The Contaminated Abdominal Wall
Robert Lim
Scott Rehrig
Introduction
A ventral hernia repair in a contaminated or clean-contaminated case should be considered as similar to a complex ventral hernia (CVH). When contemplating abdominal wall reconstruction for the repair of CVH, three basic questions must be considered: (1) What is the definition of a complex hernia, (2) how to best repair the abdominal wall to restore form and function, and (3) how to best handle the abdominal wall skin to prevent flap necrosis.
There are several clinical instances, which seem to identify certain hernia repairs as “complex.” They are those that result from damage control laparotomy and the serial closing of the abdominal fascia. Hernias that involve contaminated wounds such as ostomies, enterocutaneous fistulas, or prior superficial or deep space infections. Hernias are associated with morbidly obese and the sequelae of the metabolic syndrome (see Table 28.1). Hernias, in these patient populations, are considered complex because their infection rates, recurrence rates, and overall complication rates are higher when compared to hernias in patients without these risk factors (see Fig. 28.1).
Regardless of the source or timing of contamination, the most common organisms responsible for prosthetic mesh infection are gram-positive species specifically Staphylococcus aureus. Belansky et al. detailed the pathogenesis as follows: The mesh is contaminated by bacteria within the first 24 to 48 hours; as the unincorporated mesh has no surrounding blood supply the bacteria gain an irreversible foothold resulting in an “impenetrable” capsule known as a biofilm. Once a biofilm occurs on the surface of a prosthetic mesh, the ability to eradicate the infection is essentially zero necessitating the need for reoperation and mesh explanation.
The undamaged and properly functioning abdominal wall yields a platform that supports pulmonary, digestive, and urologic function as well the locomotion of the thoraco-abdominal musculature. The net effect of a damaged and weakened abdominal musculature is the imbalance of intraabdominal and abdominal wall pressures leading to a hernia defect. In the undamaged abdominal wall, muscles contract isometrically to counter intraabdominal forces causing an equilibration of pressures. In the damaged abdominal wall, the muscles contract isotonically leading to a non-uniform of forces that have the net effect of progressively expanding the abdominal wall defect. Prosthetic
mesh repairs serve as simple patches that function to hopefully redistribute abdominal wall forces evenly according to Pascal’s principle. In cases where hernia defects are small—less than 10 cm—patching a defect with mesh may be adequate; but in complex hernias that are large, have severely weakened tissues, and/or are prone to recurrence or infection, what is required is to restore the abdominal musculature to its proper position and function.
mesh repairs serve as simple patches that function to hopefully redistribute abdominal wall forces evenly according to Pascal’s principle. In cases where hernia defects are small—less than 10 cm—patching a defect with mesh may be adequate; but in complex hernias that are large, have severely weakened tissues, and/or are prone to recurrence or infection, what is required is to restore the abdominal musculature to its proper position and function.
Table 28.1 Factors that Make Ventral Hernias Complex | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||
Mesh Placement in Contaminated and Potentially Contaminated Environment
The major controversy regarding the use of mesh to repair abdominal wall defects are the risk of infection compared to the risk of recurrence. The literature suggests that recurrence rates are significantly lower with the routine use of mesh as compared to tissue only repairs; paradoxically, the risk of infection is significantly higher in those patients treated with mesh due to the presence of a foreign body. The incidence of infection in mesh hernia repair in clean-contaminated and contaminated cases can be as high as 40%. Other factors like steroid use, smoking, and prolonged operative times further increase the risk of infection.
Until recently, the true risk of morbidity associated with the contaminated abdominal wall was unclear. Choi et al. from Mount Sinai in New York reviewed nearly 34,000 patients who underwent ventral hernia repairs with mesh using National Surgical Quality Improvement Program (NSQIP) data. The authors compared clean-contaminated
and contaminated to clean cases using an odds ratio method. Not surprisingly, they found superficial site infections (SSI) occur 2.5 and 3.8 times more often in clean-contaminated and contaminated cases respectively compared to clean cases. Moreover, deep space surgical infection was over 6 times more likely to occur with the use of permanent mesh. The findings of this study are important as they clearly suggest that the use of prosthetic mesh for ventral hernia in the setting of any level of contamination is associated with prohibitive risk. With the increasing emphasis on patient safety and scrutiny of clinical outcomes, it behooves the surgeon to look to evidence-based guidelines when attempting to manage these complex patients. No level one data is currently available but guidelines are slowly being developed.
and contaminated to clean cases using an odds ratio method. Not surprisingly, they found superficial site infections (SSI) occur 2.5 and 3.8 times more often in clean-contaminated and contaminated cases respectively compared to clean cases. Moreover, deep space surgical infection was over 6 times more likely to occur with the use of permanent mesh. The findings of this study are important as they clearly suggest that the use of prosthetic mesh for ventral hernia in the setting of any level of contamination is associated with prohibitive risk. With the increasing emphasis on patient safety and scrutiny of clinical outcomes, it behooves the surgeon to look to evidence-based guidelines when attempting to manage these complex patients. No level one data is currently available but guidelines are slowly being developed.
