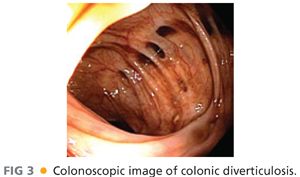
■ Colonoscopy (FIG 3) is advisable for evaluation of diverticulitis and colovesical fistula in order to exclude a perforated malignancy, which can have similar clinical presentation and radiographic appearance. The presence of malignancy would warrant an oncologic mesenteric lymphadenectomy and en bloc resection of involved bladder wall, whereas the fistula may simply be divided in cases with benign inflammatory etiology.
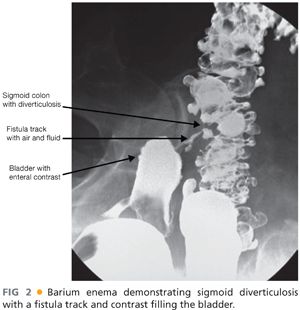
SURGICAL MANAGEMENT
Preoperative Planning
■ When patients present with diffuse peritonitis and sepsis, urgent operation may be required. Broad-spectrum antibiotics should be administered and the patient should be well resuscitated with intravenous fluid prior to surgery.
■ Every effort should be made before surgery to mark acceptable sites on the abdominal skin for stoma creation bilaterally. Consideration may be given to placement of ureteral stents preoperatively if it can be performed without undue delay.
■ Decision making should be centered on the patient’s clinical condition, the severity of pelvic sepsis, the suitability for colorectal anastomosis, and the ability to safely resect the inflammatory segment.
■ Options for surgical approach in the emergency setting include
■ Proximal diversion without resection
■ Resection with end colostomy and rectal stump closure (Hartmann procedure)
■ Resection with primary anastomosis, with or without diverting loop ileostomy
■ Laparoscopic lavage and drainage
■ Complete colonoscopy in the elective setting should be performed in order to exclude a perforated malignancy that presented as perforated diverticulitis or synchronous malignancy.
■ Cystoscopy and urine cytology may be considered in cases of colovesical fistula if there is suspicion for a primary bladder malignancy.
■ Consideration should be given to prophylactic placement of ureteral catheter(s) to assist with identification of the ureter, if it appears to be involved with the inflammatory segment.
■ Laparoscopic approaches to complex diverticular disease and colovesical fistula are appropriate in hemodynamically stable patients among surgeons with adequate laparoscopic colorectal surgery skills and training. Hand-assisted and straight laparoscopic techniques have similar short- and long-term reported outcomes.
■ If inflammation is severe and there is intention to perform a Hartmann procedure with end colostomy or a colorectal anastomosis with diverting loop ileostomy, the patient should undergo preoperative evaluation and counseling by an enterostomal therapist, including marking suitable locations for a stoma on the abdomen, either unilaterally or bilaterally, if the operative plan will depend on intraoperative findings.
■ Mechanical bowel preparation with or without oral antibiotics is a controversial topic. There is no definitive evidence for or against bowel preparation. However, if there is intention to use a circular end-to-end stapling device placed per anus, mechanical bowel preparation or rectal enema should be administered to clear stool from the rectum. If a colorectal anastomosis and diverting loop ileostomy is planned, mechanical bowel preparation is recommended to avoid leaving a column of stool between the ileostomy and the downstream anastomosis.
■ Prophylactic antibiotics to cover skin flora, enteric gram negatives, and anaerobic bacteria should be administered before making the incision.
■ Appropriate pharmacologic and/or mechanical prophylaxis for venous thromboembolism is recommended.
Positioning
■ The patient is placed in modified lithotomy position (FIG 4A) or supine with legs abducted on a split-leg table (FIG 4B) to provide access to the anus.
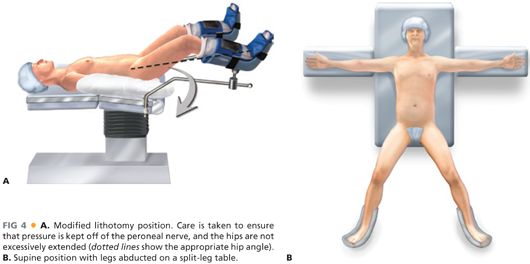
■ If a laparoscopic approach is used, consideration may be given to a position-assisting device, such as a beanbag, to prevent the patient from sliding during extreme positioning changes.
■ If stoma markings were performed preoperatively, these should be redrawn with a marker that will remain visible after skin preparation.
TECHNIQUES
LAPAROSCOPIC ELECTIVE SIGMOID COLECTOMY, BLADDER REPAIR
Abdominal Access and Port Placement
■ The abdomen may be accessed via a percutaneous Veress needle or an open Hasson cannula technique.
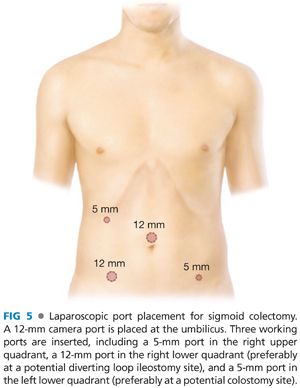
■ Typical port placement is depicted in FIG 5. A 12-mm camera port is placed at the umbilicus. Three working ports are inserted, including a 5-mm port in the right upper quadrant, a 12-mm port in the right lower quadrant (preferably at a potential diverting loop ileostomy site), and a 5-mm port in the left lower quadrant (preferably at a potential colostomy site).
Isolation and Division of the Inferior Mesenteric Artery Pedicle
■ In order to gain access to the proximal inferior mesenteric artery (IMA), the gastrocolic omentum is elevated over the transverse colon, into the upper abdomen, with the patient in steep Trendelenburg position, with the operating table tilted toward the right. The small bowel is brought to the right upper quadrant and out of the pelvis.
■ Elevation of the rectosigmoid mesentery with a grasper through the left lower quadrant port toward the left lower quadrant, demonstrates the IMA as a ridge in the sigmoid colon mesentery and exposes a plane between the mesentery and the retroperitoneum on the medial peritoneal fold of the mesentery. With cautery attached to Endo Shears brought through the right lower quadrant port, a long incision is made in the medial peritoneal fold of the mesentery along a clear space seen between the IMA and the retroperitoneum (FIG 6).
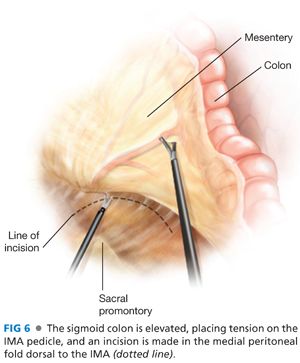
■ Through this incision, the left ureter, gonadal vessels, and retroperitoneal tissues are identified and dissected down off of the vessel and the mesentery (FIG 7A). If the left iliac artery and/or left psoas muscle are exposed, the plane of dissection must be brought more anteriorly. The left ureter is identified definitively by visualizing peristalsis.
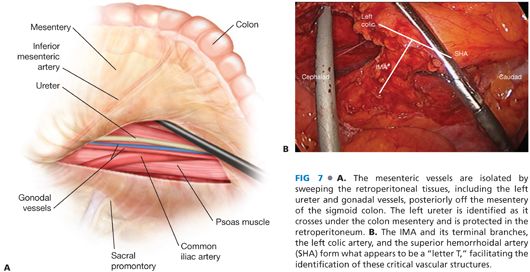
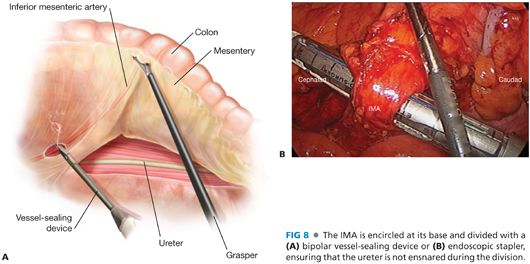
■ Once the left ureter has been identified and protected in the retroperitoneum, the IMA is encircled. The IMA and its terminal branches, the left colic artery, and the superior hemorrhoidal artery form what appears to be a “letter T,” facilitating the identification of these critical vascular structures (FIG 7B). The IMA is then divided. I prefer a bipolar vessel-sealing device (FIG 8A) but choices include vascular clips, endoscopic staplers (FIG 8B), or endoloops. When using an energy device for division, it is advisable to have endoloops available in the room as a backup to control bleeding from the divided pedicle in case of device failure.
Mobilization of the Descending Colon and Splenic Flexure
■
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








