Stomach
Every pathologist’s worst fear is missing a subtle signet ring cell carcinoma on a gastric biopsy, and all honest pathologists have indeed done so or will do so. One hopes to have his or her initiation during training and feel transiently ashamed rather than miss a lesion in clinical practice and be shamed. A good rule is to evaluate any sclerotic areas at high magnification. When encountering gastric polyps, it is good practice to evaluate the surrounding mucosa, which may show clues concerning the etiology of the polyp. The approach to high-grade neoplasms should be humble and include a panel of immunohistochemical stains to separate carcinomas, lymphomas, melanomas, and mesenchymal tumors.
POLYPS
Endoscopically and pathologically, gastric polyps are defined simply as projections above the adjacent mucosal surface. The polyp, itself, may be reactive/inflammatory, hamartomatous, or neoplastic. Gastric polyps can arise from, or be present in, tissue anywhere in the layers of the stomach wall. However, polyps that are endoscopically biopsied are most commonly epithelial lesions.
The classification of gastric epithelial polyps can be challenging histologically and can have important implications both for the clinical management of the polyp itself and for the remainder of the patient’s gastric mucosa. The first consideration in classification is to determine whether the polyp is dysplastic (pathologically equivalent to neoplastic) or nonneoplastic. The second consideration is to understand what implications that particular type of polyp might have for the patient’s remaining gastric mucosa. Unlike colonic polyps (most of which are isolated findings in an otherwise normal background mucosa), many gastric polyps arise in association with either inflammatory/atrophic gastritis or in association with inherited polyposis syndromes. Therefore, correct classification of gastric polyps, even innocuous-appearing polyps, may sometimes provide important clues to abnormalities in the surrounding stomach and elsewhere in the patient’s body. Unfortunately, some polyps defy current classification schemes and must simply be reported
descriptively with a comment as to whether they appear to have a risk of progression to carcinoma.
descriptively with a comment as to whether they appear to have a risk of progression to carcinoma.
HYPERPLASTIC POLYPS
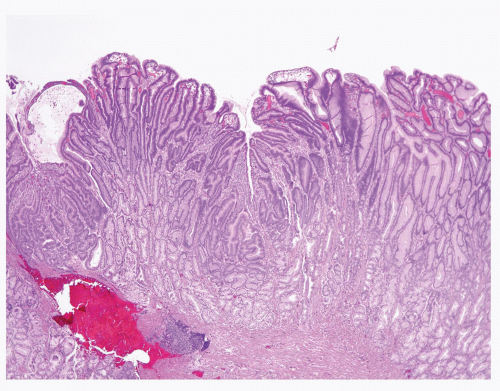
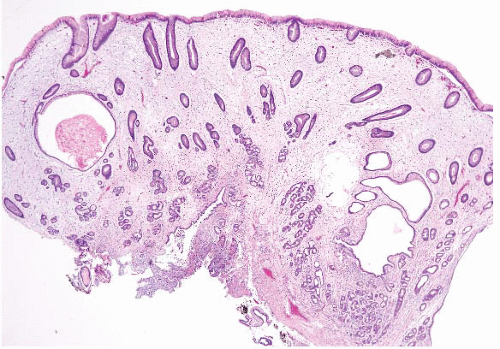
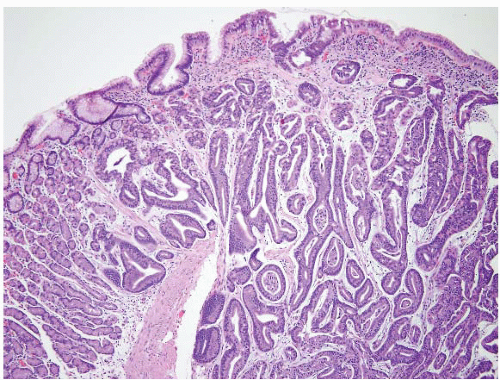
Hyperplastic polyps are among the most common gastric epithelial polyps (1). These polyps can range from a few millimeters to many centimeters (one hyperplastic polyp resected at our hospital was 9 cm in diameter) and, as such, may be mistaken endoscopically for carcinoma. They are composed of characteristic hyperplastic, elongated, and dilated foveolae within an edematous, inflamed stroma (Fig. 2.1, e-Figs. 2.1-2.7). Hyperplastic foveolae are generally lined with mature gastric mucin cells; foci of intestinal metaplasia are found in about 15% of polyps. The lining cells can be markedly reactive in appearance (especially when surface erosions are present), but true dysplasia arising in hyperplastic polyps is uncommon (Fig. 2.2). Dysplasia in hyperplastic polyps (Fig. 2.3, e-Fig. 2.7) is reported in <2% to nearly 20% of cases in the literature (2,3). In our review of 160 patients with gastric hyperplastic polyps, dysplasia was found in only 4% (4). Similarly, adenocarcinomas occasionally arise in these polyps (Fig. 2.4, e-Figs. 2.8 and 2.9), but this is unusual; Abraham et al. found adenocarcinoma within a hyperplastic polyp in only one (0.6%) of 160 patients (4). However, because of the risk of adenocarcinoma, we examine gastric hyperplastic polyps carefully.
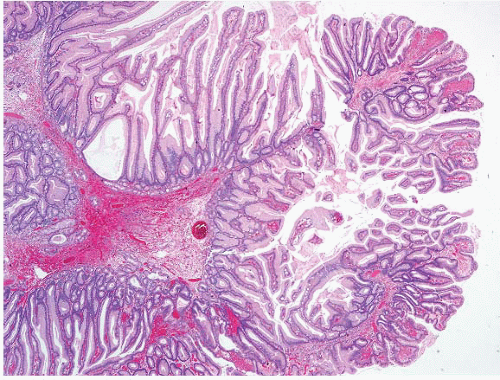 FIGURE 2.1 Gastric hyperplastic polyp. Hyperplastic polyps are characterized by elongated and dilated foveolae lined with mature gastric mucin cells within an edematous, inflamed stroma. |
Hyperplastic polyps may arise anywhere in the stomach, with only a slight preference for the antrum, and appear in multiples in approximately 20% of cases. The term “sentinel nodule” or “sentinel fold” is an old term for a hyperplastic polyp occurring at the gastroesophageal junction
(e-Figs. 2.10 and 2.11). Though initially considered to occur as a result of inflammation due to reflux, a recent study by Melton and Genta (5) that included 330 of such polyps and 120, 487 controls found no significant association of these lesions with inflammatory or neoplastic processes. Considered to be nonneoplastic lesions (although a range of molecular studies have focused on those examples that harbored neoplasia (6)), one of the major reasons to diagnose hyperplastic polyps histologically is their association with a wide range of background gastric mucosal abnormalities. They are most strongly associated with atrophic gastritis of either autoimmune or environmental (e.g., Helicobacter pylori-associated) types. But, many types of acute and chronic gastritis have been reported with hyperplastic polyps, occurring in the postantrectomy state, following therapy for gastric antral vascular ectasia (“watermelon stomach”), or with chemical (reactive) gastropathy (e-Fig. 2.12). They can be a response to any of a variety of injuries including caustic ingestion (e-Fig. 2.13). As a result, patients with hyperplastic polyps are at an increased risk for synchronous or metachronous adenocarcinomas arising in stomach tissue surrounding the polyp (6).
