(1)
Department of Obstetrics and Gynecology, Women’s Health Institute, Cleveland Clinic, 9500 Euclid Avenue, A-81, Cleveland, OH 44195, USA
Abstract
Laparoscopic urethropexy was introduced in the early 1990s, and the first robot-assisted sacral colpopexy was reported in 2004. Over the past 10–15 years, laparoscopic and robot-assisted laparoscopic techniques have been applied to many prolapse and incontinence procedures. After the United States Food and Drug Administration approved its use in gynecologic surgery in 2005, the da Vinci Surgical System (Intuitive Surgical, Inc.; Sunnyvale, CA) gave gynecologic surgeons another minimally invasive option for surgeries that had been previously performed by laparotomy, vaginally, or by the traditional laparoscopic technique.
Laparoscopic urethropexy was introduced in the early 1990s, and the first robot-assisted sacral colpopexy was reported in 2004 [1]. Over the past 10–15 years, laparoscopic and robot-assisted laparoscopic techniques have been applied to many prolapse and incontinence procedures. After the United States Food and Drug Administration approved its use in gynecologic surgery in 2005, the da Vinci Surgical System (Intuitive Surgical, Inc.; Sunnyvale, CA) gave gynecologic surgeons another minimally invasive option for surgeries that had been previously performed by laparotomy, vaginally, or by the traditional laparoscopic technique.
In the field of female pelvic reconstructive surgery, robotic-assisted laparoscopy is most widely used for sacrocolpopexy. Retrospective cohort studies show that robotic-assisted sacrocolpopexy is associated with less intraoperative blood loss, earlier hospital discharge, and better short-term anatomic outcomes when compared with open sacrocolpopexy [2, 3]. Additionally, robotic-assisted laparoscopy may enable surgeons who have not been extensively trained or are not appropriately skilled in traditional laparoscopic techniques to perform complex abdominal surgery by minimally invasive access, as there is some evidence that the learning curve may be shorter [4–6]. Finally, although this has not been widely studied in live surgery, robotic-assisted laparoscopy may offer ergonomic advantages over traditional laparoscopy [7–10].
There are many advantages of robotic sacrocolpopexy when compared with open sacrocolpopexy; however, there are several potential barriers to adopting robotic-assisted laparoscopic technology. Surgeons, surgical assistants, and operating room teams must be comprehensively trained, and patient-centered outcomes of surgical cases should be tracked. Surgeons must be wary of extending patient anesthesia time, especially during the early robotic learning curve. Finally, instrumentation cost and robotic maintenance fees must be considered in the adoption and maintenance of robotic technology at a particular institution.
Robotic technology has expanded the use of minimally invasive prolapse techniques, most especially in sacrocolpopexy. As robotic techniques for female pelvic floor disorders are taught and refined, we must continue to be cognizant of other minimally invasive surgery options, patient and societal costs, and most importantly, patient safety and satisfaction.
20.1 Perioperative Considerations
Similar criteria are used to select patients for both laparoscopic and robotic-assisted laparoscopic pelvic reconstructive procedures. Patients should be able to tolerate pneumoperitoneum and the steep Trendelenburg position needed to facilitate cephalad bowel retraction for optimal visualization of pelvic anatomy. Consequently, patients with certain cardiopulmonary conditions may not be optimal candidates for robotic or laparoscopic pelvic floor procedures. In addition, unlike traditional laparoscopic procedures, use of the surgical robot prohibits use of operative table movement during cases. Consequently, patients are usually placed in the maximally required Trendelenburg position (often 30° from horizontal) and are maintained in this position for the duration of the robotic portion of the case. This position can cause difficulty in ventilating the patient and can contribute to intraoperative hemodynamic changes [11]. Prolonged Trendelenburg position increases chest wall resistance and dead space with a consequent decrease in the alveolar-arterial diffusion of oxygen. Pulmonary compliance and functional residual capacity are reduced; these effects are often more pronounced in obese patients [12–14].
The surgeon also needs to carefully consider the effects of intra-abdominal CO2 insufflation and its hemodynamic and metabolic effects in patients with chronic obstructive pulmonary, cardiovascular, and chronic renal diseases [13–16]. Be aware of patients with contraindications to increases in intracranial pressure and patients who are potentially hypovolemic preoperatively; a laparoscopic or robotic procedure may be contraindicated in these patients. These concerns are particularly amplified in prolonged minimally invasive cases [17, 18]. Patients with such underlying cardiopulmonary conditions should be preoperatively counseled as to the need for a possible conversion to an open procedure if indicated by intraoperative physiologic parameters.
Robotic sacrocolpopexy and concomitant procedures frequently take over 3 h to perform; therefore, a patient may be exposed to prolonged general anesthesia and increased risks for thromboembolism, hypothermia, and nerve injury. Because prolongation of surgery is known to be associated with certain degrees of morbidity, a robotic surgeon should be mindful of surgical case progression. We often use time goals whereby a trainee is given a set amount of time to perform a portion of the surgery while at the surgeon console. If the time goal is met, they continue to sit at the console. If not, the attending surgeon assumes the role of the console surgeon. This technique is useful when teaching resident and fellow surgeons portions of a complex surgery.
20.2 Operating Room Set-up and Patient Positioning
We typically use either the da Vinci S or Si Surgical System (Intuitive Surgical, Inc.; Sunnyvale, CA). The Dual Console Si system is helpful when teaching a resident or fellow surgeon as long as an experienced bedside assistant is present. Our surgical team is typically composed of the robotic surgeon(s) at the surgeon console, a bedside assistant standing on the patient’s right near the assistant port, a vaginal assistant who operates the vaginal and rectal sizers, a scrubbed surgical nurse or technician, and a circulating nurse.
Figure 20.1 demonstrates the robotic room set-up for sacrocolpopexy and reconstructive pelvic surgery. There is typically one table for laparoscopic and robotic abdominal instruments, one table for vaginal instruments and cystoscopy equipment, and a large Mayo stand upon which the robotic endoscopic camera sits (after it has been removed from the warmer) until it is placed into the peritoneal cavity. The Vision cart is usually on the left side of the operating table so that the bedside assistant on the patient’s right can have an optimal viewing angle.
Patient positioning for robotic sacrocolpopexy is similar to that for laparoscopic sacrocolpopexy (see Chap. 7) in that the patient is placed on an antislip pad, foam egg crate pad, or bean bag. After induction of anesthesia and placement of an orogastric tube for stomach decompression, the patient is moved down the operating room table and placed in the dorsal lithotomy position with the buttocks slightly beyond the end of the table to facilitate movement of the vaginal and rectal sizers. The arms are tucked in by the patient’s sides, and the hands and all bony prominences are padded for neural safety. We also typically place a padded chest strap at the nipple line to further secure the torso. After the patient is prepped and draped, a three-way Foley catheter is placed. We routinely recheck for optimal positioning of the patient during the case.
After intraperitoneal access has been gained, the bed is placed in the maximal Trendelenburg position, and the 8-mm robotic ports and laparoscopic assistant ports are placed (see section below). Once this has been safely accomplished, the bedside assistant temporarily stands on the patient’s left and supervises the parallel docking of the robot on the patient’s left side.
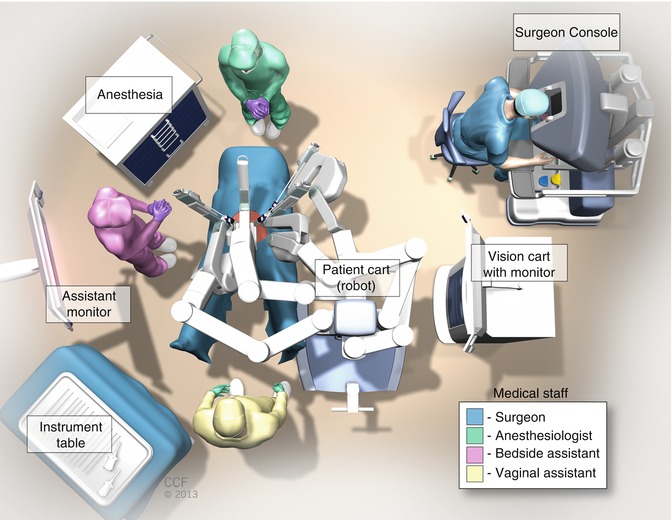

Fig. 20.1
Robotic room set up for sacrocolpopexy and reconstructive pelvic surgery
20.3 Prolapse Procedures
20.3.1 Sacrocolpopexy
Robotic sacrocolpopexy is performed using a technique similar to the laparoscopic sacrocolpopexy (see Chap. 7). The da Vinci Surgical System is currently the only widely used robotic surgical system in the United States, and the four-armed da Vinci S or da Vinci SI systems are currently the most commonly used. The robotic approach to sacrocolpopexy differs from the laparoscopic approach on a few parameters: trocar locations, docking the robotic patient cart, and use of intracorporeal knot tying, although this is also an option for the standard laparoscopic technique.
Figure 20.2b demonstrates robotic trocar placement compared with laparoscopic (Fig. 20.2a) trocar placement. Although there are a few ways in which the robotic and laparoscopic trocars can be positioned, we advocate using five trocars placed in a shallow “W” formation: two of the 8-mm robotic ports are placed bilaterally, 9 cm lateral and inferior from the umbilicus, and the third robotic trocar is placed in the left upper quadrant, 9 cm lateral to the more medial left-sided port. A 12-mm umbilical trocar is used for the robotic laparoscope, and a 10- or 8-mm assistant trocar is placed 9 cm lateral or medial to the right-sided robotic trocar. The 8-mm assistant trocar allows for the introduction and removal of suture with SH needles and does not require fascial closure, minimizing the risk of postoperative pain. The robotic trocars are placed approximately 9–10 cm apart to minimize the risk of robotic arm collision. If a patient has a short torso, a shallow “Z” configuration with the right robot port in the upper quadrant and the right lower quadrant accessory port three finger breaths cephalad and medial to the right anterosuperior iliac spine will decrease arm collision.
The robotic patient cart is then docked with the operating table in a 30-degree Trendelenburg position (Fig. 20.3). After the robotic trocars are safely placed and the patient is placed in the maximal Trendelenburg position (about 30°), the robotic patient cart is docked under the instruction of the bedside surgeon.
Although many methods of robotic patient cart docking have been described, we feel that parallel docking on the patient’s left side allows easy access for vaginal manipulation during vaginal and rectal dissections in sacrocolpopexy and results in minimal issues with robotic arm collision (Fig. 20.4).
After first affixing the camera arm, the other robotic arms are connected to the robotic trocars with care taken to position them to minimize the risk of collisions (Fig. 20.5). A 30-degree angle between the instruments’ arms and the camera is good, but a 45-degree angle is usually better. Positioning the fourth robotic arm (arm 3) at the most left lateral trocar is usually done last because of the need for its almost horizontal docking and often, its inferior angle to the patient.
We then place the appropriately calibrated robotic endoscope in the camera trocar. We typically use a 0-degree robotic endoscope when performing the vesicovaginal and rectovaginal dissection, but a 30-degree upward-facing endoscope can aid in the rectovaginal and perineal dissections and suture placement. A 30-degree downward-facing endoscope can be particularly helpful for the presacral dissection. We typically place the robotic monopolar scissors in arm 1, a bipolar instrument (either PK Dissecting Forceps [Gyrus Medical; Maple Grove, MN] or bipolar forceps) in arm 2, and a ProGrasp (Intuitive Surgical, Inc.; Sunnyvale, CA) in arm 3 for the initial dissection. If a hysterectomy is being performed, the Tenaculum forceps can be placed in arm 3 rather than the ProGrasp; however, this is rarely used in order to control costs. Once the initial dissection for the sacrocolpopexy is done (as discussed in Chap. 7), we typically use a SutureCut needle driver (Intuitive Surgical, Inc.; Sunnyvale, CA) in arm 1, a Needle Driver in arm 2, and a ProGrasp in arm 3 to suture robotically with 8-inch monofilament 2-0 or 0 polypropylene and polydioxanone. All knot tying in robotic sacrocolpopexy is performed using an intracorporeal technique. Suture and polypropylene graft placement do not differ between laparoscopic and robotic sacrocolpopexy (Fig. 20.6).
There are a few points of caution for robotic-assisted laparoscopic sacrocolpopexy. The lack of haptic feedback is important to acknowledge when distinguishing robotic from laparoscopic sacrocolpopexy. Consequently, the console surgeon has to pay close attention to visual cues when placing tension on tissues or sutures and judging the depth of suture placement. This is particularly important when determining where the sacral promontory is located for the presacral dissection. After identifying the right ureter, aortic bifurcation at the L4–L5 level, common iliac vessels, and retracting the sigmoid laterally, we typically have the bedside assistant palpate the promontory laparoscopically. Caution is also taken when placing sutures in the anterior longitudinal ligament at the level of S1; care must be taken not to penetrate the vertebral periosteum or intravertebral disk with deep suture placement. Finally, we also try to minimize robotic manipulation of the sigmoid and epiploica with the ProGrasp in arm 3 by initially retracting the bowel cephalad and laterally with a bowel grasper in the right upper quadrant assistant port. We then use the ProGrasp, with its closed or slightly open tips angled toward the sacrum, to maintain gentle, lateral traction on the sigmoid. Alternatively, suture can also be passed through several sigmoid epiploica and brought through the left lower quadrant lateral to the left upper and lower quadrant port sites (arms 2 and 3, respectively) with a Carter Thomason suture carrier (Cooper Surgical; Trumbull, CT). Both suture ends are secured with minimal tension at the skin surface with a hemostat clamp, retracting the sigmoid laterally.
Other points of caution for robotic sacrocolpopexy include the following: (1) Once the robotic system is docked, the patient bed position cannot be changed without first removing instruments and undocking the robotic arms. (2) The tip of the robotic endoscopic camera becomes very hot and must be cleaned outside of the peritoneal cavity. (3) The abilities to clutch, exchange instruments, focus the camera, and to use monopolar and bipolar energy modalities differ between the different generations of da Vinci Robotic Surgical Systems. Consequently, a surgeon should be comfortable with the features of the particular robotic system prior to its use.
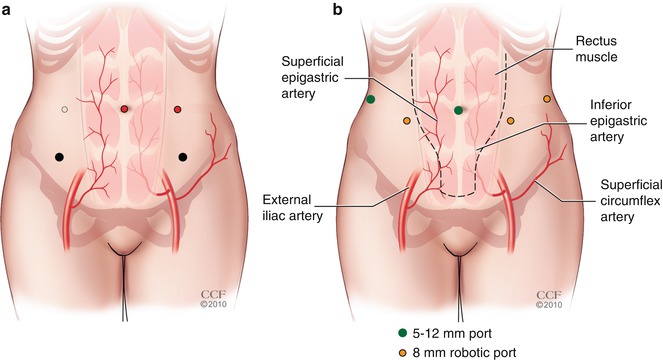
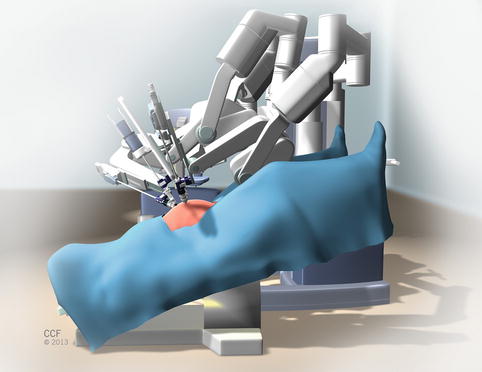
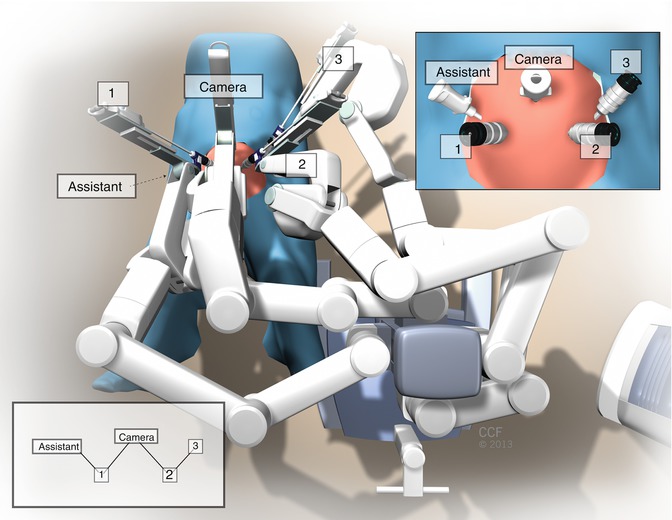
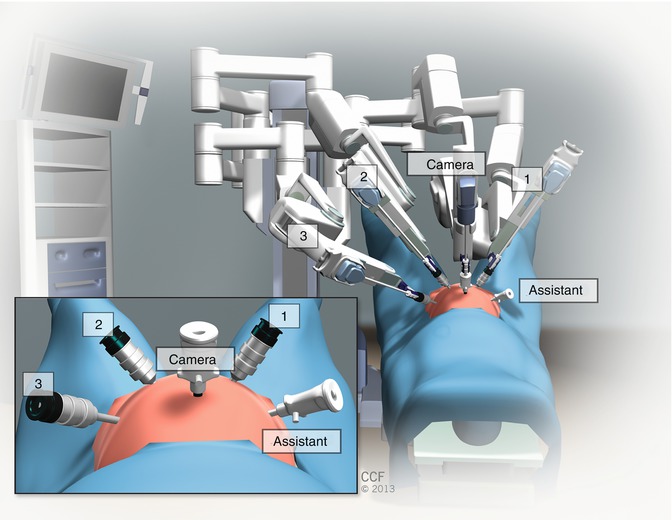

Fig. 20.2
(a) Laparoscopic trocar placement compared with (b) robotic trocar placement

Fig. 20.3
Robotic patient cart docked with operating table in 30° Trendelenburg position

Fig. 20.4
Parallel docking of robotic patient cart

Fig. 20.5
Robotic arms connected to the robotic trocars

Fig. 20.6
Suture and polypropylene graft placement for sacrocolpopexy
20.3.1.1 Robotic Sacrocolpopexy Outcomes and Complications
In the recent update of the Cochrane review of surgical management of pelvic organ prolapse, Maher and coworkers [19] stated that abdominal sacrocolpopexy had lower rates of recurrent vaginal apex prolapse (3.5 % versus 15 %; RR 0.23, 95 % CI 0.07–0.77), a reduced grade of residual prolapse (5.7 % versus 20 %; RR, 95 % CI 0.09–0.97), and less dyspareunia (16 % versus 36 %; RR 0.39, 95 % CI 0.18–0.86) when compared with vaginal sacrospinous colpopexy. Abdominal sacrocolpopexy, however, was associated with a longer operative time (mean difference [MD] 21 min, 95 % CI 12–30), longer time to recovery (MD 8.3 days; 95 % CI 3.9–12.7), and was more expensive (weighted MD USD $1,334; 95 % CI $1,027–$1,641) than the non–mesh augmented vaginal approach. Well- designed randomized trials included in the meta-analysis by Paraiso and colleagues [20], Freeman and colleagues [21], and Maher and associates [22] compared laparoscopic sacrocolpopexy with either robotic [20], open [21], or total vaginal mesh [22].
Only a few well-designed comparative studies for robotic sacrocolpopexy exist, and many have varying objective and subjective outcomes. One single-center, blinded, randomized trial from our institution randomized women with posthysterectomy Stages 2–4 vaginal apex prolapse to either laparoscopic or robotic sacrocolpopexy groups [20]. The primary outcome was total operative time from incision to closure, but secondary outcomes included postoperative pain, functional activity, bowel