing to progress” need to be promptly evaluated for an anastomotic leak.
Making a Timely Diagnosis
Key Concept: While several different tests and scoring systems are available to aid in the early diagnosis of leak, the most important factor is the surgeon’s clinical awareness and acumen.
The importance of a timely diagnosis was shown in the study by Alves and colleagues [45] where the mortality rate increased from 0 to 18 % if the diagnosis was made after the fifth postoperative day. Leaks can be difficult to diagnose in the early postoperative period because signs and symptoms take time to progress. The use of water-soluble contrast enema or computed tomography is not sensitive enough to be used to screen for leaks. At the present time, there is ongoing research into other methods to accurately predict which patients have an anastomotic leak with the hope that this will prompt an earlier diagnosis. C-reactive protein (CRP) appears to be a very promising marker for anastomotic leaks. Almeida et al. [116] showed that serum CRP levels were elevated in all patients immediately postoperatively on and after the third day in all patients who had leaks. A total of four studies have all shown persistently elevated CRP levels after postoperative days 2–4 in colorectal patients diagnosed with anastomotic leaks [116, 117]. A CRP level of 190 mg/L or more on postoperative day 3 that fails to decrease in the following days is a very accurate predictor of anastomotic leak in colorectal patients [118]. High levels of sensitivity (>95 %) and diagnostic accuracy (88.5 %) were seen in esophageal leaks when using the scoring system, as seen below, based on the postoperative levels of CRP, WBC, and albumin [119].
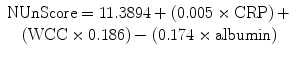
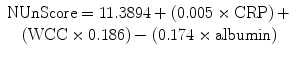
Another scoring system that used 15 different clinical and laboratory parameters decreased the delay in diagnosing anastomotic leaks among colorectal patients [120]. While these studies are promising, the clinical use of these markers and scoring systems has not been widely established. Currently, the surgeon must rely on a heightened sense of awareness to signs and symptoms that, when present, should prompt further workup. Computed tomography with rectal contrast is proven to be better in identifying anastomotic leaks than water-soluble contrast enema and also allows for accurate identification of any abscess that may be amenable to percutaneous drainage [74]. Some surgeons advocate that contrast should be injected down the distal limb of the ostomy as opposed to through the rectum to prevent further disunion of the anastomosis [121]. Some patients will present with peritonitis and/or and septic shock and require an urgent laparotomy before any diagnostic studies can be performed. All efforts should be made to try and evaluate the anastomosis preoperatively, since intraoperative evaluation can be difficult especially when the leak creates an inflammatory mass that surrounds the anastomosis. In cases with no preoperative evaluation, the anastomosis must be grossly inspected during laparotomy and with the endoscope. If there is no evidence of any dehiscence during this inspection, the anastomosis should then be tested for a leak by insufflating air through an endoscope within the anal canal or by injecting Betadine into the rectum via the endoscope. Not all cases of postoperative sepsis are due to a leaky anastomosis, and it is important to correctly identify and control the source of infection to prevent recurrent sepsis. While it is also helpful to identify these cases preoperatively to avoid negative exploratory laparotomy, when there is a strong suspicion, the operating room is almost always the right call (even if no leak is found).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