(e-Figs. 2.10 and 2.11). Though initially considered to occur as a result of inflammation due to reflux, a recent study by Melton and Genta (5) that included 330 of such polyps and 120, 487 controls found no significant association of these lesions with inflammatory or neoplastic processes. Considered to be nonneoplastic lesions (although a range of molecular studies have focused on those examples that harbored neoplasia (6)), one of the major reasons to diagnose hyperplastic polyps histologically is their association with a wide range of background gastric mucosal abnormalities. They are most strongly associated with atrophic gastritis of either autoimmune or environmental (e.g., Helicobacter pylori-associated) types. But, many types of acute and chronic gastritis have been reported with hyperplastic polyps, occurring in the postantrectomy state, following therapy for gastric antral vascular ectasia (“watermelon stomach”), or with chemical (reactive) gastropathy (e-Fig. 2.12). They can be a response to any of a variety of injuries including caustic ingestion (e-Fig. 2.13). As a result, patients with hyperplastic polyps are at an increased risk for synchronous or metachronous adenocarcinomas arising in stomach tissue surrounding the polyp (6).
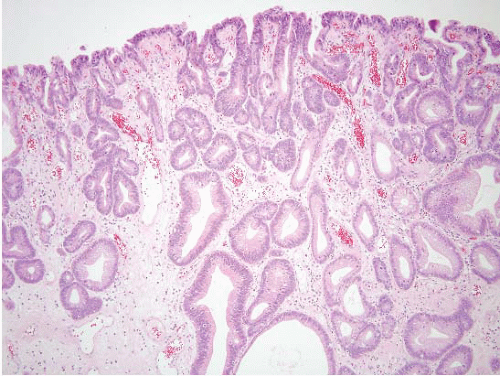 FIGURE 2.3 Gastric hyperplastic polyp with dysplasia. Though uncommon, dysplasia can be seen in association with hyperplastic polyps, as in this case. |
When we diagnose a large hyperplastic polyp in a patient who has not had corresponding biopsies of the surrounding flat mucosa, we often add a note in the report that underscores the relationship between hyperplastic polyps and background gastric atrophy or inflammatory gastritis. We also indicate that biopsies of the flat antrum and body may be helpful in further assessment. The frequent association between hyperplastic polyps and autoimmune gastritis has been extensively documented (e-Figs. 2.14-2.16) (7). As noted in Volume 1, Chapter 2, the diagnosis of autoimmune gastritis is suggested histologically when biopsies show corpus-predominant gastritis,
glandular atrophy, and intestinal metaplasia. To confirm this, we routinely perform immunostains for gastrin and chromogranin on the body biopsy for two purposes: (a) the lack of gastrin-labeled G cells confirms that the biopsy was, in fact, from the gastric body rather than transitional zone or mislabeled antrum, and (b) the presence of significant enterochromaffin-like (ECL) cell hyperplasia on the chromogranin immunostain reflects the hypergastrinemia typically present in autoimmune gastritis. Small well-differentiated neuroendocrine (carcinoid) tumors, like ECL cell hyperplasia, are also the consequence of excessive gastrin production by antral G cells that are stimulated under conditions of decreased gastric acidity. The diagnosis of autoimmune gastritis is of clinical consequence in this regard because the well-differentiated neuroendocrine tumors (WDNETs) (carcinoid tumors) that arise in response to prolonged hypergastrinemia (e.g., secondary to autoimmune gastritis or Zollinger-Ellison syndrome) differ in their behavior, prognosis, and management from sporadic gastric neuroendocrine tumors. These tumors are smaller, less infiltrative, are often multiple, almost never metastasize, and (in the case of autoimmune gastritis) respond to antrectomy to remove the source of gastrin stimulation.
glandular atrophy, and intestinal metaplasia. To confirm this, we routinely perform immunostains for gastrin and chromogranin on the body biopsy for two purposes: (a) the lack of gastrin-labeled G cells confirms that the biopsy was, in fact, from the gastric body rather than transitional zone or mislabeled antrum, and (b) the presence of significant enterochromaffin-like (ECL) cell hyperplasia on the chromogranin immunostain reflects the hypergastrinemia typically present in autoimmune gastritis. Small well-differentiated neuroendocrine (carcinoid) tumors, like ECL cell hyperplasia, are also the consequence of excessive gastrin production by antral G cells that are stimulated under conditions of decreased gastric acidity. The diagnosis of autoimmune gastritis is of clinical consequence in this regard because the well-differentiated neuroendocrine tumors (WDNETs) (carcinoid tumors) that arise in response to prolonged hypergastrinemia (e.g., secondary to autoimmune gastritis or Zollinger-Ellison syndrome) differ in their behavior, prognosis, and management from sporadic gastric neuroendocrine tumors. These tumors are smaller, less infiltrative, are often multiple, almost never metastasize, and (in the case of autoimmune gastritis) respond to antrectomy to remove the source of gastrin stimulation.
The pathogenesis of hyperplastic polyps is not known, but they are thought to represent an exaggerated, regenerative response to mucosal injury (e-Figs. 2.12-2.16). The histologic appearance of hyperplastic polyps overlaps significantly with (a) conditions of generalized gastric mucosal hyperplasia (Ménétrier disease) (Fig. 2.5, e-Figs. 2.17-2.19 and Volume 1 Fig. 2.59, e-Figs. 2.126 and 2.127), Cronkhite-Canada syndrome, and
(b) hamartomatous polyps and syndromes involving the stomach. As noted in Volume 1, Chapter 2, Ménétrier disease shows marked foveolar hyperplasia with abundant mucus production, glandular atrophy, and an edematous but typically uninflamed lamina propria, changes that are most commonly limited to the body and fundus. Knowledge of the endoscopic appearance of giant folds, the clinical scenario of hypoproteinemia and peripheral edema, and lack of intervening normal mucosa can help distinguish Ménétrier disease from hyperplastic polyp. However, the changes may be histologically indistinguishable based on a single isolated biopsy.
(b) hamartomatous polyps and syndromes involving the stomach. As noted in Volume 1, Chapter 2, Ménétrier disease shows marked foveolar hyperplasia with abundant mucus production, glandular atrophy, and an edematous but typically uninflamed lamina propria, changes that are most commonly limited to the body and fundus. Knowledge of the endoscopic appearance of giant folds, the clinical scenario of hypoproteinemia and peripheral edema, and lack of intervening normal mucosa can help distinguish Ménétrier disease from hyperplastic polyp. However, the changes may be histologically indistinguishable based on a single isolated biopsy.
HAMARTOMATOUS/SYNDROMIC POLYPS IN THE STOMACH
Hamartomas are polypoid lesions formed from disorganized tissue elements that are native to that site. Hamartomatous syndromes that may involve the stomach include Peutz-Jeghers syndrome, juvenile polyposis, and, less commonly, Cowden disease. Peutz-Jeghers is an autosomal-dominant condition caused by germline mutations in the LBK1/STK11 gene on chromosome 19p13.3, and is characterized by polyposis and distinctive melanin pigmentation around the lips, buccal (cheek) mucosa, and sometimes eyelids and hands. Because the pigment may fade after puberty, the syndrome is not excluded—even if pigment is absent—in an adult presentation. The polyps of Peutz-Jeghers syndrome primarily occur in the small bowel (65%) and are slightly less common in the colon and stomach (8,9). Importantly, unlike the small bowel polyps that show prominent arborization of the muscularis mucosae, gastric Peutz-Jeghers polyps are composed mostly of dilated or branching mucus-filled pits and may have relatively inconspicuous smooth muscle (Fig. 2.6, e-Figs. 2.20-2.23). Occasional examples of gastric Peutz-Jeghers polyps have the classic arborizing architecture with strands of smooth muscle (e-Figs. 2.22 and 2.23), but most have less specific features (but some degree of smooth muscle proliferation) (e-Figs. 2.20 and 2.21). Essentially, they are best distinguished from hyperplastic polyps by correlation with the history, a reality that is quite humbling for the pathologist (10). However, the background gastric mucosa is usually normal in patients who have Peutz-Jeghers polyps (in contrast to the frequently abnormal background mucosa in patients with hyperplastic polyps). While dysplasia is rare in these polyps, patients with Peutz-Jeghers syndrome are at a significant risk for gastric and other adenocarcinomas developing outside of the hamartomas (8). Since the stakes of a diagnosis of Peutz-Jeghers syndrome are high, and the polyps are diagnosed unreliably on gastric biopsies, we would not base a diagnosis of Peutz-Jeghers polyposis on the findings of a gastric lesion alone (10).
Juvenile polyposis is a genetically heterogeneous condition in which some families have autosomal dominant germline mutations in the DPC4 gene on chromosome 18q21. The polyps in juvenile polyposis can be limited to the colon or can be generalized, involving the colon, small bowel,
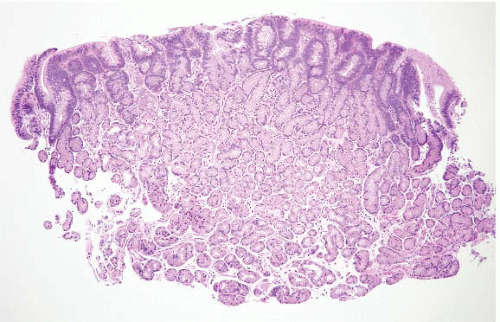
and stomach. There are additional reports of patients who appear to have juvenile polyposis predominantly confined to the stomach. Juvenile polyps involving the stomach frequently show a rounded surface contour with superficial mucosal erosions and an abundant, edematous, and inflamed lamina propria (Fig. 2.7, e-Figs. 2.24-2.30). The foveolae are frequently hyperplastic and dilated. Superimposed epithelial dysplasia, also termed mixed adenomatous/juvenile polyps, occurs in up to one-third of juvenile polyps. Cowden disease is an autosomal-dominant condition that is relatively poorly characterized, but some families have germline mutations in the PTEN gene on chromosome 10q22-23. The gastrointestinal (GI) polyps in Cowden disease are usually a minor component of the syndrome (affected individuals typically show more prominent facial trichilemmomas and oral papillomas, as well as being at an increased risk for breast and thyroid carcinomas). Detailed descriptions of gastric polyps in Cowden disease have not been made, but they are reported to be histologically indistinguishable from small hyperplastic polyps (11). Dysplasia has not been reported in these polyps.
and stomach. There are additional reports of patients who appear to have juvenile polyposis predominantly confined to the stomach. Juvenile polyps involving the stomach frequently show a rounded surface contour with superficial mucosal erosions and an abundant, edematous, and inflamed lamina propria (Fig. 2.7, e-Figs. 2.24-2.30). The foveolae are frequently hyperplastic and dilated. Superimposed epithelial dysplasia, also termed mixed adenomatous/juvenile polyps, occurs in up to one-third of juvenile polyps. Cowden disease is an autosomal-dominant condition that is relatively poorly characterized, but some families have germline mutations in the PTEN gene on chromosome 10q22-23. The gastrointestinal (GI) polyps in Cowden disease are usually a minor component of the syndrome (affected individuals typically show more prominent facial trichilemmomas and oral papillomas, as well as being at an increased risk for breast and thyroid carcinomas). Detailed descriptions of gastric polyps in Cowden disease have not been made, but they are reported to be histologically indistinguishable from small hyperplastic polyps (11). Dysplasia has not been reported in these polyps.
There are some clinical and pathologic clues that can help distinguish between gastric hyperplastic polyps and hamartomatous polyps: (a) the patient may have a previously characterized polyposis syndrome; (b) there may be biopsies of the nonpolypoid gastric mucosa showing an atrophic or inflammatory gastropathy of the type associated with the development of hyperplastic polyps; (c) hyperplastic polyps frequently show a more
lobulated or villiform surface, as compared to the often rounded surface of juvenile polyps; and (d) hyperplastic polyps often contain a more prominent edematous, inflamed lamina propria when compared with Peutz-Jeghers polyps, which can sometimes (but not always) show smooth muscle arborization. However, we do not believe that it is possible to distinguish between a hyperplastic or hamartomatous polyp based solely on the histologic features of a polyp that is resected or biopsied. In the absence of clinical information and knowledge of the histology of the background gastric mucosa, we state in the diagnostic report that the polyp is histologically consistent with a hyperplastic polyp and give the differential diagnosis listed above.
lobulated or villiform surface, as compared to the often rounded surface of juvenile polyps; and (d) hyperplastic polyps often contain a more prominent edematous, inflamed lamina propria when compared with Peutz-Jeghers polyps, which can sometimes (but not always) show smooth muscle arborization. However, we do not believe that it is possible to distinguish between a hyperplastic or hamartomatous polyp based solely on the histologic features of a polyp that is resected or biopsied. In the absence of clinical information and knowledge of the histology of the background gastric mucosa, we state in the diagnostic report that the polyp is histologically consistent with a hyperplastic polyp and give the differential diagnosis listed above.
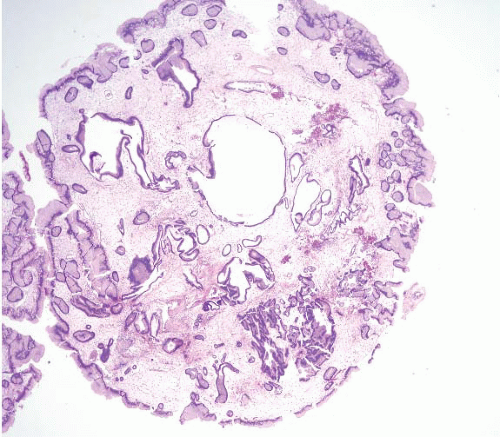 FIGURE 2.7 Gastric juvenile polyp. Note the rounded surface contour with an abundant, edematous, and inflamed lamina propria. The foveolae appear hyperplastic and dilated. |
Cronkhite-Canada Polyps
Cronkhite-Canada syndrome (12) is characterized by diffuse polyposis occurring in patients with unusual ectodermal abnormalities, including alopecia, onychodystrophy (this means fingernails that are falling apart), and skin hyperpigmentation. Europeans and Asians are most frequently affected, with a mean age of onset at 59 years. Several hundred cases of Cronkhite-Canada syndrome have been reported worldwide, the majority of these reports originating from Japan, probably as a result of the popularity of case reports in Japan. The male to female ratio is 3:2. Potentially
fatal complications, such as malnutrition, GI bleeding, and infection, often occur, and the mortality rate has been reported to be as high as 60%. Neither a familial association nor a genetic defect is known.
fatal complications, such as malnutrition, GI bleeding, and infection, often occur, and the mortality rate has been reported to be as high as 60%. Neither a familial association nor a genetic defect is known.