With the application of laparoscopy to the repair ventral hernia, infection rates, hospital length of stay, and postoperative pain have all decreased compared to open repair. Surprisingly no difference in recurrence rates exists between laparoscopic and open techniques. Analogous to the increased incidence of bile duct injury in laparoscopic cholecystectomy, compared to open cholecystectomy, the bowel injury rate in laparoscopic ventral hernia repair is up to two times that of open repair techniques attesting to the technical challenges of laparoscopic lysis of adhesions. For patients with CVH not due to a contaminated environment, a laparoscopic repair with permanent mesh is acceptable. However, in planned ventral hernia repairs where concomitant gastrointestinal, biliary, or genitourinary procedures are performed, the complication rates are too high to safely use permanent mesh. Therefore, we recommend that a laparoscopic approach be avoided since it requires the implantation of prosthetic mesh. CVH repairs that occur concomitantly with fistula repair or ostomy takedown have the highest recurrence and infection rates, which holds true for both permanent and biologic mesh. In these patients, a conservative approach is to do a staged repair whereby the surgeon treats the fistula or performs an anastomosis in one setting accepting a ventral hernia and plans the definitive abdominal wall repair with mesh at a later time. This, of course, would expose the patient to another anesthetic event, and possibly a loss of abdominal domain making the subsequent surgery more challenging.
In laparoscopic hernia repairs with unplanned contamination due to gastrointestinal, genitourinary, or biliary sources, the safest option is to repair the visceral injury and not implant prosthetic mesh at the same time. Instead, one would plan to return to the operating room in a few weeks for the definitive hernia repair. Another option would be to convert to an open procedure performing a Rives–Stoppa (RS) or component separation (CS) technique with a biologic mesh.
Emergency surgery situations associated with hernias also confer increased risk for prosthetic mesh infection. CVH is best treated with a biologic mesh placed in a retro-rectus position if the patient can tolerate the longer operation. If the patient is too unstable, then placement of an absorbable mesh, like polyglactin (Vicryl), as an interposition and temporizing measure should be considered for its expedience. The use of absorbable mesh is associated with a very high incidence of infection; but this may be more due to the patient’s underlying condition that lead to the emergency, rather than the mesh itself.
The efficacy of biologic mesh is still being studied. No randomized controlled trials exist demonstrating its superiority compared to the use of permanent mesh for CVH repair. Furthermore, few prospective studies extend beyond 2 years to suggest that long-term recurrence is significantly less. Most believe that the infection rate is significantly less with the use of biologic mesh. Interestingly, however, the largest multi-center, albeit retrospective, study shows that the surgical site infection (SSI) rate is not lower than those hernias repaired with permanent mesh. The recurrence rate is still better than those hernias repaired with tissue only. Fistula development and mesh explantation requirement, though, are higher after permanent mesh use.
There are several types of mesh currently available. (See Table 28.2) The current data does not show that a certain type of biologic mesh is superior to another. Biologic meshes are significantly more expensive than permanent ones.
Table 28.2 Common Mesh Types | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Treatment of Infected Mesh
When a contaminated prosthesis is encountered the consensus is to remove the infected mesh completely. Options for managing the resultant hernia defect again include the RS and CS repairs. Again there is some data that suggests that augmenting that repair with mesh improves the outcomes but there are no long-term and randomized studies to confirm this. Currently, there seems to be a trend towards an RS repair with mesh to reinforce the repair. Again, biologic mesh has not been compared to permanent mesh in this instance, nor has a CS repair been compared to an RS one in this setting.
Abdominal Wall Reconstruction in the Military During the Recent Conflicts
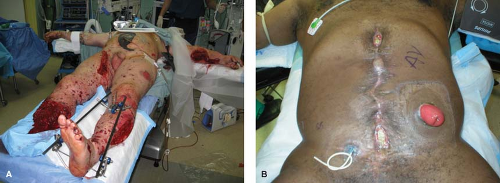
With the initiation of the wars in Iraq and Afghanistan, military surgeons have adopted civilian trauma techniques in order to salvage service members (SMs) who have undergone devastating multi-system blast injuries. Chief amongst these techniques has been the use of damage control resuscitation and laparotomy, which subsequently has generated a large cohort of severely wounded SMs with an open abdomen. Military surgeons like their civilian counterpart have increasing employed abdominal wall reconstruction techniques to definitely manage these complex cases. Vertrees et al. reported on 86 military patients with a mean Injury Severity Score of 30 evacuated to Washington DC with open abdomen between 2003 and 2007. Fortunately, 67% of the cohort successfully underwent early delayed abdominal closure via a silo technique where gortex mesh was placed and sequentially tightened over weeks until fascial closure was obtained (see Fig. 28.2). In cases where the primary closure was not possible patients underwent abdominal wall reconstruction supplemented with early on prosthetic (62%) and later biologic mesh (31%). Long-term hernia recurrence was not reported.
Abdominal Wall Reconstruction Techniques
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree