Fig. 33.1
(a) Side-to-side four-stapled technique. The linear cutting stapler is introduced through an enterotomy made on the antimesenteric side of the enterectomy staple line. (b) Side-to-side four-stapled technique. The anastomosis is created by stapling the antimesenteric bowel of the small intestine and colon using a linear cutting stapler. (c) Side-to-side four-stapled technique. A linear non-cutting stapler is used to close the enterotomy created by the surgeon to introduce the linear cutting stapler
Fig. 33.2
Ideal “B”-shaped staple configuration
The decision to close the mesenteric defect following a small bowel resection or right colon anastomosis is mostly a personal preference (Fig. 33.3). The goal is to prevent an internal hernia postoperatively, which may be seen more often after laparoscopic colon resection [29]. This is theoretically due to the lack of adhesion formation after minimally invasive surgery and the ease with which the bowel may herniate through the mesenteric defect. Retrospective studies offer some insight into this infrequent complication. Cabot and colleagues reported on 530 consecutive patients who had laparoscopic right colon resection and found a 0.8 % incidence of small bowel obstruction due to herniation through an unclosed mesenteric defect [30]. No randomized head-to-head comparison of closure versus no closure of the mesenteric defect exists, though a higher complication rate with mesenteric closure has been reported in a retrospective review of patients undergoing a large bowel resection [31]. In this series by Causey et al., 133 patients had a colectomy (right, 36 %; sigmoid colon, 33 %; and left, 11 %); 52 % of the mesenteric defects were closed. Postoperative complications were attributed to the mesenteric defect in 6 % of the patients; with closure of the mesentery, the only significant factor identified in multivariate analysis (OR = 5.5; 95 % CI 1.069–28.524, P = 0.041). While this study is by no means definitive, it points out the flaws of assuming that all complications can be avoided by closing the defect. There are no doubt pros and cons of both approaches, and the choice is likely a result of an individual surgeon’s training and experience. Those of us who choose not to close the defect fear compromising the mesenteric blood flow or creating a smaller mesenteric defect that may be more prone to incarcerate should a hernia occur. If the mesentery is closed, care should be taken to avoid the intestinal blood supply as this can result in a hematoma or worse—anastomotic ischemia. If a hematoma develops, it can usually be managed with simple pressure applied by gently squeezing the leaf of the mesentery between the operator’s fingers for a few minutes with the goal of controlling the bleeding and limiting the size of the hematoma. It is probably not necessary to open the peritoneum as this will result in further bleeding and control will be more difficult. Suture ligation of the hematoma should also be used with caution, and typically avoided. The risk is that sutures used to ligate mesenteric bleeding could compromise the blood supply to an anastomosis that has already been fashioned and will be difficult to assess.
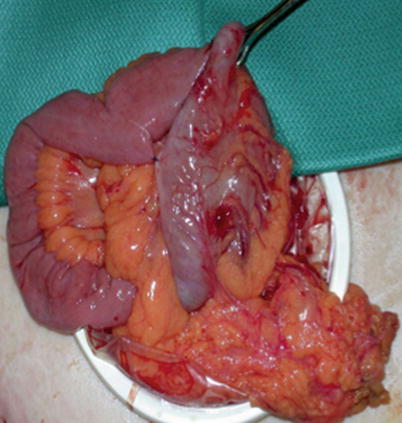

Fig. 33.3
Right colon anastomosis with mesentery closed
When performing a right colectomy laparoscopically, it is important that the orientation of the mesentery and the anastomosis is confirmed prior to closing the fascia as it is possible to twist the ileal side 360° after transection (Fig. 33.4). This is more likely to occur with laparoscopy when performing an extracorporeal anastomosis through a small midline incision. We avoid this twist by starting at the ligated vascular pedicle and following the cut edge of both the small bowel and colonic mesentery completely to the bowel wall. We then place atraumatic graspers on both ends only after these are confirmed to be straight and use Babcock clamps with extracorporealization maintaining this orientation. If there is any concern, the laparoscope can confirm that no twist is present.
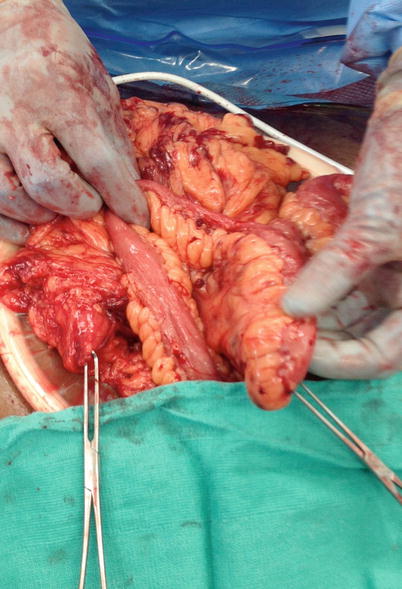

Fig. 33.4
The ileal mesentery is mobile and could be easily twisted 360°. This is readily apparent in this picture and obvious—however, through a small laparoscopic incision, it may not be evident without reevaluating with the camera
Left-Sided and Rectal Resection
Key Concept: Left–sided and rectal anastomoses have their own unique set of requirements for success and potential complications. Being familiar with single versus double stapled, hand sewn, and end to end versus modified end to side will allow several options in the face of difficulties.
Left colon and rectal resections with anastomosis represent a higher degree of complexity, as reliance on stapling, collateral blood supply and mesenteric length are all critical to a safe anastomosis. All staplers have a certain incidence of failure, but the consequence of device failure when stapling low in the pelvis can be much more significant. Mechanical staplers have been in use for decades, but new products continue to come to the operating room as well as ongoing modifications of old devices. In some instances, these new products represent a significant leap forward and offer surgeons opportunities to do things that previously were not possible, while others attempt to improve upon technology that is already effective. Adoption of these devices requires a clear understanding of the advantages and performance characteristics of each and mandates a thoughtful approach to incorporation into practice.
Regardless of the manufacturer, all of these devices have a failure rate, and more importantly, when device failure occurs, the surgeon must have a plan to salvage the anastomosis. The exact incidence of failure is difficult to establish, but in 2007, Mardestein et al. reported on 1,188 stapler misfires reported to the FDA during a 12-month period [32]. Of the misfires, 588 occurred during colorectal procedures with failure to form staples and inability to remove the stapler as the most common problem. From these adverse events, 266 occurred during rectal resections and 80 were considered major, resulting in 23 unplanned permanent ostomies. Stapler misfire during a laparoscopic procedure was associated with a 43 % conversion rate. This high rate of conversion to open surgery following stapler misfire was confirmed by Pandya et al. in their analysis of 200 consecutive laparoscopic colectomies [33].
It is unknown how many of these failures were surgeon related, but it is imperative that everyone involved in the case has intimate familiarity with the proper use of the device. The primary surgeon may not be the person deploying the stapler, and errors can occur when there is an assumption that a co-surgeon or assistant knows how to properly deploy a given stapler. In our operating room, the surgery resident is often responsible for deploying the stapler, and it is not unusual for him or her to be doing so for the first time. We have avoided this situation by focused education on the proper use of the various staplers prior to the operating room for trainees. This information can also be included into the time out or preoperative briefing procedure so that proper orientation can occur.
We routinely employ a double-stapled technique when performing a rectal anastomosis by transecting the rectum with a linear stapler distally and placing the purse-string suture on the proximal side to secure the anvil of the circular stapler. The handle of the end-to-end anastomotic stapler is then advanced through the anus to the top of the rectum and the spike deployed usually adjacent or through the transverse staple line. The anvil and spike are then united and compressed either manually or to a predetermined height, depending on the stapler manufacturer and tissue thickness. Once deployed, the anvil is extracted through the lumen of the newly created anastomosis along with rings of tissue incorporated into the head of the device. While little evidence exists to support one staple diameter over another, it is our practice to use the largest stapler that will safely fit into the conduit and negotiate the rectal stump. For most adults this is usually 29 mm; and we rarely use the 33-mm or 25-mm diameter stapler. There is some evidence to suggest that when stapling an ileal pouch to the anus, symptomatic strictures occur more frequently when a 29-mm stapler is used compared to a 33 mm [34]. Others have suggested that stenosis is a function of mechanical circular stapling regardless of the diameter [35]. We have not seen this in our practice and maintain like others that symptomatic stenosis is rare following stapled end-to-end anastomoses [36, 37] or side-to-end anastomosis, especially when careful attention to preservation of blood supply is maintained.
The double-stapled technique can be modified by placing a purse string on both the proximal and distal side and using the circular stapler to create the anastomosis—the single-stapled technique. This can prove difficult in the setting of a low rectal anastomosis, and studies have shown that there is no clinical difference between the two techniques [38].
The Mechanical Stapler
Key Concept: Preparing the rectum prior to introducing the stapler may avoid difficulties with reaching the apex of the rectal stump. Additionally, having an algorithm for the “stuck” stapler will allow rapid identification of the cause and determine the optimal next step.
One of the most common and frustrating stapler complications occurs when the handle of the end-to-end stapler won’t reach to the top of the rectal stump. There are a number of reasons this might occur, and most times can be prevented. First, it is important to ask yourself if your distal transection line is as low as you think it is (or need it to be). In some cases of a rectosigmoid anastomosis, a knuckle of sigmoid colon may give the false appearance that you are at the level of the extraperitoneal rectum, when in fact distal sigmoid remains. Barring this, there are several other instances where this can occur. Inspissated mucous in a chronically diverted rectal stump may physically prevent the stapler from reaching the top, and a preoperative endoscopic evaluation of the Hartmann stump can help clear these remnants and prevent this problem. This can also be prevented with preoperative or intraoperative rectal irrigation using a rigid proctoscope. If the rectum is soft and there is no mucous or other debris obstructing the stapler, a well-lubricated sizer can be used to gently dilate the lumen of the rectum starting small and working up to the larger sizes (29 or 33 mm). If the sizer will not reach the top of the rectum, it is unlikely that the stapler ever will. Care should be taken to avoid inadvertently pushing the sizer through the staple line. Occasionally, insufflation of the rectum with air (with a rigid proctoscope or flexible endoscope) or lubricant will distend the top of the rectum sufficiently to allow passage of the stapler. If nothing works and a hand-sewn anastomosis is not possible or desirable, then a stapled end-to-side anastomosis can be performed (Fig. 33.5 and Video 33.2). This can be done by bringing the spike of the end-to-end stapler through the anterior wall of the rectum [39] or a side to end with the anvil purse stringed into the rectum with the handle introduced through the side of the conduit (usually left colon) [40]. The so-called Baker and modified Baker type of anastomosis are effective, although data is limited. Our preference is to use the modified approach when the stapler won’t reach, but is within a few centimeters of the transverse staple line. We are careful to maintain a distance roughly corresponding to the diameter of the stapler head from the rectal transection line so as not to induce an ischemic segment between the circular anastomosis and the transverse staple line.
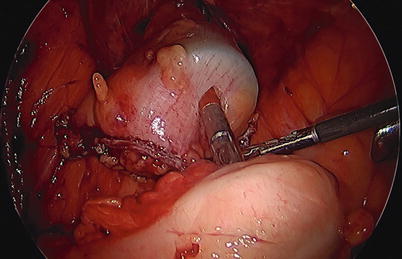

Fig. 33.5
An end-to-side colorectal anastomosis
There are also times when the handle of the stapler cannot be advanced above a relative stricture at the peritoneal reflection; this stricture can be caused by conditions such as perforated diverticulitis, which causes fibrosis of the peritoneum in the cul de sac. This fibrosis can prevent the stapler from traversing the second rectal valve. Forcing the stapler in this situation can cause a laceration, or worse a perforation, of the rectal stump. The laceration may not be readily apparent if it is located in the posterior aspect of the rectal wall and into the mesorectum. If a laceration or perforation occurs, then the surgeon should consider resecting the rectum below this point and performing the anastomosis at this level. Instead of forcing the stapler in this situation, it is our practice to abandon the stapled technique in favor of a hand-sewn end-to-end anastomosis.
Another less common scenario occurs when the stapler becomes stuck, a situation that occurs more frequently in our experience with an end-to-end anastomotic device. After deploying the stapler and creating the anastomosis, it is recommended that the anvil be opened, usually a full turn of the handle in an effort to release the newly formed anastomosis from the head of the device. Opening the device too much can result in the anvil separating from the head, causing it to lodge in the proximal side of the anastomosis. If this occurs, an endoscope can be used to retrieve the anvil [41] while allowing the surgeon an opportunity to inspect the anastomosis and determine its integrity. If the stapler can’t be removed, the surgeon must assess the situation to determine if the stapler has properly deployed. If the knife blade has failed to create the lumen, it won’t be possible to remove the stapler and no amount of manipulation will dislodge it. The outside of the anastomosis should be inspected (if possible) to determine if the staples have deployed and deformed appropriately as failure to do so will indicate a catastrophic stapler failure and a need to redo the anastomosis—a situation that when low in the pelvis may prove difficult or impossible. One approach in this situation is to mobilize further to gain additional proximal length and attempt a hand-sewn coloanal. If the staples have deployed but the stapler cannot be removed, it is possible to slide a red rubber catheter into the rectum along the stapler handle and insufflate with air, which can help determine if a lumen has been created and possibly facilitate dislodging the circular staple line from the head of the stapler. Kyzer et al. reported difficulty extracting the stapler in 3 out of 215 stapled end-to-end anastomosis and described a technique of placing stay sutures around the anastomosis in an effort to lift it out of the stapler head [36]. If all else fails, the stapler may need to be removed by dividing the bowel above and below the anastomosis and pulling the head and tissue of the failed anastomosis through the rectum. If there is enough length distally, a second attempt at a stapled end-to-end anastomosis can be made versus a hand-sewn anastomosis or (worse case) diversion.
The Anastomotic Donut and Leak Testing
Key Concept: All left–sided anastomosis should undergo leak testing!
Complications of the circular stapler can also include incomplete anastomotic donuts (i.e., rings), a situation that may imply inadequate tissue incorporation (Fig. 33.6). An air leak test will confirm the presence or absence of an incomplete anastomosis. If the donuts are incomplete but there is no air leak, it is our practice to treat the anastomosis as if it were intact. However, the finding of incomplete donuts is part of the decision-making process regarding the potential need for proximal diversion.
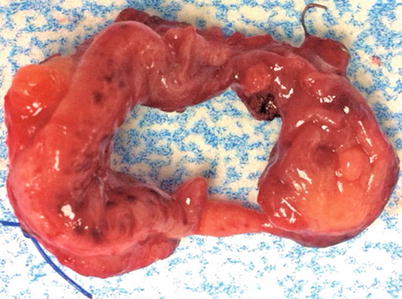

Fig. 33.6
An incomplete anastomotic donut with no serosa or mucosa noted on the inferior aspect
We routinely perform an air leak test on all left-sided anastomoses regardless of the donut integrity or whether the anastomosis was stapled or sewn in an effort to identify and prevent anastomotic leaks [42–44]. This practice is supported by the work of Ricciardi et al. who reviewed the outcomes of 825 left-sided resections and found evidence that 8 % of those tested were positive for an air leak [44]. Postoperative leaks occurred in 7.7 % of anastomoses that tested positive, in 3.8 % of those that tested negative, and in 8.1 % of those that were not tested (P < 0.03). Furthermore, this simple maneuver can provide insight as to potential outcomes based on how this situation is handled. The authors found the anastomotic leak rate was 12.1 % when an anastomosis that was initially positive for an air leak was suture repaired so that they were air tight, compared to 0 % when they were completely redone or were diverted proximally (P = NS). Beard et al. performed a randomized trial of 145 patients undergoing left-sided and rectal resections to intraoperative air leak testing or nothing. In the test group, 25 % of anastomoses leaked air and were repaired. Clinically, relevant anastomotic leaks occurred in 4 % of the test group and in 14 % in the no test group (P = 0.043) [42]. While these benefits have not been uniformly demonstrated [45], no authors have shown that air leak testing is harmful, and likely never will. Given the potential benefit and the ease of performing this test, surgeons should consider it a routine part of their practice for left-sided and rectal resections. While some surgeons advocate air leak testing of right-sided anastomosis, we do not routinely do this.
Offodile et al. reported a 19 % incidence of technical errors associated with 349 circular stapler deployments which included a positive air leak test (n = 19), difficulties inserting or extracting the stapler (n = 18), incomplete or thin donuts (n = 13), tissue damage (n = 10), and others (n = 7). Technical errors associated with the circular stapler were associated with a higher incidence of proximal diversion (34 % vs. 17 %, P < 0.0003) and conversion to open in laparoscopic cases (22 % vs. 13 %, P < 0.045), in part due to the level of the anastomosis. Overall, there was no difference with regard to leaks, reoperation, suture line strictures, and hospital stay—likely a reflection of proper surgical judgment following the initial difficulties [46].
Complications associated with the linear stapler can also occur, and in our experience, the most common complication is failure of the device to deploy and deform the staples. This can represent a true device failure or be the result of surgeon failure to deploy the stapler properly prior to transecting the bowel. The end result is the same—a distal rectal stump that is gaping. One obvious indication that this has occurred is the amount of bleeding that ensues, often the first clue especially when this occurs deep in the pelvis. In this instance, the surgeon can either attempt to get a stapler across the rectum a second time, assuming enough length, or convert the anastomosis to a double purse-string technique by attempting to place a purse string on the rectal stump. Both are quite difficult, especially in a narrow pelvis. This can be attempted transabdominally or transanally. To facilitate a transanal approach, a Lone Star (Lone Star Medical Products®, Stafford, TX, USA) can be used to evert the anal canal (Fig. 33.7), and an anal retractor can be inserted such as a Hill-Ferguson or Sawyer. The purse string in this instance can be tied on the anal side leaving a small aperture to introduce the anvil, or the sutures can be passed into the abdomen and tied down around the spike of the stapler after it is brought up through the anus. A linear stapler that won’t release after firing represents a mechanical failure and usually cannot be salvaged. In this instance, it will need to be released by cutting the bowel distally.
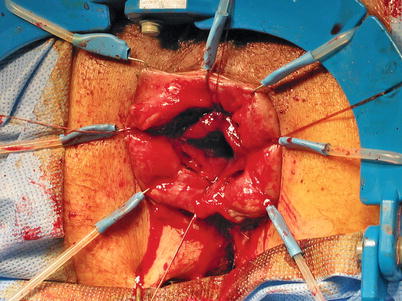

Fig. 33.7
Hand-sewn coloanal anastomosis
Gaining Enough Length
Key Concept: You need to be familiar and comfortable with each of the several methods used to gain additional length for left–sided and rectal anastomoses to avoid tension.
Another critical aspect of a successful colorectal anastomosis is ensuring adequate length of the conduit so that there is no tension. The choice of proximal transection will depend largely on the pathology and the condition of the bowel, as it is imperative that the two ends be healthy. Length can be achieved by completely mobilizing the attachments of the left colon to the retroperitoneum and flexure. The other critical aspect to obtaining adequate bowel length is mobilizing the mesentery, which will tether the left colon into the abdomen unless it is freed. There are several decisions the surgeon will need to make starting with the most important—where to divide the bowel both distally and proximally. For diverticular disease, the entire sigmoid should be removed along with any proximal colon that is inflamed or hypertrophied. In this regard, it is critical to review the most recent CT findings to ensure that any proximal inflammation is resected to mitigate the risk of recurrence. The distal transection line should always be to the top of the rectum, identified where the taenia coli splay on the anterior surface. If the disease is isolated to the sigmoid, it is not always necessary to mobilize the splenic flexure, and studies reviewing the selective approach to mobilizing the flexure have been favorable [47–50]. Key points are that the conduit be free of disease and of adequate length. When the flexure must be mobilized, several techniques are available to accomplish what can be a very difficult and variable surgical procedure. All techniques can be done either laparoscopically or open, although we find a medial to lateral approach starting underneath the inferior mesenteric artery (IMA) to be difficult to do open as it is an awkward place to see without the aid of a 30° laparoscope.
In general, if we are performing an open procedure, the dissection is performed lateral to medial. During the first step, the omentum is dissected off of the transverse colon by dividing the gastrocolic ligament, entering the lesser sac. One error in this situation is to try and mobilize the flexure through a small midline incision. This creates a situation whereby the surgeon and the assistant are not able to adequately see the field at the same time, increasing the risk of injuries to the colon, spleen, and mesentery. A nice rule of thumb is that the midline incision should extend above the umbilicus and the retractors should be set so that there is maximal pull on the left subcostal retractor. It will be necessary to reset the retractors for adequate pelvic exposure, as it is rare that retraction that allows visualization of the splenic flexure will also allow optimal visualization of the pelvis. If the field is too small, then the surgeon is often left in the dark, literally, and a headlamp can facilitate illumination of the left upper quadrant. With the surgeon retracting medially and inferiorly, the assistant divides the peritoneal attachments. If the assistant is not able to visualize the plane, consider moving him or her between the patient’s legs so that they can get a more natural look at the anatomy. Another option in a tough dissection is to get into the lesser sac along the greater curve of the stomach leaving the omentum on the colon. This may help release the flexure and open up the space particularly if the flexure is very high or in the hilum of the spleen. It is important to avoid excessive traction on the spleen as this may result in splenic injury (see Video 31.5). In a review of 975,825 patients who underwent colorectal resection during a 2-year period, Masoomi et al. reported a rate of splenic injury of 0.96 %, of which 85 % were treated with complete splenectomy (splenorrhaphy, 13 %; partial splenectomy, 1.7 %) [51]. The most common procedure associated with splenic injury was transverse colectomy (3.4 %). Using multivariate regression analysis, the investigators found that transverse colectomy (adjusted odds ratio [AOR], 5.30), left colectomy (AOR, 5.08), total colectomy (AOR, 2.85), open operation (AOR, 2.68), malignant tumor (AOR, 2.11), diverticulitis (AOR, 1.93), teaching hospital (AOR, 1.73), male sex (AOR 1.20), peripheral vascular disease (AOR, 1.14), and emergent admission (AOR, 1.06) were associated with a higher risk of splenic injury. Excessive traction and poor visualization can also result in an inadvertent colotomy. If this occurs, spillage should be controlled with a temporary mass closure suture and the flexure mobilization completed. This will allow better assessment of the injury and a more definitive repair. It is usually not necessary to resect the injury unless the blood supply was also compromised. If the IMA has been ligated along with the marginal blood supply, then the entire conduit may become ischemic and resection will likely be necessary. This is a catastrophic complication, often making it extremely difficult to have more proximal colon reach the pelvis, and should be avoided by appropriate visualization through an adequate incision with good lighting.
When approaching the flexure laparoscopically, a medial to lateral dissection is preferred; it is possible to mobilize the left colon all the way to the flexure so that only a thin layer of peritoneum remains for the lateral dissection [52]. Another option is to start by entering the retroperitoneum at the inferior mesenteric vein (IMV) and proceed medially from there. This will lead the surgeon to the area under the splenic flexure and can be used alone or in conjunction with the more traditional approach. Care should be taken to avoid inadvertently dissecting underneath the pancreas when using this approach.
Once the left colon and splenic flexure are fully mobilized, additional length for tension-free low colorectal anastomosis will be gained by appropriate mobilization of the mesentery. If a cancer resection is being performed, it is desirable to ligate the inferior mesenteric artery (IMA) at its origin, regardless of reach, to ensure an adequate nodal harvest. There is some controversy regarding the necessity of ligating the IMA flush to the aorta (high tie) when compared to preservation of the left colic branch (low tie). The oncological necessity of a high tie is predicated on the fact that lymph nodes (LN) at the origin of the IMA can harbor malignant cells and recurrences following low tie are more frequent [53]. The impact of the high tie may be more important for advanced carcinomas [54], and the effect of radiation may mitigate this benefit further. The oncologic benefits of a high tie have not been uniformly seen [55]. Taking the IMA flush on the aorta and mobilizing the left colon mesentery just lateral to the ligament of Treitz will offer the most length but may cause the conduit to become ischemic in the absence of adequate collateral circulation. If the descending or transverse colon is to be used as the proximal half of the anastomosis, the marginal blood supply should be adequate, but must be carefully preserved. The marginal blood supply originating from the middle colic artery may not be adequate if the sigmoid colon is to be used as the proximal half of the anastomosis, as the conduit will likely be too long [56, 57]. If it is necessary to use the sigmoid colon as part of the colorectal or coloanal anastomosis, then the left colic artery should be preserved [58, 59]. The surgeon will have to decide if length is achieved more adequately by keeping the conduit long (preserving the sigmoid colon) or fully mobilizing the mesentery (dividing the left colic). While the safety of using the sigmoid colon as the proximal conduit in rectal resections has been demonstrated [60], there are certain risks that come with its use. Hall et al. measured the tissue oxygen tension (pt02) of the left colon before and after ligation of the IMA in 62 patients undergoing anterior resection [61]. Baseline data demonstrated that the pt02 varied significantly between the sigmoid, the descending, and transverse colon. After the IMA was ligated, the pt02 was significantly reduced in the sigmoid colon when compared to the left or transverse colon. This difference was observed regardless of a high or low tie. This data suggests that it is the site of colon transection and not the site of arterial ligation that impacts the integrity of the anastomotic blood supply.
To gain additional length, the inferior mesenteric vein (IMV) must be ligated adjacent to the IMA, and a second time at the inferior border of the pancreas just lateral to the ligament of Treitz. Ligating the vein twice while carefully preserving the marginal artery at the splenic flexure will add several centimeters to the length of the conduit while preserving arterial blood supply. A common error in an effort to gain length is to divide the colonic mesentery up toward the splenic flexure of the colon, with the end result cutting off the blood supply to the distal conduit, which is now based on the middle colic artery. If the marginal blood supply is compromised due to inadvertent injury while mobilizing the flexure or wandering too close the mesenteric border during ligation of the mesentery, the conduit will become ischemic and very likely unusable.
In the final analysis, the importance of a tension-free anastomosis cannot be overemphasized. In reality, you will often need to employ a combination of lengthening maneuvers. Interestingly, while the importance of avoiding tension to help ensure the integrity of an anastomosis is uniformly accepted, the degree of “acceptable” tension has been poorly studied, as most experimental models of leaks rely on the assessment of bursting pressure and not stretch [62]. An exception is the study by Shikata and colleagues who characterized the blood flow of various intestinal segments before and after the application of a tensile force following anastomosis. They used an experimental canine model and found that the effects of tension on the submucosal blood flow were much better tolerated in the small bowel when compared to the colon [63]. This data helps to corroborate the clinical assertion that an anastomosis under tension is more likely to fail as it is less likely that a small bowel resection and anastomosis, given the laxity of the small bowel mesentery, will leak when compared to a left-sided colonic resection that is more likely to be on stretch. Common sense would also indicate an anastomosis that is taut is in danger of failing for the additional reasons of mechanical forces that attempt to pull the newly anastomosed bowel away from each other [64].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