The most common presenting symptoms include diarrhea, weight loss, nausea, vomiting, hypogeusia, and anorexia. Paraesthesias, seizures, and tetany, apparently related to electrolyte abnormalities, have also been reported. Mucoid diarrhea results in the depletion of the patient’s protein reserves such that the patient loses his (usually) hair and nails. Nail dystrophy with thinning, splitting, and separation from the nailbeds are the typical nail features. Both scalp and body hair alopecia may be present. Diffuse hyperpigmentation of the skin, manifested by light to dark brown macular lesions, is seen most frequently on the extremities, face, palms, soles, and neck. Microscopic examination of biopsied skin reveals abnormally increased melanin deposition with or without increased melanocyte proliferation.
Cronkhite-Canada syndrome is distinguished by the diffuse distribution of polyps throughout the entire GI tract, except for characteristic sparing of the esophagus. The question of whether polyps in Cronkhite-Canada syndrome possess malignant potential remains controversial.
A number of complications may occur with Cronkhite-Canada syndrome and can contribute to poor outcomes in patients with this disease. These include potentially fatal GI bleeding, intussusception, and prolapse. Electrolyte abnormalities, dehydration, protein-losing enteropathy, and other nutritional deficiencies due to malabsorption can complicate the course of the disease. Cronkhite-Canada syndrome patients are prone to recurrent infections, but it is not known whether this is related to malnutrition or is a primary immunological deficiency.
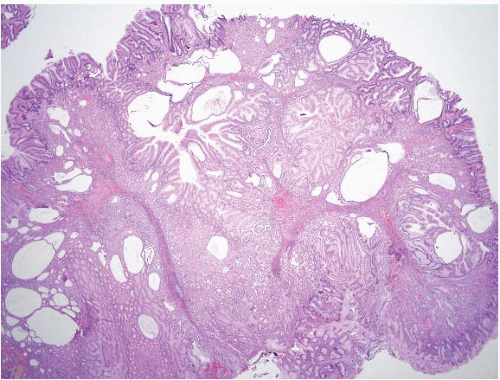
The Cronkhite-Canada polyp is characterized by its broad sessile base, expanded edematous lamina propria, and cystic glands (13) (Fig. 2.8, e-Figs. 2.31-2.34). Similar features are found in the lesions of juvenile polyposis. The only distinguishing feature reported between Cronkhite-Canada and colonic juvenile polyposis was the pedunculated growth of the latter; a feature that did not hold for gastric lesions. Unlike Cronkhite-Canada polyps, juvenile polyps sometimes have areas of dysplasia, but this is not typical. Therefore, the diagnosis of Cronkhite-Canada polyps (especially in the stomach) requires correlation with the presence of the ectodermal changes characteristic of this syndrome. The most helpful histologic feature is that the flat mucosa is abnormal and edematous (it is usually normal in the gastric mucosa of patients with gastric juvenile polyps). This is yet another condition for which we strongly encourage our gastroenterology colleagues to be vigilant about sampling flat as well as polypoid mucosa.
Fundic Gland Polyps
Fundic gland polyps (FGPs) occur in two forms: sporadic and those associated with familial adenomatous polyposis (FAP). FGPs were originally described in patients with FAP (e-Fig. 2.35) and were believed to be a
manifestation of that syndrome, but they are now recognized to be one of the most common gastric polyps (along with hyperplastic polyps) in individuals without FAP. Sporadic FGPs are found in 1% to 2% of routine upper endoscopic examinations, and are most common in middle-aged females. They are typically small (a few millimeters and only rarely >1 cm), sessile, and dome shaped. Sporadic FGPs may be single, but are commonly multiple (usually a few polyps). Rarely, patients without FAP have carpeting of the body and fundus by numerous FGPs, in a manner that resembles a polyposis syndrome (e-Fig. 2.36). Unlike hyperplastic polyps, though, FGPs are not particularly associated with any type of inflammatory or atrophic background mucosal pathology. Their presence is inversely correlated with H. pylori infection (14). They are usually an incidental finding. No symptoms or clinical manifestations (e.g., GI bleeding) can be ascribed to them. However, some studies do indicate a temporal association between the use of proton pump inhibitors and the development of FGPs (15). This potential link is controversial and has not been demonstrated in any controlled prospective trial (16).
manifestation of that syndrome, but they are now recognized to be one of the most common gastric polyps (along with hyperplastic polyps) in individuals without FAP. Sporadic FGPs are found in 1% to 2% of routine upper endoscopic examinations, and are most common in middle-aged females. They are typically small (a few millimeters and only rarely >1 cm), sessile, and dome shaped. Sporadic FGPs may be single, but are commonly multiple (usually a few polyps). Rarely, patients without FAP have carpeting of the body and fundus by numerous FGPs, in a manner that resembles a polyposis syndrome (e-Fig. 2.36). Unlike hyperplastic polyps, though, FGPs are not particularly associated with any type of inflammatory or atrophic background mucosal pathology. Their presence is inversely correlated with H. pylori infection (14). They are usually an incidental finding. No symptoms or clinical manifestations (e.g., GI bleeding) can be ascribed to them. However, some studies do indicate a temporal association between the use of proton pump inhibitors and the development of FGPs (15). This potential link is controversial and has not been demonstrated in any controlled prospective trial (16).
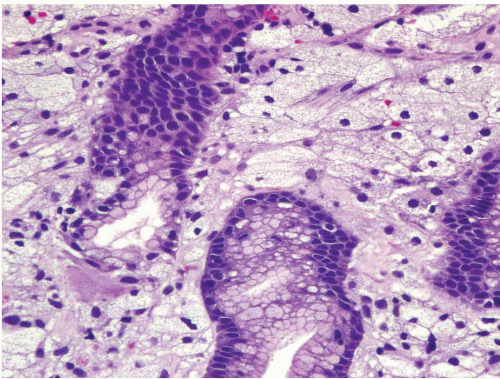
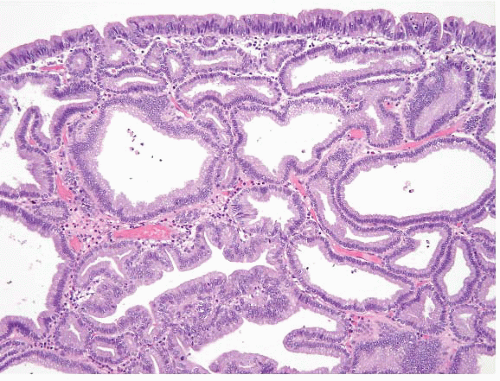
The morphology of both FGPs and proton pump inhibitor effects overlaps considerably. An endoscopic nodule correlates with dilated oxyntic glands, some of which contain cells with apocrine-like snouts (Fig. 2.9, e-Figs. 2.37-2.40). Because it is sometimes difficult to distinguish between these two processes, correlation relies chiefly on the presence of an endoscopic lesion.
FGPs associated with FAP syndrome differ from sporadic FGPs on several epidemiologic and clinicopathologic points. For example, FAP-associated FGPs occur in a majority of patients with FAP (reported
frequencies range from 12.5% to 84% of FAP patients, depending on their age at endoscopy), and show a more equal gender distribution. They are more numerous than sporadic FGPs, and hence patients with FAP are more likely to have fundic gland “polyposis.” FAP-associated FGPs also occur at younger ages, including children (FGPs are vanishingly rare in the non-FAP pediatric population; a retrospective search for all FGPs biopsied in children without FAP using a computerized pathology database spanning 16 years revealed only three cases at our hospital). In addition, approximately 25% of FAP-associated FGPs demonstrate low-grade epithelial dysplasia (Fig. 2.10, e-Figs. 2.41 and 2.42) (17). Dysplasia in sporadic FGPs can occur but is distinctly unusual and has never been associated with progression to carcinoma (14). A retrospective histologic evaluation of several hundred sporadic FGPs at our hospital revealed <1% with lowgrade dysplasia, and one of these cases was subsequently determined to be a member of an attenuated FAP family (17).
frequencies range from 12.5% to 84% of FAP patients, depending on their age at endoscopy), and show a more equal gender distribution. They are more numerous than sporadic FGPs, and hence patients with FAP are more likely to have fundic gland “polyposis.” FAP-associated FGPs also occur at younger ages, including children (FGPs are vanishingly rare in the non-FAP pediatric population; a retrospective search for all FGPs biopsied in children without FAP using a computerized pathology database spanning 16 years revealed only three cases at our hospital). In addition, approximately 25% of FAP-associated FGPs demonstrate low-grade epithelial dysplasia (Fig. 2.10, e-Figs. 2.41 and 2.42) (17). Dysplasia in sporadic FGPs can occur but is distinctly unusual and has never been associated with progression to carcinoma (14). A retrospective histologic evaluation of several hundred sporadic FGPs at our hospital revealed <1% with lowgrade dysplasia, and one of these cases was subsequently determined to be a member of an attenuated FAP family (17).
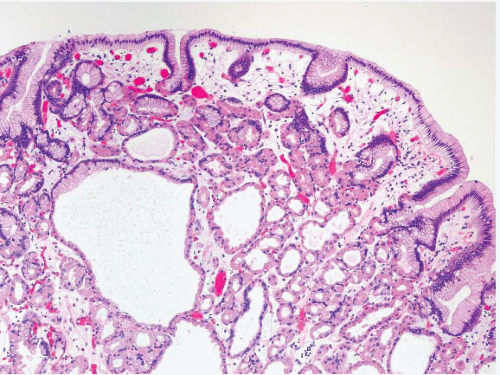 FIGURE 2.9 Fundic gland polyp (FGP). FGPs show dilated oxyntic glands, some of which contain cells with apocrine-like snouts. |
The natural history of FGPs is interesting because both sporadic and FAP-associated FGPs can increase, decrease, or remain constant in number, as seen when patients are followed-up with serial upper endoscopic examinations. Sporadic FGPs, even fundic gland polyposis, have never been reported to progress to gastric adenocarcinoma. Several cases of adenocarcinoma associated with fundic gland polyposis in patients with FAP exist, but this is a reportable occurrence (18,19). Currently, upper endoscopic surveillance in patients with FAP is performed mainly to address the increased risk for duodenal polyposis (>300-fold) and particularly periampullary adenomas and adenocarcinomas (20). The presence
of dysplastic or nondysplastic FGPs is regarded as an incidental finding and would virtually never require surgical resection. Even surveillance of dysplastic FGPs remains controversial.
of dysplastic or nondysplastic FGPs is regarded as an incidental finding and would virtually never require surgical resection. Even surveillance of dysplastic FGPs remains controversial.
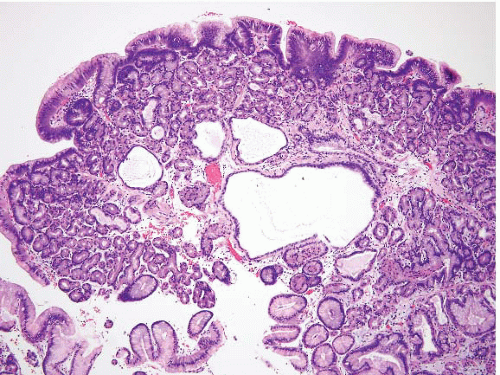 FIGURE 2.10 Fundic gland polyp with low-grade dysplasia. Dysplasia in sporadic fundic gland polyps is unusual but is more common in polyps occurring in the setting of FAP. |
As their name implies, FGPs develop only in the gastric body and fundus; they do not arise in the antrum. In patients with FAP, FGPs are more common than gastric adenomas, despite the fact that the colorectal and duodenal polyps in these patients are adenomas. When gastric adenomas do develop in the FAP setting, they are usually antral. Despite the increased frequency of dysplastic FGPs and gastric adenomas in FAP, the risk of gastric adenocarcinoma is only slightly increased (approximately twofold in one study) and is not significantly increased statistically in Western FAP patients (20). This contrasts with studies of Asian patients with FAP, among whom the risk of gastric adenocarcinoma is increased approximately 10-fold. This low risk in Western FAP patients also contrasts with the much higher risk of gastric adenocarcinoma in non-FAP patients who have adenomas. This most likely reflects the fact that germline mutations in the APC gene on chromosome 5q21 leading to FAP do not predispose patients to background gastritis or gastric atrophy. Most Western patients with FAP have normal gastric mucosa outside of any antral adenomas or body FGPs. Most patients with sporadic gastric adenomas, in contrast, have chronic atrophic gastritis (particularly H. pylori-mediated) that predisposes to gastric adenocarcinoma in the intestinalized background gastric mucosa outside of the adenomas.
Pancreatic Acinar Cell Metaplasia/Heterotopia
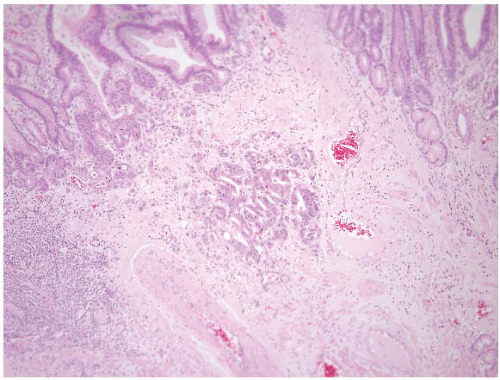
This finding is generally incidental in the esophagus, as well as in the stomach, duodenum, and jejunum. But, we have occasionally seen it mistaken for a well-differentiated neuroendocrine (carcinoid) tumor, and the contributing pathologists are confused by the lack of expression of neuroendocrine markers. Occasionally, heterotopic pancreatic tissue can produce a symptomatic mass in the gastric antrum that results in gastric outlet obstruction (21). Endoscopically, the lesion may have central umbilication with associated mucosal erosion. Other than this occurrence, it is of little clinical significance. The heterotopic tissue may contain an admixture of pancreatic acinar tissue, ducts, and islet cells in varying proportions (Fig. 2.11, e-Figs. 2.43-2.51) (22). Since the lesion is often submucosal, superficial biopsies (containing only the overlying mucosa) may be nondiagnostic. As mentioned in Volume 1, encountering pancreatic metaplasia in oxyntic mucosa should prompt the pathologist to consider a diagnosis of autoimmune metaplastic atrophic gastritis (AMAG) as it has been observed in 50% of patients with AMAG and is rare in other gastritides (23).
GASTRIC XANTHOMAS
Gastric xanthomas are pale yellow nodules or plaques, usually <3 mm in size, located in the gastric mucosa. They are frequently found in groups, most often along the lesser curvature and pyloric regions. They consist of lipid-laden histiocytes in the lamina propria, occasionally extending into
the submucosa (Fig. 2.12, e-Figs. 2.52 and 2.53). There is no correlation between gastric xanthomas and hypercholesterolemia. The lesion is of little significance, except that it may be misdiagnosed as carcinoma (24). However, in any concerning case, performing a keratin and CD68 stain can be reassuring. Some authors have associated gastric xanthomas with atrophic gastritis and Helicobacter gastritis (25).
the submucosa (Fig. 2.12, e-Figs. 2.52 and 2.53). There is no correlation between gastric xanthomas and hypercholesterolemia. The lesion is of little significance, except that it may be misdiagnosed as carcinoma (24). However, in any concerning case, performing a keratin and CD68 stain can be reassuring. Some authors have associated gastric xanthomas with atrophic gastritis and Helicobacter gastritis (25).
GASTRIC ADENOMAS AND GASTRIC DYSPLASIA
Intestinal and Gastric Foveolar-type Gastric Adenomas
Much literature in this area has been difficult to interpret. Although there is massive experience with gastric cancer and its precursors in Japan, pathologists there have used different diagnostic criteria from those in the West as pointed out by Lauwers et al. in 1999 (26). The observation that Japanese pathologists did not require invasion to diagnose carcinoma (and “invasion” was not listed as a criterion in the 1990 World Health Organization [WHO] classification) presumably has informed their far better cure rates for early carcinomas, compared to results in Western countries. Based on these observations, an international panel convened in Vienna published consensus definitions of gastric epithelial neoplasia in 2000 (Table 2.1) (27), which distinguished between noninvasive lesions and invasive ones.
Currently, if such a lesion produces a polyp, it is referred to as an adenoma (Fig. 2.13, e-Figs. 2.54-2.60) and the dysplasia is graded, whereas flat lesions are termed “dysplasia” and are graded using criteria similar to those in the esophagus. This is not particularly scientific, but is a convention
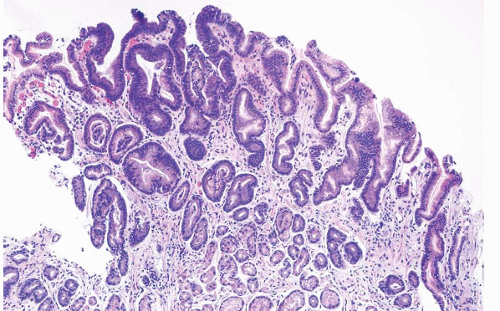
that has worked. Both types of lesions confer risk, and both require sampling of the entire stomach to exclude invasive carcinoma. As in the case of gastric hyperplastic polyps, the background pathology is important. To illustrate this, Abraham et al. studied (61) gastric adenomas from 51 patients between 1985 and 2001 (28). The adenomas were classified as intestinal-type, containing at least focal goblet cells and/or Paneth cells
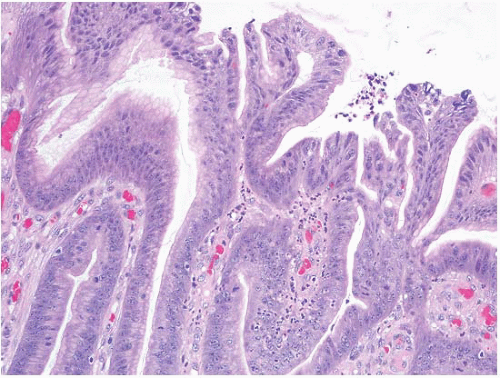
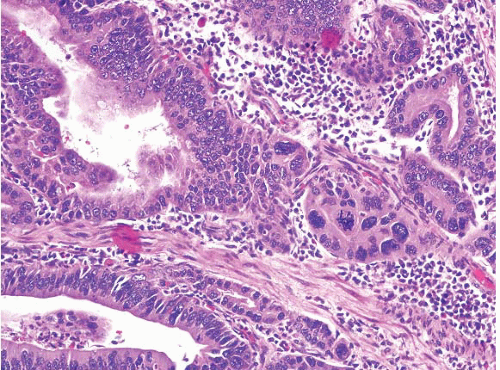
(Fig. 2.13, e-Figs. 2.54-2.60); gastric foveolar type, lined entirely by gastric mucin cells, as shown on periodic acid Schiff/Alcian blue staining (PAS/AB) (Fig. 2.14, e-Figs. 2.61-2.64); or indeterminate. The histologic features of both the adenomas (location, multiplicity, degree of dysplasia, and presence of adenocarcinoma within the polyp) and the surrounding gastric mucosa (presence of gastritis, intestinal metaplasia, and adenocarcinoma) were evaluated. Gastric adenomas were distributed equally throughout the stomach, were most frequently solitary (82%), and contained adenocarcinoma in nine cases (14.8%). There were 34 intestinal-type adenomas (56%) in 31 patients, 25 gastric foveolar-type adenomas (41%) in 18 patients (including 10 patients with FAP), and two indeterminate type (3%). Intestinal-type adenomas were significantly more likely than gastric foveolar-type adenomas to show high-grade dysplasia (p < 0.0001) (Fig. 2.15, e-Figs. 2.65-2.68), adenocarcinoma within the polyp (p = 0.016), intestinal metaplasia in the surrounding stomach (p < 0.000001), and gastritis (p = 0.002). Patients with intestinal-type adenomas were also more likely to have separate adenocarcinomas, as seen in five cases (100%), although this did not reach statistical significance. Youn Park et al. found that foveolar-type gastric dysplasia is more commonly associated with an endoscopically flat lesion compared to adenomatous-type dysplasia in a study that included 69 cases (31 adenomatous, 23 hybrid [adenomatous and foveolar], and 15 foveolar type). In the same study, adenomatous-type dysplasia was more often located in the gastric body/fundus, was larger, and was more likely to be multifocal. These differences, however, were not
statistically significant. In contrast to the work of Abraham and colleagues, the same group found that foveolar and hybrid-type dysplasia (defined by at least 10% of a second phenotype) were significantly more likely to display high-grade morphology than the adenomatous-type (p = 0.046 and 0.008, respectively). In addition, intestinal metaplasia was identified in the background mucosa in all cases of foveolar, hybrid, and adenomatous dysplasia. Furthermore, foveolar-type dysplasia showed more recurrence than the hybrid or adenomatous types (29). These dissimilar findings between the two studies are probably best explained by differences in the populations studied (United States vs. Korean) and exclusion criteria. In the Abraham study, for example, the patients with gastric foveolar-type gastric adenomas lacked intestinal metaplasia in their background flat mucosa. Regardless of the type of dysplasia, complete removal of the adenoma should be performed as a fraction of these progresses to high-grade dysplasia or invasive cancer. A retrospective study of 27 gastric adenomas found 8 lesions with progression: 1 adenoma with high-grade dysplasia and 3 with low-grade dysplasia progressed to invasive cancer, and 4 with low-grade dysplasia progressed to noninvasive high-grade dysplasia (30). For pathologists practicing in the United States, the Abraham criteria are most applicable and allow the pathologist to assign risk categories for gastric adenomas; since the “gastric foveolar-type adenoma” is rare, unassociated with background pathology and appears as an isolated sporadic lesion akin to a colonic sporadic adenoma, the patients are at low risk (28,31).