and bladder symptoms, quality of life, anatomic vaginal support, and cost from a health care perspective. Total operative time was significantly longer in the robotic group (227 ± 47 vs 162 ± 47 min, p < .001), with docking only accounting for an average additional 14 min. In addition, sacrocolpopexy suture tying was longer for the robotic group (98 ± 22 versus 68 ± 16 min, p < .001). Although pain scores were not significantly different on postoperative day 1, the robotic group reported more pain at rest and with normal activities at several points during the 6-week postoperative period. We believe that increased pain in the robotic group was caused by muscular pain associated with manipulation and fascial closure of the right paracolic gutter accessory port. Hence, we have changed port size from 10 or 12 mm to 8 mm. At 6 and 12 months follow-up, anatomic and quality of life outcomes did not differ between the two groups. There were no significant differences in intraoperative and perioperative complications between robotic and laparoscopic sacral colpopexy [20]. The most frequent complication was in the area of urinary tract infections, of which there were three in the laparoscopic and five in the robotic groups (9 % versus 14 %, respectively, p = .71). There were two cystotomies recognized intraoperatively in both groups and one enterotomy in the robotic group. The robotic group had two patients with a mesh erosion (6 % versus 0 %, p = .49), and three with abdominal wall pain necessitating trigger point injections (9 % versus 0 %, p = .24).
The majority of other studies comparing robotic and laparoscopic sacrocolpopexy with open sacrocolpopexy are retrospective cohorts from either one or two institutions, and the length of follow-up included in these studies ranged from 3 to 44 months. Overall, these studies show that both anatomic and subjective cure rates are comparable between robotic and laparoscopic sacrocolpopexy. Similar to the randomized trial by Paraiso and coworkers [20], Antosh’s retrospective cohort trial comparing robotic and laparoscopic sacrocolpopexy did not show a significant difference in perioperative and postoperative complications [23]. There was no difference in the respective number of cystotomies (three versus one, p = 1.0) or blood transfusions (one versus two, p = 0.17) in either group. There were no conversions to laparotomy in either group. There were also no significant differences in urinary tract infection (nine versus six cases, p = .20), fever (one case in both groups, p = .46), wound infection/abscess (two versus one case, p = 1.0) or mesh erosion (two versus 0 cases, p = 1.0).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