that has worked. Both types of lesions confer risk, and both require sampling of the entire stomach to exclude invasive carcinoma. As in the case of gastric hyperplastic polyps, the background pathology is important. To illustrate this, Abraham et al. studied (61) gastric adenomas from 51 patients between 1985 and 2001 (28). The adenomas were classified as intestinal-type, containing at least focal goblet cells and/or Paneth cells
(Fig. 2.13, e-Figs. 2.54-2.60); gastric foveolar type, lined entirely by gastric mucin cells, as shown on periodic acid Schiff/Alcian blue staining (PAS/AB) (Fig. 2.14, e-Figs. 2.61-2.64); or indeterminate. The histologic features of both the adenomas (location, multiplicity, degree of dysplasia, and presence of adenocarcinoma within the polyp) and the surrounding gastric mucosa (presence of gastritis, intestinal metaplasia, and adenocarcinoma) were evaluated. Gastric adenomas were distributed equally throughout the stomach, were most frequently solitary (82%), and contained adenocarcinoma in nine cases (14.8%). There were 34 intestinal-type adenomas (56%) in 31 patients, 25 gastric foveolar-type adenomas (41%) in 18 patients (including 10 patients with FAP), and two indeterminate type (3%). Intestinal-type adenomas were significantly more likely than gastric foveolar-type adenomas to show high-grade dysplasia (p < 0.0001) (Fig. 2.15, e-Figs. 2.65-2.68), adenocarcinoma within the polyp (p = 0.016), intestinal metaplasia in the surrounding stomach (p < 0.000001), and gastritis (p = 0.002). Patients with intestinal-type adenomas were also more likely to have separate adenocarcinomas, as seen in five cases (100%), although this did not reach statistical significance. Youn Park et al. found that foveolar-type gastric dysplasia is more commonly associated with an endoscopically flat lesion compared to adenomatous-type dysplasia in a study that included 69 cases (31 adenomatous, 23 hybrid [adenomatous and foveolar], and 15 foveolar type). In the same study, adenomatous-type dysplasia was more often located in the gastric body/fundus, was larger, and was more likely to be multifocal. These differences, however, were not
statistically significant. In contrast to the work of Abraham and colleagues, the same group found that foveolar and hybrid-type dysplasia (defined by at least 10% of a second phenotype) were significantly more likely to display high-grade morphology than the adenomatous-type (p = 0.046 and 0.008, respectively). In addition, intestinal metaplasia was identified in the background mucosa in all cases of foveolar, hybrid, and adenomatous dysplasia. Furthermore, foveolar-type dysplasia showed more recurrence than the hybrid or adenomatous types (29). These dissimilar findings between the two studies are probably best explained by differences in the populations studied (United States vs. Korean) and exclusion criteria. In the Abraham study, for example, the patients with gastric foveolar-type gastric adenomas lacked intestinal metaplasia in their background flat mucosa. Regardless of the type of dysplasia, complete removal of the adenoma should be performed as a fraction of these progresses to high-grade dysplasia or invasive cancer. A retrospective study of 27 gastric adenomas found 8 lesions with progression: 1 adenoma with high-grade dysplasia and 3 with low-grade dysplasia progressed to invasive cancer, and 4 with low-grade dysplasia progressed to noninvasive high-grade dysplasia (30). For pathologists practicing in the United States, the Abraham criteria are most applicable and allow the pathologist to assign risk categories for gastric adenomas; since the “gastric foveolar-type adenoma” is rare, unassociated with background pathology and appears as an isolated sporadic lesion akin to a colonic sporadic adenoma, the patients are at low risk (28,31).
TABLE 2.1 Vienna Classification of GI Epithelial Neoplasia | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
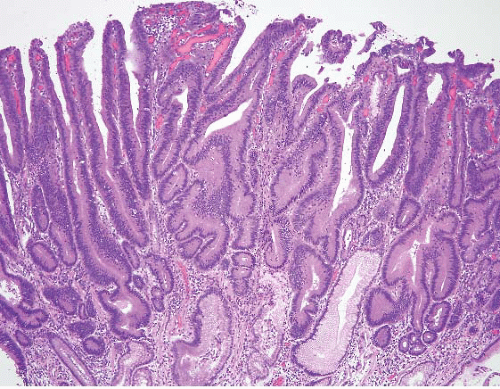 FIGURE 2.13 Gastric adenoma. An example of gastric adenoma, intestinal type, showing low-grade dysplasia is seen here. |
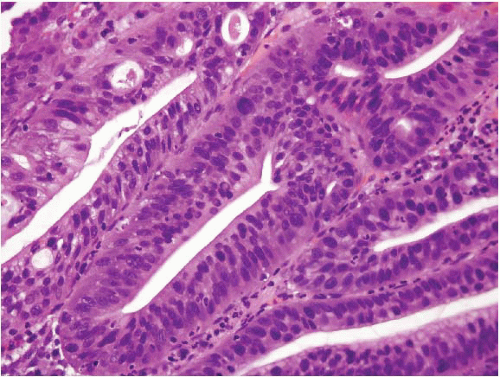 FIGURE 2.15 Gastric adenoma, high-grade dysplasia. Gastric intestinal-type adenoma showing high-grade dysplasia. |
Since gastric adenomas are rarely true “sporadic” lesions (the vast majority in daily practice in the United States are of the intestinal type and
associated with background intestinal metaplasia), thorough sampling of the surrounding gastric mucosa is essential to understand the clinicopathologic context of the adenoma. A Korean study also found an increased risk of colorectal adenomas (48.3% vs. 33.3% in control group, p = 0.022) in patients with gastric adenomas, arguing for screening colonoscopies in patients with this type of gastric polyp (32).
associated with background intestinal metaplasia), thorough sampling of the surrounding gastric mucosa is essential to understand the clinicopathologic context of the adenoma. A Korean study also found an increased risk of colorectal adenomas (48.3% vs. 33.3% in control group, p = 0.022) in patients with gastric adenomas, arguing for screening colonoscopies in patients with this type of gastric polyp (32).
Flat dysplasia is typically encountered incidentally also in the setting of atrophy as a result of long-standing injury due to Helicobacter infection or autoimmune atrophic gastritis. There are no guidelines regarding patient management in this situation as the issue of flat dysplasia is difficult to address since these are endoscopically undetectable lesions (Fig. 2.16, e-Fig. 2.69). Low-grade dysplasia has been reported to “regress,” persist, and progress to a higher grade dysplastic lesion/adenocarcinoma in 53.3%, 31.1%, and 6.6%/8.8% of cases, respectively. High-grade lesions, however, are unlikely to regress and are associated with a significant risk of progression to invasive carcinoma, reported at 69% (11/16 patients) by Rugge et al. (in this study, however, it was unclear if the studied cases represented flat or polypoid lesions) (33). Of course, “regression” in the setting of flat lowgrade dysplasia is a term that must be used with caution as these lesions are not grossly evident and are unlikely to be “spotted” endoscopically. In the study by Rugge et al. “regression” required (a) at least two “negative” endoscopic examinations and (b) follow-up of at least 12 months after the first negative biopsy. As a result of its low likelihood of progression, it is difficult to justify surgical intervention in a patient with low-grade dysplasia. These patients are typically followed endoscopically and their stomachs mapped with multiple biopsies. High-grade dysplastic lesions are typically managed with endoscopic mucosal resection (EMR) or surgical resection.
Pyloric Gland Adenoma
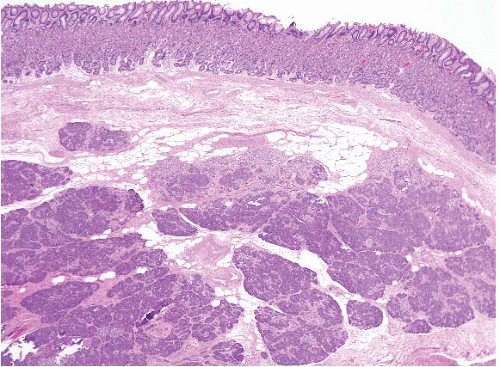
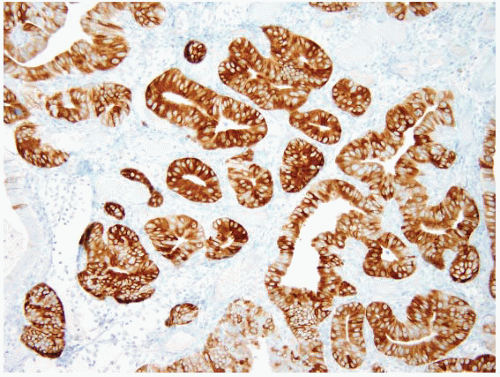
These lesions have been mentioned briefly over the years and in the 1990 WHO classification of gastric neoplasms but were fully characterized in 2003 by Vieth et al. (34). Pyloric gland adenoma (PGA) is a neoplastic polyp known to occur in the stomach, gallbladder, duodenum, and main pancreatic duct. These polyps show a preference for the gastric corpus, account for 2.7% of all gastric polyps, and are typically seen in older patients (median age 73 years); gastric examples show a remarkable female predominance (31,34). More than one-third occur in patients with AMAG (31,34) and account for 10% of polyps found in patients with AMAG (7). H. pylori or chemical gastritis may also be present in the background mucosa (34). Histologically, these polyps are composed of closely packed pyloric-type glands with cuboidal to low columnar epithelium showing pale or eosinophilic, “ground glass” cytoplasm (Figs. 2.17 and 2.18, e-Figs. 2.70 and 2.71). Nuclei are round without prominent nucleoli. Foci of dysplasia/carcinoma are commonly encountered. Low-grade and highgrade dysplasia are seen in 12% and 39% of the cases, respectively (31), while invasive carcinoma is associated with 12% to 47% of the lesions, depending on the authors’ criteria for carcinoma; using Western criteria, the figure is probably closer to 10% to 15% (31,34) (Fig. 2.19 and 2.20, e-Figs. 2.72-2.77). PGA’s show coexpression of MUC6 (Fig. 2.18, e-Fig. 2.71) (marker of pyloric gland mucin) and MUC5AC (marker of foveolar mucin) and lack expression of MUC2 (marker of intestinal mucin) and
CDX2. While foveolar-type gastric adenomas show MUC5AC expression, they lack expression of MUC6 and MUC2(31). Some cases, however, show areas of transition from gastric to intestinal differentiation and these foci may show immunolabeling with MUC2 and CD10 (35). As with other
types of adenomas, complete excision of PGA with biopsy of the background flat mucosa is appropriate in these patients.
CDX2. While foveolar-type gastric adenomas show MUC5AC expression, they lack expression of MUC6 and MUC2(31). Some cases, however, show areas of transition from gastric to intestinal differentiation and these foci may show immunolabeling with MUC2 and CD10 (35). As with other
types of adenomas, complete excision of PGA with biopsy of the background flat mucosa is appropriate in these patients.
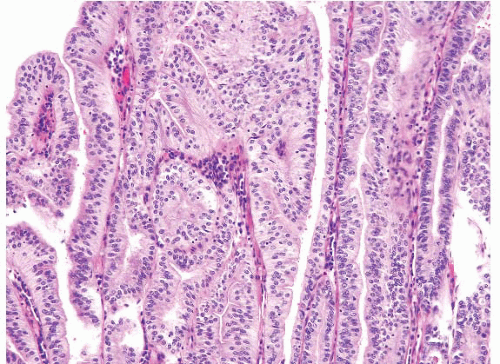 FIGURE 2.19 High-grade dysplasia in pyloric gland adenoma. Though the nuclei are still small, there is nuclear stratification and some loss of polarity and this would be regarded as high-grade dysplasia in a pyloric gland adenoma. Compare this image to that in Figure 2.17, in which the nuclei are arranged in a monolayer. |
Oxyntic Gland Polyp/Adenoma
Encountered in the literature as “chief cell hyperplasia with structural and nuclear atypia” and “chief cell proliferation of the gastric mucosa,” this peculiar lesion was initially described in 2003 by Müller-Höcker and Rellecke and later in 2005 by Matsukawa et al. as an unusual variant of fundic gland polyp occurring in the cardia/corpus (36,37). Initial reports described anastomosing cords of irregularly branched tubules composed of monotonous epithelial cells with central, round nuclei and amphophilic cytoplasm associated with oxyntic and foveolar microcysts. The case described by Matsukawa et al. reports nuclear atypia in the form of nuclear stratification, suggestive of a tubular carcinoma. However, mitotic figures and Ki67 labeling index were low in both reports. Chief cell origin for these proliferations is supported by ultrastructural findings and positive immunohistochemical staining for pepsinogen-I. However, scattered parietal cells were observed within these proliferations. In both of these reports an adjacent fundic gland polyp could be identified (36,37). More recently, Ueyama et al. described similar lesions with the term “gastric adenocarcinoma of fundic gland type.” In their report of 10 cases, the Ki67 labeling index was also low and none of the patients died or suffered recurrences during the 10- to 70-month follow-up period (38). We have encountered similar polyps (Fig. 2.21, e-Figs. 2.78-2.83) and diagnosed them as “oxyntic gland polyp.” Because of their rarity and lack of
long-term studies, we suggest endoscopic follow-up to ensure that the lesion has been completely removed but would not suggest additional intervention.
long-term studies, we suggest endoscopic follow-up to ensure that the lesion has been completely removed but would not suggest additional intervention.
ADENOCARCINOMA
Gastric adenocarcinoma is the second leading cause of cancer death and the fourth most common cancer worldwide after lung, breast, colon, and rectum (39). According to SEER data, the year 2010 would see an estimated 21,000 new cases of gastric cancer in the United States, with 10,570 of those patients dying of their disease. There is wide geographic variation and high-risk areas include China, Japan, Eastern Europe, and parts of South and Central America (39). Reasons for this geographic variation are uncertain, but environmental factors (H. pylori infection, dietary factors) seem to play a significant role as migrants from high- to low-risk areas show a decrease in gastric cancer risk. Patients with AMAG are also at risk for gastric neoplasia and the crude prevalence of adenocarcinoma in patients with AMAG has been estimated at 2% (7). Over the past several decades, the epidemiological profile of gastric cancer has changed dramatically. For example, a clinicopathologic study from a high-risk area (40) found that the mean age at the time of diagnosis increased from 53.4 to 57.4 over a 20-year period. Similarly, early gastric cancer (T1) increased from 24.8% to 48.9% and the overall 5-year survival rate increased from
64% to 73.2% in patients aged 40 years and older during the same time period. While the incidence of gastric adenocarcinoma outside the gastric cardia is decreasing, the incidence of gastric cardia adenocarcinoma is increasing (40).
64% to 73.2% in patients aged 40 years and older during the same time period. While the incidence of gastric adenocarcinoma outside the gastric cardia is decreasing, the incidence of gastric cardia adenocarcinoma is increasing (40).
Survival after resection of gastric adenocarcinoma has been studied in multiple series, with patients stratified by stage of disease, Lauren tumor type (diffuse vs. intestinal) (41,42), tumor location, time period, and administration of adjuvant therapy. All studies uniformly show an association between stage and survival. The Lauren classification correlates significantly with survival, in that intestinal-type tumors are associated with longer survival than diffuse-type tumors (42). The Lauren classification, viewed simply, classified gastric cancers into (a) those that have intestinal differentiation, form a large mass, and arise in a backdrop of intestinal metaplasia (intestinal type) and (b) those that apparently arise de novo




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree